Abstract
Many cryptic species have been recently recognized in the European butterfly fauna, and climatic determinants have been investigated to explain the usually parapatric distribution of these species. Cryptic species of Leptidea, however, are often sympatric throughout Europe, with variable ecological interactions and segregation patterns. Focusing on the highly diverse landscape between the Alps and the Adriatic Sea, we evaluated the climatic and geomorphological correlates of the fine distribution of L. sinapis and L. juvernica, by means of discriminant analysis on 151 sites in a representative region. In addition to reliable records selected from collections and from the literature, we identified 104 specimens from 89 sites by analysing the morphometric variation of male and female genitalia and filtering for consistency between all alternative identification methods hitherto developed for this species complex. We found that L. sinapis is widespread from the coastal plain to the inner mountains, up to 1800 m above sea level (a.s.l.), whereas L. juvernica is restricted to the mountainous areas, inhabiting also the bottom of pre-alpine valleys, as low as 200 m a.s.l. Despite that the two species are often syntopic, L. juvernica is more frequent in areas with more intense rainfall and steeper slopes, whereas L. sinapis may occur in a broader range of conditions, also in warmer sites with a wider temperature range.
Introduction
Cryptic species have been detected and distinguished during the last few decades even in supposedly well-known animal groups, like butterflies in comparison with most other invertebrates. This has occurred also within the European fauna, which is one of those most thoroughly investigated (Dincă et al. Citation2011). As a consequence, biogeographical and evolutionary insights need revision upon a substantially improved understanding of the actual continental biodiversity (Vodă et al. Citation2014), and the conservation value of regional populations needs re-assessment taking into account a more complex pattern of differentiation than previously thought (Dapporto et al. Citation2012).
Also for European butterflies, evidence is accumulating that phenotypically similar species that have remained hitherto undistinguished, may nevertheless differ significantly in ecological tolerance, particularly with respect to climatic factors (e.g., Friberg et al. Citation2008a; Zinetti et al. Citation2013; Vodă et al. Citation2015). Understanding how climatic conditions may affect the geographical distribution of different cryptic species is an urgent goal, especially when considering the recent acceleration of climatic changes (Mallet et al. Citation2011; Devictor et al. Citation2012).
Differences in climatic niches between cryptic species of European butterflies have been investigated mainly by contrasting parapatric species (e.g., Zerynthia polyxena and Z. cassandra, Zinetti et al. Citation2013; Aricia agestis and A. cramera, Polyommatus icarus and P. celina, Vodă et al. Citation2015), because cryptic species rarely coexist in broad areas and are mutually exclusive on islands (Vodă et al. Citation2014). Pairs of cryptic species in the genus Leptidea, however, are sympatric in wide regions, and often syntopic, offering the possibility to test whether differences in climatic tolerance play a role in maintaining a sympatric scenario.
The species complex including Leptidea sinapis (Linnaeus, 1758), L. juvernica Williams, 1946 and L. reali Reissinger, 1990 has recently become a model system for research on speciation and on the role of ecological differentiation and behavioural interactions in the coexistence of cryptic species (Freese & Fiedler Citation2002; Friberg et al. Citation2008a, Citation2008b; Friberg & Wiklund Citation2009; Dincă et al. Citation2013). As far as is known, L. sinapis is widespread in Europe and broadly sympatric with either L. juvernica or L. reali, whereas the latter two species are parapatric (Dincă et al. Citation2013). While L. juvernica and L. reali can be distinguished from each other only by means of karyological or molecular analyses (Dincă et al. Citation2011), L. sinapis can be sorted out effectively from syntopic L. juvernica or L. reali also based on the average smaller size of male and female copulatory apparatuses (e.g., Lorković Citation1993; Freese & Fiedler Citation2004; Fumi Citation2008), as corroborated by genetic analyses (Verovnik & Glocovčan Citation2007; Dincă et al. Citation2011).
The ecological separation between sympatric species of Leptidea is variable throughout Europe (Friberg et al. Citation2008b, Citation2013) and up to now it has been investigated for a few areas in the Pyrenees (Vila et al. Citation2003), French Prealps (Amiet Citation2004), Czech Republic (Beneš et al. Citation2003) and central Sweden (Friberg et al. Citation2008a). Our study focused on the Alpine–Adriatic area, i.e. the region extending from the Eastern Alps to the coast of the northern Adriatic Sea. In contrast with its narrow extent, this area is characterized by strong diversity in geomorphological features and local climatic conditions. Previous samplings of Leptidea populations have provided morphometric and molecular evidence that this area is colonized by L. sinapis and L. juvernica (Dincă et al. Citation2013), and suggest some geographical and ecological separation between these species (Fracasso Citation2002). Our aim was to evaluate whether and how the two cryptic species segregate in this area with respect to the climatic diversity, and which are the major factors explaining their relative distribution.
Material and methods
Study area and sampled sites
We considered a total of 151 sites inhabited by Leptidea populations, scattered over the entire territory of the Veneto region (Italy). This region was selected as suitable for our aims because its environmental diversity, especially with respect to geomorphological and climatic conditions, is representative of a broader Alpine–Adriatic area. Additionally, diffuse field surveys have been recently carried out within a more general research program on the butterfly fauna (Bonato et al. Citation2014), which allowed us to sample quite uniformly throughout the territory.
For 89 of the 151 sites, we directly examined 1–2 specimens, for a total of 104 specimens (71 males and 33 females). Sampling on multiple sites has been preferred to sampling multiple specimens per site, in order to maximize the geographical and environmental diversity in the sample. Most specimens (70%) were collected in the field during the last decade; the remainder was selected from institutional collections (including all major butterfly collections hosted in civic museums of natural history; see Acknowledgements). Among all other records of Leptidea species, either published or not, for which we could not examine specimens directly, we selected only those referring to specimens identified by experienced entomologists upon the same morphometric parameters and criteria that we adopted (described below).
When the location of a site was not acquired directly on the field by a global positioning system (GPS) instrument, it was estimated from the toponym, referring to the “Cartografia Tecnica Regionale Numerica” produced by the Veneto regional administration. All of the sites were located with a precision of at least 1 km and were > 1 km apart. Data were managed in a geographic information system (GIS), by means of the software “QGIS”.
Species identification by morphometric analysis
The copulatory apparatuses were macerated in a 25% water solution of sodium hydroxide (NaOH) for 15 min at 70°C, then dissected, cleaned and eventually included in ethylene glycol, without any cover to avoid shape alterations. Lateral view photos were taken by means of a digital camera (Leica DFC 420) through a stereoscope (Leica DM-LB). The following morphometric parameters were measured on the photos by a single person (F. Gallo), to the nearest 0.01 mm, by means of the software “Uthscasa Image Tool”: length of ductus bursae in the females; length of aedeagus, saccus, uncus and width of vinculum in the males (terminology according to Higgins Citation1975).
Each of the five morphometric parameters was considered separately for species identification only when the frequency distribution of the measures was found to be obviously bimodal, with a “gap” separating two discrete intervals, each one encompassing a mode. As a conventional criterion, the “gap” was defined as the widest interval between the two modes including no more than a single isolated record, i.e. a record separated from the other records by > 0.05 mm. Following a conservative approach, a specimen with an isolated value included in the gap was not attributed to a species. For the males, the species identification obtained from the analysis of single parameters was checked for consistency with the identification obtained from all other alternative methods employed by other authors on Leptidea, i.e. (i) graphical evaluation of the bivariate distribution of the lengths of aedeagus and saccus (e.g. Verovnik & Glocovčan Citation2007), and (ii) calculation of the discriminant function obtained by Fumi (Citation2008) on 320 specimens of Leptidea from central Italy and based on the measures of aedeagus, saccus, uncus and vinculum. Fumi’s discriminant function was actually developed by comparing specimens of L. sinapis with specimens of L. reali, not L. juvernica; nevertheless, it is expected to be effective to distinguish L. sinapis from both other species, because no morphometric differences have been found to date in the copulatory apparatuses between L. reali and L. juvernica. Following a conservative approach, males were identified as either L. sinapis or L. juvernica only when no incongruence resulted from all three of these alternative methods (single morphometric parameters, combination of aedeagus and saccus, and discriminant function).
Discriminant analysis of climatic parameters
To describe the local climatic conditions, the following 11 variables were estimated for each site: annual rainfall; minimum, average and maximum annual temperatures; minimum and maximum summer temperatures; temperature range in the entire year, in spring and in summer; slope and orientation (angular direction from the north), in a circular area of about 600 m2 around the site. For the first nine variables, we considered mean values estimated between more years (Barbi et al. Citation2011, Citation2013). For the latter two variables, we considered maximum values calculated from the Digital Terrain Model of the Veneto region, by means of the software “QGIS” and “GRASS”.
To evaluate differences in the climatic conditions between the sites inhabited by L. sinapis and those inhabited by L. juvernica, a discriminant analysis was performed on all 11 variables. The analysis was run (i) on all sites in the entire territory of Veneto, and (ii) only on the sites in the hilly and mountainous part of Veneto, to the exclusion of the lowland sites. To cope with the assumption of normality for the frequency distribution of the variables, the following transformations were applied: [] for rainfall; [
] for temperatures and ranges; [
] for slope; no transformation for orientation, because the frequency distribution was found approximately normal. Data were also standardized. The discriminant analysis was performed with the software “STATISTICA 8.0”.
Results
Morphometric distinction between Leptidea species
Among the morphometric parameters measured on the copulatory apparatuses, we obtained an overtly bimodal frequency distribution only for the length of ductus bursae in the females and the length of aedeagus in the males, with a gap adequate to sort out the specimens into two morphotypes (according to the criteria described in the Material and methods). For the ductus bursae we found a gap in the range 0.74–0.89 mm (n = 33), and for the aedeagus a gap in the range 1.80–1.96 mm (n = 71), in both cases with a single isolated record within the gap. Consequently, the ductus bursae allowed us to identify 32 out of 33 females, whereas the aedeagus suggested a species identity for 70 out of 71 males. Male identifications were confirmed by the graphical analysis of the combined variation of the lengths of aedeagus and saccus (). For the same males, Fumi’s (Citation2008) discriminant function resulted in a bimodal frequency distribution, with two completely separated groups, also with respect to the threshold value (1.084) estimated by Fumi between L. sinapis and L. reali, which is undistinguishable from L. juvernica in morphometric traits. The species identity suggested by the discriminant function was different for a single male from that suggested from the aedeagus alone and the combined aedeagus and saccus ().
Figure 1. Length of aedeagus and saccus in males of Leptidea from the Veneto region. The species identity refers to the consensus between all the three methods employed (see Material and methods).
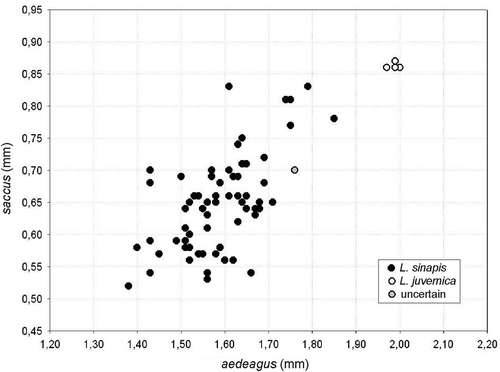
Overall, the morphometric analysis of the copulatory apparatuses allowed us to identify 102 out of 104 specimens, including 94 specimens of L. sinapis and 8 specimens of L. juvernica, with a similar efficacy between males and females (χ2 = 0.34; d.f. = 1; p = 0.56).
Climatic niche separation between Leptidea species
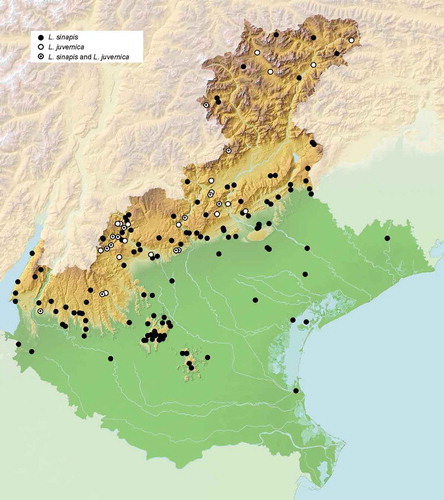
By considering all 151 sites from where specimens of Leptidea were identified at the species level, either by us or by others (), L. sinapis was found more frequently (in 133 sites) than L. juvernica (in 31 sites).
Sites inhabited by L. sinapis are between 0 and 1800 m above sea level (a.s.l.), and those inhabited by L. juvernica are between 200 and 1900 m a.s.l. L. sinapis was found in both the lowland and the hilly and mountainous territory, whereas L. juvernica was detected only within the latter, mainly along valleys running longitudinally through the hilly and montane prealpine belt, to the exclusion of the warmer and more isolated groups of hills, such as the Berici and the Euganei hills.
The analysis of variance of 11 climate-related variables (see Material and methods) revealed that the sites inhabited by L. sinapis differ significantly on average from those inhabited by L. juvernica for all variables with the exception of the orientation (F (1, 162) = 0.21; p = 0.65). Accordingly, the latter parameter was excluded from the discriminant analysis. On average, the temperatures are higher and span a broader range in the sites inhabited by L. sinapis, whereas the rainfall is more abundant and the slopes are steeper in the sites inhabited by L. juvernica (). In general, the average climatic conditions were found to be significantly different between the sites colonized by the two species (Wilks’ λ = 0.85; F (10, 153) = 2.75; p = 0.004). The discriminant analysis performed on the 10 variables produced a function with moderate discriminant power (eigenvalue = 0.18; canonical correlation R = 0.40), which accounts for 38% of the overall variance (). The correlation coefficients between the discriminant function and the variables () indicated that the discriminant function correlates positively with rainfall and slope, and negatively with temperatures and temperature ranges.
Table I. Sites inhabited by Leptidea sinapis and L. juvernica in the Veneto region: differences in climatic variables and results of the discriminant analysis. SD = standard deviation; T = temperature.
Figure 3. Frequency distribution of the values of the discriminant function obtained from the climatic variables (see Material and methods), in the sites inhabited by Leptidea sinapis and in those inhabited by L. juvernica.
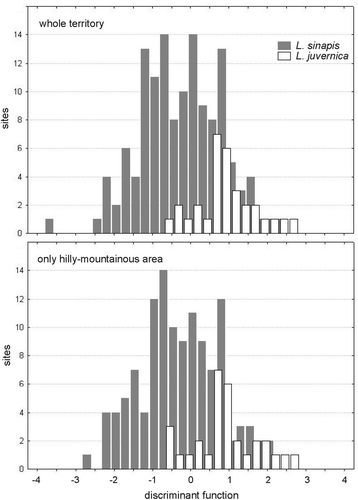
We obtained very similar results when considering only the 139 sites in the hilly–mountainous part of Veneto: the analysis of variance highlighted similar differences between the sites inhabited by the two species; the climate-related variables were significantly different between the two species with the single exception of the orientation (F (1, 150) = 0.41; p = 0.52); the multivariate analysis of the 10 variables confirmed that the sites inhabited by the two species differ significantly in the overall climatic conditions (Wilks’ λ = 0.85; F (10, 141) = 2.44; p = 0.010); the discriminant function had a similar discriminant power, even though slightly lower (eigenvalue = 0.17; R = 0.38; 27% of the total variance), with similar correlations with the climatic variables ().
Discussion
Morphometric differences between Leptidea species
Sorting out individuals in the species complex including L. sinapis, L. reali and L. juvernica is a non-trivial task if one needs to rely only on anatomical characters. Nevertheless, phenotype-based identification is often preferable to a genetic screening, and is sometimes the only feasible approach, especially when dealing with older specimens or a large number of specimens, because molecular techniques may be poorly effective on tissues that have not been preserved adequately, and costs increase significantly with the number of samples. As a consequence, different authors have tested the diagnostic value of different phenotypic characters regarding wing colour (e.g., Freese & Fiedler Citation2004), anatomy of genitalia (e.g., Lorković Citation1993; Mazel & Leestmans Citation1999) and even characters exhibited in the egg, larva or pupa (e.g., Friberg Citation2007). Up to now, no single character or combination of characters was found to be completely effective to discriminate the Leptidea triplet, but morphometric measures of the copulatory organs have been demonstrated to allow us to sort out most specimens of L. sinapis from sympatric and sometimes syntopic L. juvernica or L. reali, and the validity of this method has been corroborated by molecular analyses (Verovnik & Glocovčan Citation2007; Dincă et al. Citation2011). However, different authors working in different regions have adopted different morphometric criteria and thresholds of measures to separate the species. Rigorous identification methods based on adequate statistical analyses have also been proposed (Freese & Fiedler Citation2002 for L. sinapis vs. L. juvernica; Fumi Citation2008 for L. sinapis vs. L. reali). However, they have been developed for populations inhabiting limited areas (northern Bavaria, Freese & Fiedler Citation2002; central Italy, Fumi Citation2008), so that their applicability to other European regions is uncertain, because some geographical variation in the size of the copulatory apparatuses is known within a single species (Lorković Citation1993; Lelo Citation2002). As a consequence, we developed an original and explicit operative protocol (see Material and methods), by taking into account all morphometric parameters so far demonstrated to be of partial diagnostic value, and – following a conservative approach – by filtering for consistency among all alternative methods adopted by different authors. Despite being conservative in principle, our approach allowed us to identify 98% of all specimens (more than 100) of both sexes.
For the morphometric parameters of the copulatory apparatuses, we estimated between-species gaps that are reasonably consistent with the other threshold values hitherto obtained and/or adopted by other authors, with varying precision and accuracy, for other areas in the Alpine–Adriatic region, as far north as Austria and as far east as Slovenia (). Conversely, our gaps were significantly different from those estimated in other regions, e.g. in Croatia (Lorković Citation1993) and Bosnia (Lelo Citation2002), where the size ranges of variation of copulatory apparatuses are shifted to lower values in both L. sinapis and L. juvernica, so that the threshold gap between the two species is shifted accordingly. Our results confirm previous evidence for within-species geographical variation in the size of copulatory apparatuses (Lorković Citation1993; Mazel & Leestmans Citation1999).
Table II. Morphometric measures of genitalia of specimens identified as either Leptidea sinapis or L. juvernica from different areas in the Alpine–Adriatic region, by different authors. All values in mm.
We found some overlap between species in the range of variation of all morphometric parameters, as already reported from other regions where L. sinapis is sympatric with either L. juvernica or L. reali. No or negligible overlaps were found in the length of the aedeagus and in the length of the ductus bursae, confirming these two parameters as the most reliable in identifying males and females respectively (e.g., Freese & Fiedler Citation2002). Nevertheless, species identity is expected to remain uncertain for a few specimens, as happened in our study, for different reasons: besides anomalous individual conditions, artefacts may occur as an effect of the preservation history of the specimens, or of their dissection and mounting; moreover, intermediate sizes of copulatory organs have been also explained by hybridization between species (Verovnik & Glocovčan Citation2007), but genetic analyses and courtship experiments suggest that hybrids are at most very rare between L. sinapis and L. juvernica, because of behavioural and biochemical pre-zygotic barriers (Freese & Fiedler Citation2002; Friberg et al. Citation2008c; Dincă et al. Citation2013).
Climatic niche separation between Leptidea species
After field and experimental research, evidence has accumulated that L. sinapis, L. reali and L. juvernica are very similar not only in morphological features but also in ecology and behaviour of adults, in developmental and reproductive phenology, and in the range of exploitable larval food plants (Freese & Fiedler Citation2002, Citation2004; Friberg et al. Citation2008a, Citation2008b, Citation2008c; Friberg & Wiklund Citation2009). As a consequence, other factors have been suspected to better explain and/or control how sympatric species coexist or segregate in an area. In particular, more subtle differences in ecological tolerance and some form of behavioural interaction or interspecific competition have been hypothesized and demonstrated to play a role in shaping the fine-scale pattern of occurrence (Freese & Fiedler Citation2002; Friberg et al. Citation2008a, Citation2008b, Citation2013).
Moreover, the differentiation of the ecological niche between two sympatric species of Leptidea is different in different regions: in some areas, L. sinapis features as a generalist species while L. juvernica or L. reali are specialists (e.g., Amiet Citation2004; Friberg et al. Citation2008a), but in other areas the roles are reversed (Beneš et al. Citation2003). In our study area, we found that L. sinapis is present from the coastal lowlands to the mountains, whereas L. juvernica is less frequent and apparently more selective, only colonizing the mountains including the bottom of deep valleys. Our analysis confirms previous observations on the relative distribution between the two species in other areas of the Alpine–Adriatic region, either anecdotal or derived from different analytical methods (Embacher Citation1996; Verovnik & Glocovčan Citation2007). A similar situation has been documented in contiguous regions, namely in the Western Alps (Amiet Citation2004) and the Balkan peninsula (Lorković Citation1993).
Our sampling strategy (1–2 specimens each from many different localities) allowed us to assess the fine-scale geographical and climatic distribution of the two species; nevertheless, it prevented us from evaluating how frequently the two species occur in syntopy. Actually, we found syntopic specimens of the two species in at least 13 sites, which account for 17% of all the sites where multiple specimens were examined. This is in agreement with reports from other parts of the Alpine region, where L. sinapis has been found in syntopy with another Leptidea species (Amiet Citation2004). Despite the fact that L. sinapis and L. juvernica may coexist locally, we found that the ecological niches exploited by the two species are partially different with respect to the local climatic regimes. In particular, we found evidence for a partial separation with respect to seasonal variations of temperature, rainfall and a geomorphological variable such as the local slope, which is expected to affect the local climate. Instead, we found a less appreciable predictive role, if any, for other climate-related geographical variables, including altitude and orientation, at odds with reports from other regions (Embacher Citation1996; Beneš et al. Citation2003; Amiet Citation2004). The geographical and ecological relations between L. sinapis and L. juvernica highlighted in the Veneto region are in general agreement with the situation recognized in other regions in southern and central Europe (where L. sinapis is more frequent in warmer and/or drier sites, whereas L. juvernica is more frequent in colder and/or wetter sites; Beneš et al. Citation2003; Verovnik & Glocovčan Citation2007), and contribute to the comprehension of the complex “niche mosaic” pattern that is emerging for the Leptidea species complex in Europe (Friberg et al. Citation2008b, Citation2013). Nevertheless, besides the local geomorphological and climatic conditions, other ecological factors, including vegetation structure and historical variation in land use, are expected to play a role and therefore deserve to be taken into account in further investigations. Additionally, recent experiments have demonstrated some effects of directs interactions between L. sinapis and L. juvernica, especially in courtship behaviour (Friberg et al. Citation2013). Moreover, analyses carried out on other cryptic systems of butterflies have suggested that other stochastic demographic processes may concur with climatic factors and competitive interactions in explaining the observed pattern of parapatric distributions (Zinetti et al. Citation2013; Vodă et al. Citation2015).
Acknowledgements
We thank all persons who provided records and specimens of Leptidea from the Veneto region, and museum curators who allowed us to study collections under their care (A. Dal Lago, Museo Naturalistico Archeologico di Vicenza; L. Latella, Museo di Storia Naturale di Verona; P. Paolucci, DAFNAE, Università di Padova; F. Pesarini, Museo di Storia Naturale di Ferrara; M. Uliana, Museo di Storia Naturale di Venezia). We also thank F. Lucati who improved the English, and an anonymous reviewer for insightful comments.
References
- Amiet JL. 2004. Séparation de niches écologiques chez deux espèces jumelles sympatriques de Leptidea (Lepidoptera, Pieridae). Revue d’Écologie 59:433‒452.
- Barbi A, Cacciatori G, Checchetto F, Chiaudani A, Delillo I, Meneghin P, Rech F, Tardivo G, Tridello G. 2011. Atlante climatico del Veneto. Temperature. Mestre: Regione del Veneto.
- Barbi A, Cagnati A, Cola G, Checchetto F, Chiaudani A, Crepaz A, Delillo I, Mariani L, Marigo G, Meneghin P, Parsi SG, Rech F, Renon B, Robert-Luciani T. 2013. Atlante climatico del Veneto. Precipitazioni. Basi informative per l’analisi delle correlazioni tra cambiamenti climatici e dinamiche forestali nel Veneto. Mestre: Regione del Veneto.
- Beneš J, Konvička M, Vrabec V, Zàmečník J. 2003. Do the sibling species of small whites, Leptidea sinapis and L. reali (Lepidoptera, Pieridae) differ in habitat preferences? Biologia, Bratislava 58:943‒951.
- Bonato L, Uliana M, Beretta S. 2014. Farfalle del Veneto: atlante distributivo [Butterflies of Veneto: distributional atlas]. Regione Veneto: Fondazione Musei civici di Venezia, Marsilio Editori, Venezia. pp. 1‒392.
- Dapporto L, Bruschini C, Dinca V, Vila R, Dennis RLH. 2012. Identifying zones of phenetic compression in West Mediterranean butterflies (Satyrinae): Refugia, invasion and hybridization. Diversity and Distributions 18:1066–1076. doi:10.1111/j.1472-4642.2012.00903.x.
- Devictor V, van Swaay C, Brereton T, Brotons L, Chamberlain D, Heliölä J, Herrando S, Julliard R, Kuussaari M, Lindström Å, Reif J, Roy DB, Schweiger O, Settele J, Stefanescu C, Van Strien A, Van Turnhout C, Vermouzek Z, Wallis DeVries M, Wynhoff I, Jiguet F. 2012. Uncertainty in thermal tolerances and climatic debt. Nature Climate Change 2:638–639. doi:10.1038/nclimate1668.
- Dincă V, Lukhtanov VA, Talavera G, Vila R. 2011. Unexpected layers of cryptic diversity in wood white Leptidea butterflies. Nature Communications 2:324.
- Dincă V, Wiklund C, Lukhtanov VA, Kodandaramaiah U, Norén K, Dapporto L, Wahlberg N, Vila R, Friberg M. 2013. Reproductive isolation and patterns of genetic differentiation in a cryptic butterfly species complex. Journal of Evolutionary Biology 26:2095‒2106. doi:10.1111/jeb.12211.
- Embacher G. 1996. Beitrag zur Verbreitung und Biologie von Leptidea sinapis (Linnaeus, 1758) und L. reali Reissinger, 1989 (Lepidoptera: Pieridae, Dismorphiinae). Zeitschrift der Arbeitsgemeinschaft öesterreichischer Entomologen 48:107‒112.
- Fracasso A. 2002. Leptidea reali (Reissinger, 1989): Note preliminari sulla distribuzione nel nord-est dell’Italia (Lepidoptera, Pieridae). Bollettino del Museo civico di Storia Naturale di Venezia 53:149‒154.
- Freese A, Fiedler K. 2002. Experimental evidence for species distinctness of the two wood white butterfly taxa, Leptidea sinapis and L. reali (Pieridae). Nota Lepidopterologica 25:39‒59.
- Freese A, Fiedler K. 2004. Unterscheidungsmerkmale von Leptidea sinapis (Linneus, 1758) und Leptidea reali Reissinger, 1989 (Lepidoptera, Pieridae) und ihre Eignung zur Artbestimmung. Nachrichten des Entomologischen Vereins Apollo 25:65‒77.
- Friberg M. 2007. A difference in pupal morphology between the sibling species Leptidea sinapis and L. reali (Pieridae). Nota Lepidopterologica 30:61‒64.
- Friberg M, Bergman M, Kullberg J, Wahlberg N, Wiklund C. 2008a. Niche separation in space and time between two sympatric sister species ‒ a case of ecological pleiotropy. Evolutionary Ecology 22:1‒18. doi:10.1007/s10682-007-9155-y.
- Friberg M, Leimar O, Wiklund C. 2013. Heterospecific courtship, minority effects and niche separation between cryptic butterfly species. Journal of Evolutionary Biology 26:971‒979. doi:10.1111/jeb.2013.26.issue-5.
- Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C. 2008b. Habitat choice precedes host plant choice – niche separation in a species pair of a generalist and a specialist butterfly. Oikos 117:1337‒1344. doi:10.1111/oik.2008.117.issue-9.
- Friberg M, Vongvanich N, Borg-Karlson A-K, Kemp DJ, Merilaita S, Wiklund C. 2008c. Female mate choice determines reproductive isolation between sympatric butterflies. Behavioral Ecology and Sociobiology 62:873‒886. doi:10.1007/s00265-007-0511-2.
- Friberg M, Wiklund C. 2009. Host plant preference and performance of the sibling species of butterflies Leptidea sinapis and Leptidea reali: A test of the trade-off hypothesis for food specialization. Oecologia 159:127‒137. doi:10.1007/s00442-008-1206-8.
- Fumi M. 2008. Distinguishing between Leptidea sinapis and L. reali (Lepidoptera: Pieridae) using a morphometric approach: impact of measurement error on the discriminative characters. Zootaxa 1819:40‒54.
- Hauser E. 1997. Leptidea sinapis (Linnaeus, 1758) und Leptidea reali Reissinger, 1989: zwei verdschiedene Arten? (Lepidoptera, Pieridae). Beiträge zur Naturkunde Oberösterreichs 5:65‒75.
- Higgins L.G. 1975. The Classification of European Butterflies. London: Collins. pp. 1‒330.
- Lelo S. 2002. Variation in exogenous and endogenous (genitalia) characteristics of butterflies of the species Leptidea sinapis Linnaeus, 1758 (Pieridae, Dismorphiinae) within populations from the area around Sarajevo. Natura Croatica 11:293‒319.
- Lorković Z. 1993. Leptidea reali Reissinger, 1989 (=lorkovicii Real, 1988), a new European species (Lepid, Pieridae). Natura Croatica 2:1‒26.
- Mallet J, Wynne IR, Thomas CD. 2011. Hybridisation and climate change: Brown argus butterflies in Britain (Polyommatus subgenus Aricia). Insect Conservation and Diversity 4:192‒199. doi:10.1111/icad.2011.4.issue-3.
- Mazel R, Leestmans R. 1999. Séparation biométriques des Leptidea sinapis L, L. morsei Fenton et L. reali Reissinger. Linneana Belgica 17:46‒52.
- Verovnik R, Glocovčan P. 2007. Morphological and molecular evidence of a possible hybrid zone of Leptidea sinapis and L. reali (Lepidoptera: Pieridae). European Journal of Entomology 104:667‒674. doi:10.14411/eje.2007.084.
- Vila R, Viader S, Jubany J. 2003. Leptidea sinapis (Linnaeus, 1758) i L.reali (Reissinger, 1989): dues espècies “bessones” a Catalunya i Andorra (Leptidoptera: Pieridae). Butlleti de la Societat Catalana de Lepidopterologia 90:25‒47.
- Vodă R, Dapporto L, Dincă V, Vila R. 2014. Cryptic matters: Overlooked species generate most butterfly beta-diversity. Ecography 37:1‒5.
- Vodă R, Dapporto L, Dincă V, Vila R. 2015. Why do cryptic species tend not to co-occur? A case study on two cryptic pairs of butterflies. PLoS ONE 10:e0117802.
- Zinetti F, Dapporto L, Vovlas A, Chelazzi G, Bonelli S, Balletto E, Ciofi C. 2013. When the rule becomes the exception. No evidence of gene flow between two Zerynthia cryptic butterflies suggests the emergence of a new model group. PLoS ONE 8:e65746.