Abstract
The importance of chemical communication in ants has been widely reported, but acoustic communication also has a significant role in those subfamilies that show this behaviour. In this study, we expand the knowledge about stridulatory organs of the subfamily Myrmicinae (Hymenoptera: Formicidae) with the first description of the stridulatory organs of five species (Aphaenogaster striativentris, Goniomma blanci, G. hispanicum, Oxyopomyrmex salulcyi and Pheidole pallidula). Subsequently, we made a morphometric study of 16 species to make comparisons of their stridulatory organs. Scanning electron microscope (SEM) photographs were taken for the morphological study, and the head and dimensions of the pars stridens, together with measurements of the striae, were used for the morphometric analysis. The five myrmicines studied show typical stridulatory organs for Formicidae. We also add two genera (Goniomma and Oxyopomyrmex) to the list of genera that are known to have stridulatory organs. The morphometric study shows a positive correlation between the body size and the size of the pars stridens and between the length and width of the pars stridens. However, a relation between the size of the stridulatory organs and the size of the striae of the pars stridens was not detected. Stridulatory organs have been shown to have significant interspecific differences in size.
Introduction
Ants are Hymenoptera belonging to a single family, the Formicidae. Because they are eusocial insects, ants need efficient communication systems, enabling them to keep the complex hierarchy of the colony and the synchronisation of tasks. The main communication system in these insects is chemical, but it is not the only one and acoustic communication also plays an important role in some subfamilies, as has been shown by many authors such as Janet (Citation1893), Markl (Citation1972) and Hölldobler and Wilson (Citation1990).
Ants have two ways of producing sounds: drumming different parts of the body against the substrate (Hölldobler & Wilson Citation1990), and stridulation. Stridulation consists in rubbing a part of the insect’s body with other parts to produce sound (Hickling & Brown Citation2000). In ants, stridulatory organs are present in the subfamilies Ponerinae, Myrmicinae, Pseudomyrmecinae (Markl Citation1968), and – anatomically different – in the monotypic Nothomyrmeciinae (Taylor Citation1978).
Ant stridulatory organs have been studied since the late 19th century, and have been described and analysed in several species. The first descriptions of the stridulatory organs were made by Lubbock (Citation1877), Janet (Citation1893) and particularly by Markl (Citation1968), being later completed by authors such as Schillinger and Baroni Urbani (Citation1985), Giovannotti (Citation1996), Pavan et al. (Citation1997), Grasso et al. (Citation1998), Hernández et al. (Citation2002), Álvarez et al. (Citation2006), Ruiz et al. (Citation2006), Álvarez (Citation2009) and Ferreira et al. (Citation2010). Ant stridulatory organs are formed by two parts: the first is a scraper named the plectrum, formed by a thickening of the posterior edge of the third abdominal segment (postpetiole). This part of the ant does not present specially modified structures. The second part is the pars stridens formed by the pretergite of the fourth abdominal segment (first gastral segment), that shows a variable area where the cuticular ridges adopt the structure of thin parallel ribs (striae), which result in a striate and regular pattern. Under the pars stridens, tegumentary reliefs with the appearance of supporting structures are found, which were described for the first time by Álvarez (Citation2009), and were named “pillars”. These pillars are tegumentary foldings that, because of their location under the pars stridens, were stated to have an amplifying function by Álvarez (Citation2009). The stridulatory sound is produced by means of dorso–ventral movements of the gaster, and was described by Spangler (Citation1967) in the species Pogonomyrmex occidentalis (Cresson, 1865). Previous studies show that the stridulatory organs are present in every caste of the species that have it, for example in Atta cephalotes (Linnaeus, 1758) (Markl Citation1973) and Messor barbarus (Linnaeus, 1767) (Hernández et al. Citation2002). In behavioural studies, stridulation has been shown to have a relation with specific behaviour in A. cephalotes, where it occurs while the ants are cutting leaves (Roces et al. Citation1993), and is synchronised with mandible vibration which results in more efficient leaf-cutting (Tautz et al. Citation1995).
Acoustic communication in Formicidae has nowadays been extensively studied and documented. Stridulatory organs of many species have been described, but there are still gaps in our knowledge of this feature, with many species still to be studied. Raignier (Citation1933) described in an extensive work the stridulatory organs of species belonging to 13 different genera: Aphaenogaster, Crematogaster, Formicoxenus, Messor, Myrmecina, Myrmica, Manica, Pheidole, Solenopsis, Stenamma, Strongylognathus, Temnothorax and Tetramorium. Although Raignier covered a large number of species, he only provided pictures of the stridulatory organs of the genus Messor. Schillinger and Baroni Urbani (Citation1985), Grasso et al. (Citation1998), Ruiz et al. (Citation2006) and Álvarez (Citation2009) added more species to those documented by Raignier. After Raignier’s compilation, detailed descriptions have increased the number of studied genera to 22: Rhytidoponera (Whelden 1956, Citation1960), Atta (Markl Citation1972), Acromyrmex (Kermarrec et al. Citation1976), Pogonomyrmex (Markl et al. Citation1977), Novomessor (Hölldobler et al. Citation1978), Streblognathus (Ware Citation1994), Ectatomma (Giovannotti Citation1996; Pavan et al. Citation1997), Pachycondyla (Giovannotti Citation1996; Pavan et al. Citation1997; Ferreira et al. Citation2010) and Paraponera (Giovannotti Citation1996).
Morphometric analyses are a powerful tool to draw conclusions about the biological relevance and function of morphological structures. These methods allow us to explore the characteristics of a structure using its measurements and to make comparisons between the different variables. Thus, it is possible to investigate inter- and intraspecific relationships with the use of stridulatory organs. Some studies have used this morphometric tool with stridulatory organs: Hernandez et al. (Citation2002) compares stridulatory organs in the different castes of Messor barbarus, and Ruiz et al. (Citation2006) perform interspecific comparisons, but within the same genus (Crematogaster auberti and C. scutellaris). Giovannotti (Citation1996) studies the relationship between different species of Ponerinae (Ectatomma quadridens, E. ruidum, E. tuberculatum, Pachycondyla apicalis and Paraponera clavata). Álvarez et al. (Citation2006) carried out comparative studies of Aphaenogaster senilis Mayr, 1853 workers from five different nests. However, the most comprehensive study is that of Álvarez (Citation2009), who described the stridulatory organs of 19 species of the subfamily Myrmicinae and performed a morphometric study to compare them.
It is important to broaden the knowledge about acoustic communication in ants if we want to understand better the complex life history of these insects, because in those subfamilies showing this form of communication, it is an important feature of their behaviour. All the reasons named before have promoted, in this research, the study of stridulatory organs in five ant species belonging to the Myrmicinae subfamily. Because stridulatory organs were not described previously in these five species, the objective is to check that they are similar to the typical structures in the Formicidae family, and to provide a detailed description of these structures. Moreover, using a morphometric analysis, we attempt to establish possible relationships between the size of the pars stridens and the average width of its striae with the average size of the individuals by measuring a variable indicative of body size, the cephalic width.
Material and methods
Morphological study
The samples of Iberian Myrmicinae for the morphological study came from different Spanish locations, listed in . Field-collected samples were preserved in 70% ethanol and were identified using the keys in Collingwood (Citation1978) and at www.hormigas.org.
Table I. Localities and coordinates where the species whose stridulatory organs have been described in this study were sampled.
For the scanning electron microscope (SEM) study of the stridulatory organs, the samples were cleaned with 70% ethanol and dried at room temperature for 24 hours for dehydration. Then, the gaster was separated from the body and this part was fixed to an electron microscopy stub with double-sided adhesive tape. Samples were then coated with a 102-Å gold layer with a SC502 Sputter Coater and studied with the microscope Hitachi S-3000N from the Servicio Interdepartamental de Investigación (SIDI) in the Universidad Autónoma de Madrid (Spain). The most relevant parts of the ants for the study were photographed: the head, which was used in the morphometric study, and the pars stridens and surrounding structures. Specimens studied under SEM were kept in the Entomology Collection of the Biology Department in the Universidad Autónoma de Madrid.
The five species of myrmicines studied were: Aphaenogaster striatriventris Forel, 1895, Goniomma blanci (André, 1881), G. hispanicum (André, 1883), Oxyopomyrmex saulcyi Emery, 1889 and Pheidole pallidula (Nylander, 1849).
Morphometric study
For the comparative study of the stridulatory organs in different species of myrmicines, we used SEM pictures from the morphological study and other pictures compiled by Álvarez (Citation2009) and Castro (unpublished). This allowed us to include the following species for the morphometric analysis: Aphaenogaster iberica, A. senilis; Crematogaster auberti Emery, 1869; C. scutellaris (Olivier, 1792); Messor barbarus; M. bouvieri Bondroit, 1919; M. capitatus (Latreille, 1798); M. lobicornis Santschi, 1939; Myrmica sabuleti Meinert, 1861; Temnothorax formosus Santschi, 1909; Tetramorium caespitum (Linnaeus, 1758); and T. lanuginosum Mayr, 1870. In total, we collected data on 16 species of myrmicines from nine genera.
We measured the following relevant morphometric variables: cephalic width (mm), width and length of the pars stridens (mm; ) and average width of the striae of the pars stridens (µm). The striae width resulted from the arithmetic average of the measurement of 10 striae. The latter measurement is important because striae width might be related with the frequency of the sound produced by the stridulatory organs. In species with a polymorphic worker caste (Messor and Pheidole), we selected individuals from the minor and major caste.
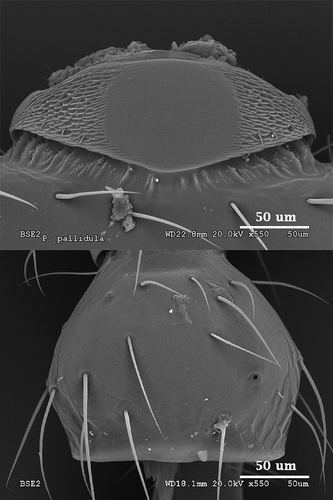
Figure 1. Scanning electron microscopy photographs of the head of Aphaenogaster senilis and the stridulatory organ of Myrmica sabuleti, showing the way measurements were taken for the morphometric study: cephalic width and pars stridens length (vertical) and width (horizontal).
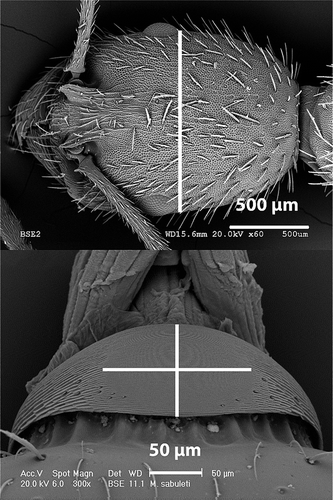
For all the variables, a normality test was made using the Kolmogorov–Smirnov test, and the correlation between different variables was tested with Pearson’s correlation. To study the interspecific variations, we used the Kruskal–Wallis test, a nonparametric method to test if a set of data came from the same population. Finally, we created a scatter plot to study the relationship of two quantitative variables, selecting in this case the relation between the length of the stridulatory organs and the cephalic width of the individual. All statistic analyses were performed with the package SPSS Statistics 19.
Results
Description of the stridulatory organs
A comparative description of the stridulatory organs was made based on SEM photographs from five species belonging to four different genera. shows the measurements (average and standard deviation) for all the species: the cephalic width (which gives an estimation of the size of the ant), length and width of the pars stridens and average width of the ridges.
Table II. Average values of the morphometric variables of the studied myrmicine species and their standard deviation (SD).
Genus Aphaenogaster Mayr, 1853
A. striativentris
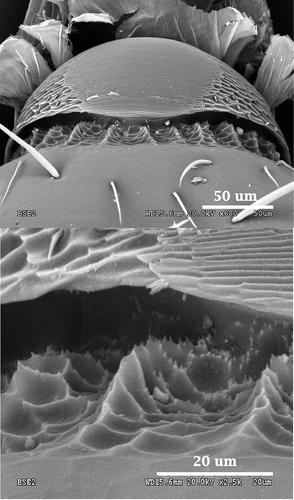
In this species, the pars stridens is oval, but in this case it is wider than long, with a length of 0.09 mm and a width of 0.14 mm (). The striation is thinner than the values obtained for A. senilis by Álvarez (Citation2009), with a ridge average width of 0.90 µm (). Pillars are more prominent than in A. senilis but, as in other species of Aphaenogaster, they are scarcely conspicuous and have a more diffuse sculpture ().
Genus Goniomma Emery, 1895
G. hispanicum
It has a pars stridens much wider than long (width 0.11 mm and length 0.06 mm; ). This is the species with the thinnest striation in the morphometric study, together with Messor capitatus, Pheidole pallidula and Tetramorium caespitum, with an average ridge width of 0.80 µm (). The outline of the pars stridens is clearly rounded. Pillars are very strong and the number of teeth on their surface is higher than in G. blanci. A geometric pattern can be observed on the base of the pillars ().
G. blanci
In this species, the picture of the pars stridens shows half of the structure because the other half was partially damaged in the single individual that could be studied. However, it shows the relevant parts of the whole structure. We can appreciate that the striation is somewhat thicker than in G. hispanicum (1.10 µm in G. blanci and 0.80 µm in G. hispanicum). Pillars are conspicuous with many teeth on their surface and with the base showing a cell-shaped pattern ().
Genus Oxyopomyrmex André, 1881
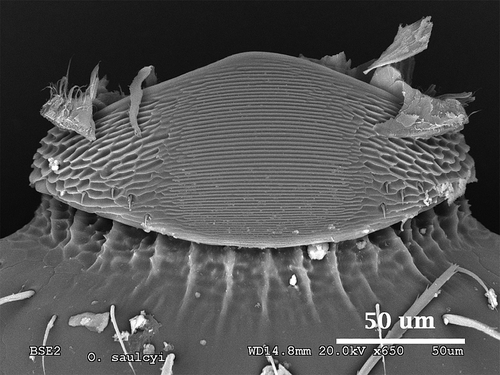
O. saulcyi
This species shows a peculiar pars stridens with a pentagonal shape, a rounded apex pointing to its anterior part and the wider side on its posterior end. Width and length have very similar values: 0.08 mm and 0.07 mm, respectively (). The edges of the pars stridens are less defined than in the rest of the studied species. Striation is very pronounced, more than in most of the species described before, with an average of 1.50 µm of ridge width (). The ridges and the interspaces get thinner as we move closer to the anterior part of the structure. Pillars are strongly marked, with several teeth and geometric patterns on their base ().
Genus Pheidole Westwoood, 1839
P. pallidula
This species also presents a pentagonal pars stridens, but in this case the pentagon is inverted, with the apex pointing to the gaster. This shape is much more marked in the major than in the minor forms. Width and length are quite similar, but it is slightly wider (0.08 mm width and 0.07 mm length; ). Striation, as mentioned before, is among the thinnest of all the considered species. Pillars are noticeable and thin, with scattered teeth situated mostly in the lateral parts of the stridulatory organs (). The plectrum is shown in so that its structure can be appreciated.
Morphometric analysis
In total, we studied 49 individuals from 16 species. The width and length of the stridulatory organs were positively correlated (Pearson’s correlation coefficient: r = 0.516, p = 0.001). Correlation between the species’ cephalic width and the average width of the pars stridens was also positive and significant (Pearson’s correlation coefficient: r = 0.604, p < 0.0001). As the length and width of the stridulatory organs are associated, we can extrapolate the results from one variable to another, showing that larger individuals have larger stridulatory organs. On the other hand, the correlation between the size of the ant (cephalic width) and the width of the ridges of the pars stridens was not significant (Pearson’s correlation coefficient: r = 0.187, p = 0.254), showing that the width of the ridges is not related to ant size.
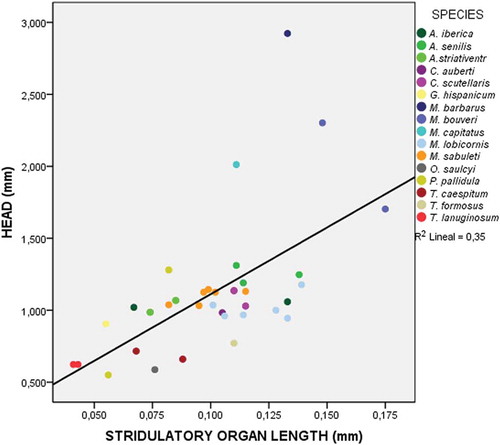
The Kruskal–Wallis test shows significant global differences between the stridulatory organs from the different species (K = 33.073; p = 0.004). Finally, the scatter plot () shows a significant correlation (R2 = 0.365; p < 0.001) between the two selected variables (length of the stridulatory organs and cephalic width per individual).
Discussion
The most generalised opinion among ant specialists is that acoustic communication has very little relevance when compared with chemical communication (Hölldobler & Wilson Citation1990). For example, Lenoir et al. (Citation2001) reviewed the importance of mechanisms that take place in the ant colony for chemical recognition of individuals within the nest and the strategies that social parasites use to break into the system. However, the fact that stridulation appears in most of the species of the Myrmicinae subfamily, and in all the castes (Markl Citation1973; Hernández et al. Citation2002), seems to indicate that it may have more importance in the communication of this group of ants than was previously thought.
Regarding the morphological details of the stridulatory organs, in the studies carried out by Grasso et al. (Citation1998) and Álvarez (Citation2009) there are setae in the lower third of the pars stridens, present in all the species. These authors propose a proprioceptive function for them. We verify the presence of these setae in all the described species, in variable numbers. These setae would generate information about the position of the pars stridens, and would not be needed in those species that lack this structure.
The research of Álvarez (Citation2009) described for the first time specific cuticular formations under the pars stridens, which support this structure, and named them “pillars”. It was suggested that the function of the pillars, together with the spaces they leave between them, is to act like a sound box when the sound is produced. We have verified the presence of pillars in all of the studied species except Messor barbarus and M. bouvieri. Also, in other species of the genus Messor, these structures are substantially reduced in size in comparison with those of other genera (see also Álvarez Citation2009). There seems to be a relation between the size of the ant and the presence of pillars, because most of the larger species from the genus Messor have reduced or absent pillars. However, in the images from Álvarez (Citation2009), the species M. lobicornis, which is smaller, shows very noticeable pillars.
The images of Goniomma blanci, G. hispanicum, Oxyopomyrmex saulcyi and Pheidole pallidula show small teeth on the pillars of stridulatory organs that do not appear in previous descriptions. These species are among the smallest ones from our study, and thus ant size may favour the presence of these small cuticular ridges on the pillars, which may have some effect on the character of the sound produced by the stridulatory organs. It would be interesting to perform a comparative acoustic analysis with other species without teeth to understand about the meaning of these structures, described here for the first time.
In previous studies, the possibility that the size of the stridulatory organs is related to the size of the individual has been discussed. Some authors found a correlation between these two variables for several species: Atta cephalotes and Acromyrmex octospinosum Reich, 1793 (Kermarrec et al. Citation1976) and Messor capitatus and M. structor (Latreille, 1798) (Schillinger & Baroni Urbani Citation1985). However, Álvarez (Citation2009) rejected this relationship in her work describing many species of the genera Aphaenogaster, Crematogaster, Messor, Myrmica, Leptothorax and Tetramorium, and a similar conclusion was reached by Ferreira et al. (Citation2010) in their study about cryptic species. Our results suggest that there is an association between the size of the individual and the size of its stridulatory organs, with larger stridulatory organs in larger species.
However, there is no relation between the size of the individuals and the width of the striae of its pars stridens, showing that this width can vary independently from ant size. In , we show that some large species like M. capitatus have one of the lowest values of striae width, while some small species like O. saulcyi have larger values. The value of striae width is directly related to the density of these structures per surface unit. Within the same genus, we also find striking differences, as can be seen in the genera Messor and Aphaenogaster. The striae width may have a relation with the frequency of the sound produced by the stridulatory organs, with wide striae probably producing low frequencies, and narrow ones high frequencies. The independence of striae width and ant size may therefore be related to the character of the sound produced, rather than a relation with the size of this trait. This would also explain why within the same genus we find variability in the size of the striae, because this would help to produce sounds that are distinct between related species.
All the results from our paper suggest that we should consider the possibility of stridulation having a greater relevance than currently recognised in the overall behaviour of myrmicine ants.
Conclusions
The study of five species of the Myrmicinae subfamily confirms that they have typical stridulatory organs for the Formicidae family. We describe for the first time the stridulatory organs of the following Iberian myrmicine ants: Aphaenogaster striativentris, Goniomma blanci, G. hispanicum, Oxyopomyrmex saulcyi and Pheidole pallidula. Moreover, we add two genera (Goniomma and Oxyopomyrmex) to the previous list of genera with described stridulatory organs.
New formations have been described on the pillars of the pars stridens as a result of our study. They consist of teeth on the surface of the pillars, and may have some relation with the production of sound.
There is a positive correlation between the size of the individual and the size of the pars stridens of the stridulatory organs, indicating that larger species have larger stridulatory organs. However, the average size of the ridges from the pars stridens does not depend on the size of the ant. There are significant interspecific differences in the size of the stridulatory organs.
Acknowledgements
José Francisco Gómez helped with the editing of photographs. Margarita González helped with statistic analyses. Esperanza Salvador, Isidoro Poveda and Enrique Rodríguez helped with the SEM photographs. Francisco M. Azcárate and Javier Seoane helped with several aspects of the work and provided specimens for the study. The following people collected samples of ants in different locations: Elia Alonso, Lidia Burguillo, Ester Castro, Pío Castro, José Francisco Gómez, Elena González de Heredia, Ana Herrero, Kirina Kubota, Sandra Padilla, Daniel Pinchete, Laura Rodríguez, Laura Ruipérez, Pilar Salazar, Rodrigo Sebastián, Beatriz Tomás, Antonio Tornero and Irene Tornero.
References
- Álvarez M. 2009. Estudio de la comunicación acústica en licénidos (Lepidoptera, Lycaenidae) y formícidos (Hymenoptera, Formicidae). PhD Thesis, Universidad Complutense de Madrid, Spain.
- Álvarez M, Martínez MD, Ruiz E, Hernández JM. 2006. Estudio comparado del Pars stridens en las obreras de cinco nidos de Aphaenogaster senilis Mayr, 1853 (Hymenoptera, Formicidae). Boletín de la Real Sociedad Española de Historia Natural 101:93–98.
- Collingwood CA. 1978. A provisional list of Iberian Formicidae with a key to the worker cast (Hym. Aculeata). Eos 52:65–95.
- Ferreira RS, Poteaux C, Delabie JHC, Fresneau D, Rybak F. 2010. Stridulations reveal cryptic speciation in neotropical sympatric ants. PLoS ONE 5:e15363.
- Giovannotti M. 1996. The stridulatory organs of five Ponerinae species. A SEM study. (Hymenoptera, Formicidae). Fragmenta entomologica 28:157–165.
- Grasso DA, Mori A, Le Moli F, Giovannotti M, Fanfani A. 1998. The stridulatory organs of four Messor ant species (Hymenoptera, Formicidae). Italian Journal of Zoology 65:167–174. doi:10.1080/11250009809386741.
- Hernández JM, Martínez MD, Ruiz E. 2002. Descripción del órgano estridulador en Messor barbarus (Linneo, 1767) (Hymenoptera, Formicidae). Anales de Biología 24:167–174.
- Hickling R, Brown RL. 2000. Analysis of acoustic communication by ants. The Journal of the Acoustical Society of America 108:1920–1929. doi:10.1121/1.1290515.
- Hölldobler B, Stanton RC, Markl H. 1978. Recruitment and food-retrieving behavior in Novomessor (Formicidae, Hymenoptera). Behavioral Ecology and Sociobiology 4:163–181. doi:10.1007/BF00354978.
- Hölldobler B, Wilson EO. 1990. The Ants. Cambridge, Massachusetts: Harvard University Press.
- Janet C. 1893. Note sur la production des sons chez les fourmis et sur les organes qui les produisent. Annales de la Société Entomologique de France 62:159–167.
- Kermarrec A, Mauléon H, Antun A. 1976. La stridulation de Acromyrmex octospinosus Reich. (Formicidae, Attini). Biométrie de l’áppareil stridulateur et analyse du signal produit. Insectes Sociaux 23:29–47. doi:10.1007/BF02283904.
- Lenoir A, D’Ettorre P, Errard C. 2001. Chemical ecology and social parasitism in ants. Annual review of Entomology 46:573–599. doi:10.1146/annurev.ento.46.1.573.
- Lubbock J. 1877. On some points in the anatomy of ants. Monthly Microscopical Journal 18:120–142. doi:10.1111/j.1365-2818.1877.tb00115.x.
- Markl H. 1968. Die Verständigung durch Stridulationssignale bei Blattschneiderameisen, II: Erzeugung und Eigenschaften der Signale. Zeitschrift für Vergleichende Physiologie 60:103–150. doi:10.1007/BF00878447.
- Markl H. 1972. Nesting ecology and the evolution of stridulation in ants. Proceedings of the 17th International Congress of Zoology, 24–30 September, 1972, Monaco:1–11.
- Markl H. 1973. The evolution of stridulatory communication in ants. Proceedings of 7th Congress of the International Union for the Study of Social Insects. Vol. 7. UK: London. pp. 258–265.
- Markl H, Hölldobler B, Hölldobler T. 1977. Mating behavior and sound production in harvester ants (Pogonomyrmex, Formicidae). Insectes Sociaux 24:191–212. doi:10.1007/BF02227171.
- Pavan G, Priano M, De Carli P, Fanfani A, Giovannotti M. 1997. Stridulatory organs and ultrasonic emission in certain species of Ponerine ants (Genus: Ectatomma and Pachycondyla, Hymenoptera, Formicidae). Bioacoustics 8:209–221. doi:10.1080/09524622.1997.9753363.
- Raignier A. 1933. Introduction critique à l’étude phonique et psychologique de la stridulation des fourmis. Brotéria, Ciências Naturais 29:51–82.
- Roces F, Tautz J, Hölldobler B. 1993. Stridulation in leaf-cutting ants: short-range recruitment through plant-borne vibrations. Naturwisenschaften 80:521–524. doi:10.1007/BF01140810.
- Ruiz E, Martínez MH, Martínez MD, Hernández JM. 2006. Morphological study of the stridulatory organs in two species of Crematogaster genus: Crematogaster scutellaris (Olivier 1972) and Crematogaster auberti Emery 1869 (Hymenoptera: Formicidae). Annales de la Société Entomologique de France (n.s) 42:99–105. doi:10.1080/00379271.2006.10697454.
- Schillinger E, Baroni Urbani C. 1985. Morphologie de l’organe de stridulation et sonogrammes comparés chez les ouvrières de deux espèces de fourmis moissonneuses du genre Messor (Hymenoptera, Formicidae). Bulletin de la Société Vaudoise des Sciences Naturelles 77:377–383.
- Spangler HG. 1967. Ant stridulations and their synchronization with abdominal movement. Science 155:1687–1689. doi:10.1126/science.155.3770.1687.
- Tautz J, Roces F, Hölldobler B. 1995. Use of a sound-based vobratome by leaf-cutting ants. Science 267:85–87. doi:10.1126/science.267.5194.84.
- Taylor RW. 1978. Nothomyrmecia macrops: A living fossil ant rediscovered. Science 201:979–985. doi:10.1126/science.201.4360.979.
- Ware AB. 1994. Factors eliciting stridulation by the ponerine ant Streblognatus aethiopicus Smith (Hymenoptera, Formicidae). African Entomology 2:31–36.
- Whelden RM. 1960. The anatomy of Rhytidoponera metallica F. Smith (Hymenoptera, Formicidae). Annals of the Entomological Society of America 53:793–808. doi:10.1093/aesa/53.6.793.