Abstract
Studies suggest there is a connection between adrenal cortisol production and social rank in many non-human primates. Behavioral observations have confirmed that Sichuan snub-nosed monkeys (Rhinopithecus roxellana) have obvious social ranks. Thus, the goal of this study was to investigate whether there is a relationship between fecal glucocorticoid metabolite (FGM) concentrations as an indicator of social stress and dominance rank in Sichuan snub-nosed monkeys. Fecal samples were collected about every 5 days for 1 year from 10 Sichuan snub-nosed monkeys (> 7 years old; n = 5 males, 5 females), and analysed for FGM with a corticosterone radioimmunoassay using machine learning and open access data. Results showed that: (1) yearly mean FGM levels were negatively correlated with individual animal behavioral social rank in both males and females; (2) we divided the whole year into warm and cold seasons (seasonally) and breeding and nonbreeding seasons physiologically. Except for cold seasons, FGM levels have a negative correlation with behavioral social ranks in males and females; (3) female rank does not clearly relate to FGM levels; (4) social ranks of snub-nosed monkeys are more collaborative traits than aggressive ones. Our findings have important implications for understanding the different physiological consequences of dominant and subordinate social status on Sichuan snub-nosed monkey societies, and it quantifies how physiological stress differs during seasons and phases, and by individuals.
Introduction
In social animals, dominance rank and stress are inherently connected. The Sichuan snub-nosed monkey (Rhinopithecus roxellana) is an example of a social animal, and their social relationships have been studied through classic behavioral observations (Zhang Citation2003; Li et al. Citation2006; Yan et al. Citation2006; Zhang et al. Citation2006; Wang et al. Citation2007; Yu et al. Citation2009b; He et al. Citation2013). In non-human primates, a number of factors, including social structure (Sapolsky Citation1992, Citation2005; Saltzman et al. Citation1994; Shively et al. Citation1997), social stability (Robert et al. Citation1983; Smith et al. Citation1997) and reproductive status (Ziegler et al. Citation1995; Ziegler & Sousa Citation2002; Weingrill et al. Citation2004) are known to affect fecal glucocorticoid metabolite (FGM) levels. The glucocorticoid metabolite is produced in response to stress caused by adverse physiological or psychological effects (Möstl & Palme Citation2002). It can be determined noninvasively by FGM analyses (Wasser et al. Citation1997; Schatz & Palme Citation2001; Dehnhard et al. Citation2003; Franceschini et al. Citation2008). In some species, such as the African wild dog (Lycaon pictus) and Ring-tailed lemur (Lemur catta), the dominant individual has higher stress levels because of the need to maintain its status (Bercovitch & Clarke Citation1995; Creel et al. Citation1996; Cavigelli Citation1999). However, in other species with a strict dominance structure, the dominant individuals frequently and aggressively maintain their dominance status over the subordinate cohort, e.g. rhesus macaques (Macaca mulatta), squirrel monkeys (Saimiri sciureus) and savanna baboons (Papio cynocephalus). The subordinate individuals have higher FGM levels because of the physical (as determined by behavior observation) and psychologically induced (as determined by stress hormone test) stress (Davis & Christian Citation1957; Manogue Citation1975; Sapolsky Citation1990; Zumpe & Michael Citation2005). Research in birds has shown that FGM levels are significantly elevated when male greylag geese (Anser anser) compete for mating priority during the breeding seasons (Kotrschal et al. Citation1998). In lemurid primates, FGM levels vary among individuals and during seasonal changes (Cavigelli Citation1999). And it is widely accepted that lower ranking individuals often avoid higher ranking individuals, especially during the breeding season (McLeod et al. Citation1996; Kotrschal et al. Citation1998; East & Hofer Citation2001; Sands & Creel Citation2004), resulting in detectable separation and fragmentation patterns within the population.
Sichuan snub-nosed monkeys (Rhinopithecus roxellana) are an endemic species of conservation concern to China. They are listed on Appendix I of the Convention on International Trade in Endangered Species (CITES). These monkeys have two types of social units: “one-male units” (OMUs) and “all-male units” (AMUs) (Chen Citation1989; Kirkpatrick Citation1995; Ren et al. Citation2000). The ranks of dominant males are generally higher than of dominant females because of their greater physical strength, bigger body size and longer, sharper teeth than females (Pereira Citation1993; Mitani et al. Citation1996). Here, the adult male is the highest-ranked individual of an OMU, whereas females have an unstable hierarchy amongst themselves (Zhang et al. Citation2003; Li et al. Citation2006; Qi et al. Citation2006). Sub-adult and juvenile individuals occupy the lowest ranks of an OMU (Kotrschal et al. Citation1998).
It is widely known that in snub-nosed monkeys, OMUs and AMUs come together to form a troop (Bales et al. Citation2005; Tan et al. Citation2007). Furthermore, lower ranked units keep wider distances apart from higher ranked units (Yu et al. Citation2009b). But, in general, Sichuan snub-nosed monkeys are considered a “friendly” species. Yu et al.’s (Citation2009a) study on “friendship behaviour” suggests that this kind of behavior mostly happens within one unit.
In Sichuan snub-nosed monkeys, Wang et al. (Citation2007) found that dominance rank was related to food abundance, specifically among adult males of all OMUs inside one troop. Food abundance is high in warm seasons and low during cold seasons. That is based on the general rule that higher ranked individuals have also more priority (Leinfelder et al. Citation2001); thus, dominance ranks have effects on resource distribution among individuals and groups. High-ranking individuals can attain more food resources and reproductive opportunities (Wranghan & Waterman Citation1981; Whitten Citation1983). Therefore, the relationship between cortisol and rank is driven, at least in part, by energetic factors (Muller & Wrangham Citation2004). Social unit membership is the basic element of the society of Sichuan snub-nosed monkeys. Social ranks among the units are decided by the head of each unit. The adult male is the dominant individual inside each unit. Female individuals occupy the middle ranks and sub-adults are the lowest ranks. Thus, in this study, we want to investigate the relationship between FGM and social ranks in the society of Sichuan snub-nosed monkeys.
We evaluated concepts potentially related to high stress levels (i.e., FGM) in Sichuan snub-nosed monkeys, applying an open-access data approach for transparency and repeatability (see Cushman & Huettmann Citation2010; Drew et al. Citation2011; and citations within). We then applied high-power data mining and machine learning techniques (e.g. Jochum & Huettmann Citation2010) to investigate whether any evidence indicates that: (1) yearly mean FGM levels are correlated with specific behavioral dominance ranks; (2) FGM levels can be explained by behavioral dominance ranks during warm and cold seasons, and during breeding and non-breeding seasons; and (3) FGM levels reflect the social types of Sichuan snub-nosed monkeys (collaborative or aggressive).
Materials and methods
Study area
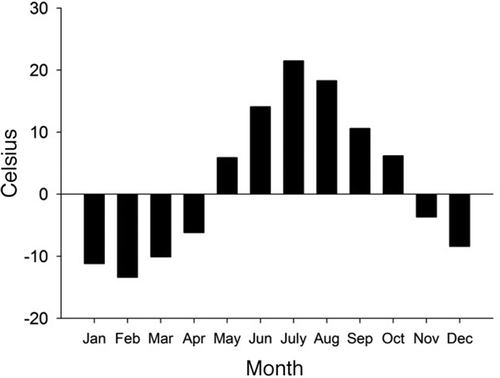
This study was conducted at an established observation site in the Dalongtan area, Shennongjia National Nature Reserve, Hubei Province (31°29ʹ663”E, 110°18ʹ205”N). The study area is located at an elevation of 2200 m above sea level, with vegetation composed of dense, mixed deciduous broadleaf and conifer trees, evergreen conifers and broadleaf trees (Tie et al. Citation2010). The annual mean temperature is 11.0–12.2°C, with the highest temperatures of approximately 21.2–26.5°C occurring in July, and the lowest temperatures of approximately −5.8 to −3.6°C occurring in January. The months of May–October are moderate in temperature, whereas January–December are cold. The mean frost-free period is 173 days per year. The year was divided into the warm season (May through October), with a mean daily temperature of at least 0°C, and the cold season (November through April; temperature below 0°C). Further details are shown in .
Study subjects
Since 2005, Sichuan snub-nosed monkeys have been fed at this site two times a day. During this time, several groups have naturally formed within an area of around 80 km2 with a radius of c. 5 km. In this area we conducted three censuses from 2008 to 2009, and counted overall 43 adult individuals belonging to four OMUs within one troop. The OMUs are referred to as the Baitou Unit, Dadan Unit, Dayang Unit and Xiaoxin Unit. A single AMU was found and named Changwei Unit. The dominance rank of each unit within the troop is closely related to the dominant individual of each unit (Zhang Citation2003). Accordingly, we studied their five dominant males, named Baitou (BT), Dadan (DD), Dayang (DY), Xiaoxin (XX) and Changwei (CW), and the dominant females, Laoni (LN), Duanwei (DW), Juzi (JZ), YingY (YingY) and Yinyin (YY), from each of the four OMUs and one AMU.
Fecal collection
We then took 2 weeks to carry out individual identification and make the monkeys habituated to the researchers, which facilitated the tracking of individual animals for fecal sample collection. When a target individual defecated, the position of the feces was noted and then the feces were picked up once the animals moved away. Any contaminating mud was immediately wiped from the fresh fecal samples, which were then placed in labeled plastic bags, transported back to camp in portable cooler boxes that can maintain samples at −20°C for 1 day, and stored frozen at −20°C. Fecal samples (n = 620 total) were collected about once every 5 days for 1 year (). Because FGM concentrations vary throughout the day (Sousa & Ziegler Citation1998), we only collected feces from 08:00–12:00 hours (GMT), for a standardized approach.
Table I. Numbers of studied individuals and samples used for this analysis.
FGM extraction and quantification
Fecal samples were dried in an oven at 70°C, pulverized and mixed, and the steroids were extracted using established methods (Brown et al. Citation2001). Approximately 0.5 g of dried fecal sample was extracted by adding 10 mL of 80% ethanol and placing samples in a water bath at 70°C with shaking for 20 min. After centrifugation (2500 × g for 15 min), the supernatant was recovered and the pellet re-suspended in an additional 5 mL of 80% ethanol. Then the samples were again vortexed in a water bath. Ethanol supernatants were combined, dried down in a water bath at 60°C, and re-suspended in 1 mL methanol. Samples were stored at −20°C until further analysis. The average recovery of unlabeled corticosterone added to feces before extraction was 85.19%.
FGM concentrations were quantified using a double-antibody 125I-corticosterone radioimmunoassay (RIA; Chemclin Biotech Co., Ltd., Beijing, China). The RIA was validated for snub-nosed monkeys by comparing parallelisms in serial dilutions of fecal extract with the corticosterone standard curve (10–500 ng/mL; y = 3.638 – 1.789x; R2 = 0.988). The assay sensitivity was 50 pg/tube at a 90% binding. Buffer blanks were below the sensitivity level. Intra-assay coefficients of variation at the 50% binding point of the standard were 3.37% (n = 10) and inter-assay coefficients of variation were 6.14% (n = 10) for the low standard, 4.89% (n = 8) for the middle standard and 11.34% (n = 8) for the high standard, respectively. Cross reactivity with corticosterone is between 6 and 30 nM with added steroid concentration between 70 and 700 nM.
Behavior observations
We applied behavior sampling following the method of Li et al. (Citation2006) to record the frequency of aggressive behaviors (AT) and submissive behaviors (ST) for each studied individual. Monkeys were observed and data recorded for 3 hours per day once every 3 days for an entire year. We defined the following aggressive behaviors: driving away, lunging, chasing, grasping and hitting, wrestling and biting. Submissive behavior is described as: avoiding, crouching, retreating and fleeing (Yan et al. Citation2006). We calculated a dominance index (DI) as DI = AT%/ST% × 100 for each individual to reflect their dominance ranks behaviorally. The DI was then assigned to each individual.
Determination and classification of seasons
The published breeding season of Sichuan snub-nosed monkeys is early September through mid-November, with the rest of the year considered the non-breeding season (Qi Citation1982; Chen Citation1989). However, according to behavioral observations over 1 year by local park rangers, these monkeys actually have a longer breeding season that begins in August and ends in November. Thus, the non-breeding season is shorter than initially reported, and so we defined the breeding season as August–November and the non-breeding season as December–July.
Data analysis
Behaviors were observed during the fecal sampling process. We obtained and present 1-year overall behavior observations. DI was calculated based on these data. We evaluated the overall behavioral ranks determined based on DI values of the studied individuals. Fecal data are expressed on a per gram dry weight basis and as the mean ± standard error (SE).
For the multivariate machine learning analysis we used TreeNet (Salford Systems Ltd), a boosting regression tree analysis, on the data to describe patterns and details of the signals, and for a more detailed and robust analysis (Cushman & Huettmann Citation2010; Drew et al. Citation2011; see Popp et al. Citation2007 for an application). Unlike other statistical approaches and software, TreeNet is non-parametric and allows the assessment of statistical interactions (Friedman Citation1999, Citation2002). Statistical interactions are usually very problematic for most linear analysis, and are currently not well resolved statistically and in traditional approaches. However, they can obscure any linear statistics results and are inherent in virtually any behavioral and wildlife data; thus, they are essential to document and to address in any more advanced and complex data analysis as provided here. The interactions we present here for the first time in such studies are two-way interactions and based on classification trees, boosting and bagging (Friedman Citation2002).
The classification scheme (5 = high rank and 1 = low rank) was set up for correlation analysis between behavior ranks and FGM levels. For descriptive purposes and for potential pre-screening purposes, we ran a general correlation analysis of the multivariate data set based on Spearman rho2, using VARCLUST in R (Hmisc package). The software packages SPSS V17.0 (SPSS Inc., 2008, Chicago, IL, USA), Salford Predictive Miner (SPM; Salford Systems, Inc., San Diego, CA, USA) and SigmaPlot V10.0 (Systat Software, Inc, San Jose, CA, USA) were used for statistical calculations and graphical presentation. Spearman correlation analysis during the breeding and non-breeding seasons between FGM levels and behavior rank was applied.
Ethical note and underlying open-access data
This study was fully endorsed by both the Center and the Reserve, and finally approved by the Chinese version of the Institutional Animal Care and Use Committee (IACUC) of the College of Nature Conservation, Beijing Forestry University. All of the sampling activities were conducted in collaboration with technical staff of the Shennongjia Nature Reserve. Maximal care was taken to avoid animal disturbance during fecal sampling and behavior observation. For instance, we spent about 2 weeks to familiarize the monkeys with our research staff, and wore camouflage clothing to reduce any stress. All data in this study are publicly available online and in Appendix I or with the authors. These data can also be found with ISO (International Organization for Standardization) metadata at dSPACE at the institutional repository dSPACE at the library of the University of Alaska Fairbanks (UAF).
Results
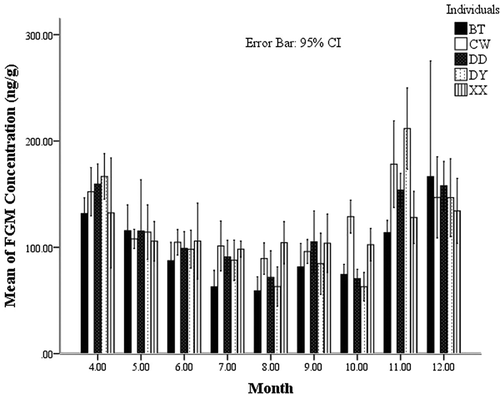
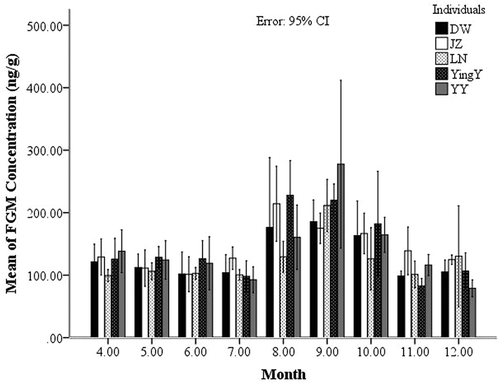
We found that high-behavioral-rank individuals have a low FGM level in both males and females (). The change gradient of FGM levels varied more in males than in females, based on the CV (Coefficient of Variation) result.
Table II. Overall ranks of male and female snub-nosed monkeys, using behavior classifications (dominance index, DI) and fecal glucocorticoid metabolite (FGM) levels.
A negative relationship between behavior ranks and FGM was identified (r = −0.156, P = 0.000). In addition, in both males and females, individuals ranking 5 (highest ranked individuals) had the lowest FGM level and those with a rank of 1 (lowest ranked individuals) had the highest FGM concentration (P = 0.000).
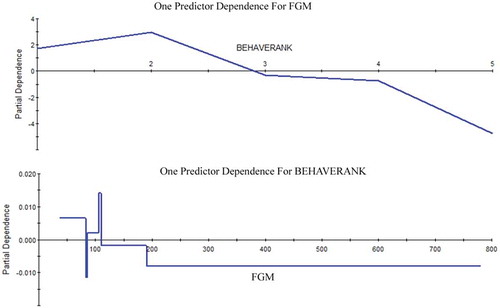
We show the nature of this relationship between behavioral rank and FGM in , as per TreeNet, in order to show the drivers of this relationship, and as an underlying mechanism.
Figure 2. Relationship of behavioral ranks vs fecal glucocorticoid metabolite (FGM), and FGM vs behavioral ranks, as determined by TreeNet.
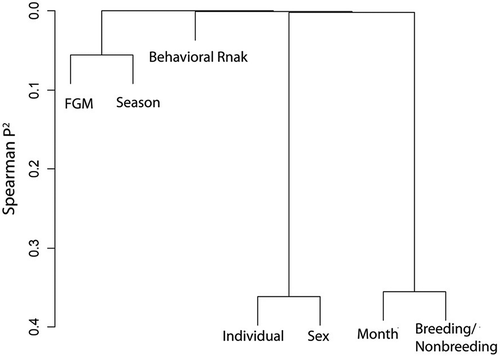
shows that individual ID had a relatively low correlation with sex. Month and breeding status correlated to each other the most. FGM ranks had a lower correlation with season and behavior rank.
Figure 3. Spearman rho2; presented as varclust.
We applied nonparametric correlations (Spearman) to analyze the correlation between FGM levels and behavior ranks (). During the cold seasons, the FGM levels were not correlated with the behavior ranks (R = −0.079, P = 0.287 > 0.05). During the warm seasons, however, FGMs were negatively correlated with behavior rank (R = −0.223, P < 0.01; ).
Table III. Climatic seasonal order of the fecal glucocorticoid metabolite (FGM) levels for dominant males and females.
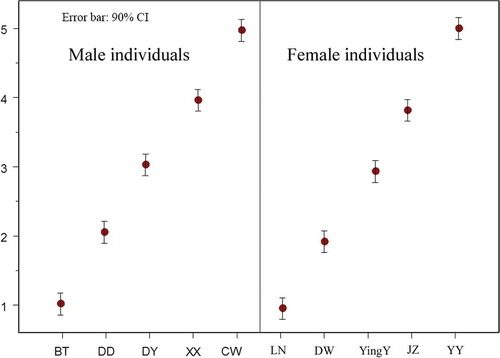
Figure 4. Behavioral ranks by male and female individuals.
Fecal FGM levels negatively correlated with behavior ranks during the non-breeding (R = −0.110, P = 0.048) and breeding (R = −0.237, P = 0.000) seasons.
The TreeNet regression analysis showed that the influence of the FGM level for dominant males and females was opposite among sexes ().
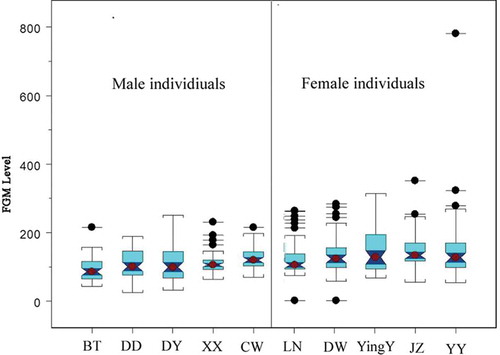
Figure 5. Boxplots of fecal glucocorticoid metabolite (FMG) levels by individuals (mean, 95% confidence intervals notches, 75% quantile boxes and outliers are shown).
Thus, for males, seasonal change was the main factor influencing the FGM variation, whereas a breeding versus non-breeding situation influenced females the most ().
Table IV. Importance ranks of the fecal glucocorticoid metabolite (FGM) variables of males/females as well as by male and by female.
Here showed the interaction presented (two-way interactions based on classification trees). Results show that individuality is a major driver for the general statistical patterns analyzed.
Table V. Two-way interactions as provided by TreeNet (Friedman Citation1999, Citation2002).
We measured the FGM levels for all five female individuals in the breeding and non-breeding seasons. These results showed that the low-ranking female YY had statistically a higher FGM than the high-ranking LN in the breeding season (P = 0.018), and a very similar FGM in the non-breeding season (p = 0.228).
We list the individuals who have a social collaborative relationship, and shows that DD, DY and DI had similar FGM levels among males; YingY, JZ and YY had close FGM levels among females.
Table VI. Levels for collaborative vs aggressive behavior.
Discussion
This is the first report of FGM levels in free-ranging Sichuan snub-nosed monkeys. We found that lower ranked individuals may be more stressed by dominant males across the entire year based on significant differences in FGM concentrations, as shows. Studies on olive baboon (Papio anubis; Sapolsky Citation1992), rhesus macaques (Macaca mulatta; Manogue Citation1975) and savanna baboons (Papio cynocephalus; Sapolsky Citation1990) have shown similar results. In captive gorillas (Gorilla gorilla), dominant females induce stress in subordinates through psychological and physical aggression (McLeod et al. Citation1996). We confirmed this hypothesis through advanced data-mining methods based on machine learning. Therefore, this is one of the first primate studies ever reporting on statistical interactions. The two-way interaction results in show a widely known relationship between complex mammals and behavior data, whereas in most other wildlife studies worldwide, interactions and such analysis are generally ignored (Braun Citation2005) and traditional analysis techniques cannot take them well into account (e.g. Manly et al. Citation2002; but see Popp et al. Citation2007); arguably, individuality is a major driver for the general statistical patterns analyzed (see also Jochum & Huettmann Citation2010). We think that with boosting and related machine-learning methods, one can start to track these interactions, visualize them, and then generalize and predict findings for a better understanding. At minimum, it represents a testable hypothesis to work from, and new information. This speaks for good progress and for a more meaningful quantitative analysis in wildlife biology and conservation than has been achieved before (Braun Citation2005; Cushman & Huettmann Citation2010; Drew et al. Citation2011). However, in the FGM levels of females, there were no differences among sampled individuals over the year, as shows. But the order of yearly means of FGM levels still correlated negatively with behavioral dominance ranks in females. Compared to the ranks of males, ranks of females were not so strict. The ranks of females were only strict during the breeding season and at Shennongjia Nature Reserve. Some studies have presented that resident males usually cooperate in mating defence (Xiang et al. Citation2014). That was true because males were responsible to protect their units and reproductive resources. However, this did not influence the ranks among adult males.
FGM levels and behavior ranks were negatively correlated in warm, breeding and non-breeding seasons, but not during the cold season. We think that food could be a reason, because Sichuan snub-nosed monkeys eat only 13 species of plants during the cold season and 53 during the warm season (Tie et al. Citation2010). Many studies have shown that a decrease in vegetation could increase FGM levels in the red colobus (Piliocolobus badius; Chapman et al. Citation2007), spider monkeys (Ateles geoffroyi yucatanensis; Rangel-Negrín et al. Citation2009) and woolly monkeys (Lagothrix ssp.; Ange-van Heugten et al. Citation2009), and ring-tailed lemurs (Lemur catta; Pride Citation2005). The troop may have suffered high physiological stress from food deficiency during the cold season. That is because FGM levels were similar to each other during this season among both male groups and female groups, as showed. Thus, increased FGM levels resulting from food limitation in the cold season could be one of the reasons why FGM levels were uncorrelated to behavior ranks.
Interacting feeding patterns were pursued to feed the Sichuan snub-nosed monkeys in Da Longtan, and all units successively gathered in the feeding area for food, with only 0.5–10 m of separation distance among units (measured by infra-red distance tools). Based on our field observations, XX and CW units (representing low-ranking males) kept a distance (10–15 m) far away from the other, higher ranking units.
CW was not involved in food and habitat competition. DD and DY may suffer more physical and physiological stress than XX and CW because they are involved in food competition. This kind of stress is associated with seasonal changes, which agrees with the result that the FGM rank of males is mostly influenced by seasons. And this also was related to the individual differences. But whether food could be a limiting factor to cause a higher FGM in CW than XX during the cold season needs further study still.
Higher-ranking females could have more superiorities than lower ones (Ren et al. Citation1991); during breeding seasons, lower ranking females had higher FGM levels because they received more aggression (Cavigelli et al. Citation2003). A study at this site showed that females increased their focus on grooming the single resident male to obtain more opportunities for mating (Yu et al. Citation2013) which could prove that female individuals actually compete for mating during breeding seasons. However, this pattern was not very strong, especially during non-breeding seasons. Some researchers found that the social behavior of this monkey expresses more collaborative traits, which means some individuals (ranks close to one) could help each other sometimes, based on the behavioral observations (Li et al. Citation2002). Here we explain it from the FGM hormone perspective. It was found that social collaboration (as expressed by similar DI values) could be reflected by the closer FGM levels between and among individuals. Thus, here we can list the individuals who have a social collaboration relationship. Elevated GC (glucocorticoid) levels are associated with high levels of aggression and high rank in some species (Creel et al. Citation1996), but not in others (Virgin & Sapolsky Citation1997). Female Sichuan snub-nosed monkeys have been described as being more collaborative and gentle than the males (Ren et al. Citation2000; Li et al. Citation2002), and the FGM correlates of rank were influenced by breeding status, not by aggressive behavior. In fact, we hardly observed aggressive behaviors among females during our field work. In general, a high FGM concentration appears to be connected to reproductive events and to the endocrine status of non-human primates (Ziegler et al. Citation1995; Albuquerque et al. Citation2001; Ziegler & Sousa Citation2002). Reproductive stressors played a key role in female physiological stress, as found in studies on common marmosets, Callithrix jacchus, and spotted hyaenas, Crocuta crocuta (Goymann et al. Citation2001). Here, we found that the ranks of FGM in females are most influenced by breeding and non-breeding seasons. FGM levels rise during reproduction, as our results show. And an FGM correlate of rank was observed during the breeding season, but not in the non-breeding season.
Because of the supplemental food provided to the monkeys, units did not really need to venture out of the territory in their search of food. When feeding began, the BT Unit, which was the most dominant one, was located closest to the feeding area, whereas the least dominant XX Unit was located the farthest away. Similar situations were found in other species (Hogstad Citation1987; Desrochers Citation1989). High-ranking dominant males are often the first to obtain food (Wranghan & Waterman Citation1981; Whitten Citation1983). They keep subordinates away via threats and aggression, which end when subordinates avoid interactions (Yu et al. Citation2009a). Thus, CW had the highest FGM of all. These may be a reason to cause variable FGM levels in different male individuals. Though dominant females had the same traits (He et al. Citation2013), they were still much more gentle physiologically and behaviorally (Li et al. Citation2002). This matches the frequently found unstable behavioral rank order in female individuals (Li et al. Citation2006).
Conclusions and wider perspective
In this study, we used machine learning (boosted regression trees) to infer generalizable patterns in FGM concentrations and behavior of snub-nosed monkeys in China from complex data. We conclude that dominant female and male individuals exhibit higher stress levels during different seasons. For males, higher stress levels occur in cold seasons, whereas for females this occurs during the breeding season.
We conclude that: (1) both the yearly mean FGM concentrations of males and females could reflect behavioral dominance ranks in Sichuan snub-nosed monkeys at Shennongjia Nature Reserve; (2) the order of FGM levels was only correlated with behavioral dominance ranks in warm, breeding and non-breeding seasons for males; (3) for females, the ordering of FGM levels with behavioral dominance ranks only occurs in cold seasons; and (4) ranks are not very rigid in the FGM levels of females, and reflect a high cooperation rather than an aggressive social relationship.
This study sets up a new method, an open-access approach and a high-powered analysis for studying social ranks in snub-nosed monkeys. As a perspective, we hope that this method and its findings could be applied in further studies on wild Sichuan snub-nosed monkey groups, and in similar applications.
Acknowledgements
Fecal samples were collected by many Shennongjia Natural Reserve staff, and population surveys were carried out by staff and students of Beijing Forestry University. We appreciate Dr. Janine Brown’s valuable advice and work on the English writing. The managers of the Shenongjia Natural Reserve allowed us to stay and do yearly records. Drs. Melissa Songer and Zhang Dong kindly commented on and revised our earlier drafts. Salford Systems Ltd. kindly allowed us to use their software for this research. This study was funded by the Special Fund for Forestry Scientific Research in the Public Interest, China’s Ministry of Science and Technology (No. 201004054) as well as the EWHALE lab, University of Alaska Fairbanks (UAF).
References
- Albuquerque ACSR, Sousa MBC, Santos HM, Ziegler TE. 2001. Behavioral and hormonal analysis of social relationships between oldest females in a wild monogamous group of common marmosets (Callithrix jacchus). International Journal of Primatology 22:631–645. doi:10.1023/A:1010741702831.
- Ange-van Heugten KD, van Heugten E, Timmer S, Bosch G, Elias A, Whisnant S, Swarts HJM, Ferket P, Verstegen MW. 2009. Fecal and salivary cortisol concentrations in woolly (Lagothrix ssp.) and spider monkeys (Ateles spp.). International Journal of Zoology 2009:1–9. doi:10.1155/2009/127852.
- Bales KL, French JA, Hostetler CM, Dietz JM. 2005. Social and reproductive factors affecting cortisol levels in wild female golden lion tamarins (Leontopithecus rosalia). American Journal of Primatology 67:25–35. doi:10.1002/(ISSN)1098-2345.
- Bercovitch FB, Clarke AS. 1995. Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques. Physiology and Behavior 58:215–221. doi:10.1016/0031-9384(95)00055-N.
- Braun CE, editor. 2005. Techniques for wildlife investigations and management. Bethesda, Maryland, USA: The Wildlife Society (TWS).
- Brown J, Bellem A, Fouraker M, Wildt D, Roth T. 2001. Comparative analysis of gonadal and adrenal activity in the black and white rhinoceros in North America by noninvasive endocrine monitoring. Zoo Biology 20:463–486. doi:10.1002/(ISSN)1098-2361.
- Cavigelli SA. 1999. Behavioral patterns associated with faecal cortisol levels in free-ranging female ring-tailed femurs, Lemur catta. Animal Behavior 57:935–944. doi:10.1006/anbe.1998.1054.
- Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. 2003. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: Ring-tailed lemurs (Lemur catta). Hormones and Behavior 43:166–179. doi:10.1016/S0018-506X(02)00031-4.
- Chapman CA, Saj TL, Snaith TV. 2007. Temporal dynamics of nutrition, parasitism, and stress in Colobus monkeys: Implications for population regulation and conservation. American Journal of Physical Anthropology 132:240–250. doi:10.1002/ajpa.20664.
- Chen FG. 1989. Progress in the studies of golden monkeys. Xi’an: Xi’an Northwest University Press (in Chinese).
- Creel S, Creel NM, Mills GLM, Monfort SL. 1996. Rank and reproduction in cooperatively breeding African wild dogs: Behavioral and endocrine correlates. Behavioral Ecology 8:298–306. doi:10.1093/beheco/8.3.298.
- Cushman SA, Huettmann F. 2010. Spatial complexity, informatics and wildlife conservation. Tokyo, Japan: Springer.
- Davis D, Christian J. 1957. Relation of adrenal weight to social rank of mice. Proceedings of the Society for Experimental Biology and Medicine 94:728–731. doi:10.3181/00379727-94-23067.
- Dehnhard M, Schreer A, Krone O, Jewgenow K, Krause M, Grossmann R. 2003. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis). General and Comparative Endocrinology 131:345–352. doi:10.1016/S0016-6480(03)00033-9.
- Desrochers A. 1989. Sex, dominance, and microhabitat use in wintering black-capped chickadees: A field experiment. Ecology 70:636–645. doi:10.2307/1940215.
- Drew CA, Wiersma Y, Huettmann F, editors. 2011. Predictive species and habitat modeling in landscape ecology. New York: Springer.
- East ML, Hofer H. 2001. Conflict and cooperation in a female dominated society: A reassessment of the “hyper-aggressive” image of spotted hyenas. Advances in the Study of Behavior 31:1–30.
- Franceschini MD, Rubenstein DI, Low B, Romero LM. 2008. Fecal glucocorticoid metabolite analysis as an indicator of stress during translocation and acclimation in an endangered large mammal, the Grevy’s zebra. Animal Conservation 11:263–269. doi:10.1111/acv.2008.11.issue-4.
- Friedman JH 1999. Greedy function approximation: A Gradient Boosting Machine. Technical report, Dept of Statistics, Stanford University.
- Friedman JH. 2002. Stochastic gradient boosting. Computational. Statistics & Data Analysis 38:367–378. doi:10.1016/S0167-9473(01)00065-2.
- Goymann W, East ML, Wachter B, Höner OP, Möstl E, Van’t Holf TJ, Hofer H. 2001. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proceedings of the Royal Society of London B: Biological Sciences 268:2453–2459. doi:10.1098/rspb.2001.1828.
- He HX, Zhao HT, Qi XG, Wang XW, Guo ST, Ji WH, Wang CL, Wei W, Li BG. 2013. Dominance rank of adult females and mating competition in Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains. China Adaptive Evolution and Conservation Ecology of Wild Animals 58:2205–2211.
- Hogstad O. 1987. Subordination in mixed-age bird flocks: A removal study. Ibis 131:128–134. doi:10.1111/j.1474-919X.1989.tb02751.x.
- Jochum K, Huettmann F. 2010. Spatial information management in wildlife conservation ecology: Adding spatially explicit behavior data to the equation? Chapter 10. Tokyo, Japan: Springer. pp. 175–192.
- Kirkpatrick RC. 1995. The natural history and conservation of the snub-nosed monkeys (genus Rhinopithecus). Biology Conserversion 72:363–369. doi:10.1016/0006-3207(94)00039-S.
- Kotrschal K, Hirschenhauser K, Möst E. 1998. The relationship between socialstress and dominance is seasonal in greylag geese. Animal Behavior 55:171–176. doi:10.1006/anbe.1997.0597.
- Leinfelder I, deVries H, Deleu R, Nelissen M. 2001. Rank and grooming reciprocity among females in a mixed-sex group of captive hamadryas baboons. American Journal of Primatology 55:25–42. doi:10.1002/(ISSN)1098-2345.
- Li BG, Li HQ, Zhao DP, Zhang YH, Qi XG. 2006. Study on dominance hierarchy of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in Qinling Mountains. Acta Theriologica Sinica 26:18–25 (in Chinese).
- Li BG, Zhang P, Watanabe K, Chia LT. 2002. Does allogerooming serve a hygienic function in the Sichuan snub-nosed monkey (Rhinopithecus roxellana). Acta Zoologica Sinica 48:707–715 (in Chinese).
- Manly BF, McDonald LL, Thomas DL, McDonald TL, Erickson WP. 2002. Resource selection by animals: Statistical design and analysis for field studies. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Manogue KR. 1975. Dominance status and adrenocortical reactivity to stress in squirrel monkeys (Saimiri sciureus). Primates 14:457–463. doi:10.1007/BF02382742.
- McLeod PJ, Alisalobel S, Taylor H, Cox JR. 1996. The relation between urinary cortisol levels and social behavior in captive timber wolves. Canadian Journal of Zoology 74:209–216. doi:10.1139/z96-026.
- Mitani JC, Gros-Louis J, Richards A. 1996. Sexual dimorphism, the operational sex ratio and the intensity of male competition in polygyous primates. The American Naturalist 47:966–980. doi:10.1086/285888.
- Möstl E, Palme R. 2002. Hormones as indicators of stress. Domestic Animal Endocrinology 23:67–74. doi:10.1016/S0739-7240(02)00146-7.
- Muller MN, Wrangham RW. 2004. Dominance, cortisol and stress in wild chimpanzees (Pantroglodytes schweinfurthii). Behavioral Ecology and Sociobiology 55:332–340. doi:10.1007/s00265-003-0713-1.
- Pereira ME. 1993. Agonistic interaction, dominance relation, and ontogenetic trajectories in ringtailed lemurs. Juvenile primates: Life history, development, and behavior. New York: Oxford University Press. pp. 285–305.
- Popp J, Neubauer D, Paciulli L, Huettmann F. 2007. Using TreeNet for identifying management thresholds of mantled howling monkeys’ habitat preferences on Ometepe Island, Nicaragua, on a tree and home range scale. Journal of Medical and Biological Sciences 1:1–14.
- Pride ER. 2005. Foraging success, agonism, and predator alarms: Behavioral predictors of cortisol in Lemur catta. International Journal of Primatology 26:295–319. doi:10.1007/s10764-005-2926-9.
- Qi JF. 1982. The breeding and reproduction of Sichuan snub-nosed monkeys. Chinese Wildlife 2:25–30 (in Chinese).
- Qi XG, Li BG, Lin YH. 2006. Maternal investment and birth sex ratio bias of the golden snub-nosed monkey (Rhinopithecus roxellana) in Qinling Mountains of China. Acta Zoologica Sinica 52:1–10 (in Chinese).
- Rangel-Negrín A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC. 2009. Stress in Yucatan spider monkeys: Effects of environmental conditions on fecal cortisol levels in wild and captive populations. Animal Conservation 12:496–502. doi:10.1111/acv.2009.12.issue-5.
- Ren RM, Yan KH, Su YJ. 2000. A field study of the society of Rhinopithecus roxellana. Beijing: Peking University Press (in Chinese).
- Ren RM, Yan KH, Su YJ, Qi HJ, Liang B, Bao WY, de Waal FBM. 1991. The reconciliation behavior of golden monkey (Rhinopithecus roxellana roxellana) in small breeding group. Primates 32:321–327. doi:10.1007/BF02382673.
- Robert M, Sapolsky RM, Krey LC, McEwen BS. 1983. The adrenocorticol stress-response in the aged male rat: Impairment of recovery from stress. Experimental Gerontology 18:55–64. doi:10.1016/0531-5565(83)90051-7.
- Saltzman W, Schultz-darke NJ, Scheffler G, Wegner FH, Abbott DH. 1994. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiology & Behavior 56:801–810. doi:10.1016/0031-9384(94)90246-1.
- Sands J, Creel S. 2004. Social dominance, aggression and fecal glucocorticoid levels in a wild population of wolves, Canis lupus. Animal Behavior 67:387. doi:10.1016/j.anbehav.2003.03.019.
- Sapolsky RM. 1990. Adrenocortical function, social rank, and personality among wild baboons. Biological Psychiatry 28:862–878. doi:10.1016/0006-3223(90)90568-M.
- Sapolsky RM. 1992. Neuroendocrinology of the stress response. In: Becker J, Breedlove S, Crews D, editors. Behavioral endocrinology. Cambridge, MA, US: The MIT Press. pp. 287–324.
- Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308:648–652. doi:10.1126/science.1106477.
- Schatz S, Palme R. 2001. Measurement of faecal cortisol in cats and dogs: A non-invasive method for evaluating adrenocortical function. Veterinary Research Communications 25:271–287. doi:10.1023/A:1010626608498.
- Shively CA, Laber-Laird K, Anton RF. 1997. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry 41:871–882. doi:10.1016/S0006-3223(96)00185-0.
- Smith TE, Schaffner CM, French JA. 1997. Social and developmental influences on reproductive function in female Wied’s black tufted-ear marmosets (Callithrix kuhlii). Hormones and Behavior 31:159–168. doi:10.1006/hbeh.1997.1380.
- Sousa MBC, Ziegler TE. 1998. Diurnal variation on the excretion patterns of fecal steroids in common marmoset (Callithrix jacchus) females. American Journal of Primatology 46:105–117.
- Tan CL, Guo S, Li BG. 2007. Population structure and ranging patterns of Rhinopithecus roxellana in Zhouzhi National Nature Reserve, Shaanxi, China. International Journal of Primatology 28:577–591. doi:10.1007/s10764-007-9147-3.
- Tie J, Zhang J, Peng LP, Wang DX, Hu DF, Zhang ZX. 2010. Feeding habits of Rhinopithecus roxellana in Shennongjia Nature Reserve of China in winter and spring. Chinese Journal of Ecology 29:62–68 (in Chinese).
- Virgin CE, Sapolsky RM. 1997. Styles of male social behavior and their endocrine correlates among low-ranking baboons. American Journal of Primatology 42:25–39. doi:10.1002/(ISSN)1098-2345.
- Wang XW, Li BG, Wu XM, He PJ, Hu YL. 2007. Dominance hierarchy of Sichuan snub-nosed monkey (Rhinopithecus roxellana) OMUs in Qin ling Mountains by feeding superiority. Acta Theriologica Sinica 27:344–349 (in Chinese). doi:10.1007/s13351-013-0302-9.
- Wasser SK, Bevis K, King G, Hanson E. 1997. Noninvasive physiological measures of disturbance in the Northern spotted owl. Conservation Biology 11:1019–1022. doi:10.1046/j.1523-1739.1997.96240.x.
- Weingrill T, Gray DA, Barrett L, Henzi SP. 2004. Fecal cortisol levels in free-ranging female chacma baboons: Relationship to dominance, reproductive state and environmental factors. Hormones and Behavior 45:259–269. doi:10.1016/j.yhbeh.2003.12.004.
- Whitten PL. 1983. Diet and dominance among female vervet monkeys (Cercopithecus aethiops). American Journal of Primatology 5:139–159. doi:10.1002/(ISSN)1098-2345.
- Wranghan RW, Waterman PG. 1981. Feeding behavior of vervet monkeys on Acacia tortilis and Acacia xanthophloea: With special reference to reproductive strategies and tannin production. The Journal of Animal Ecology 50:715–731. doi:10.2307/4132.
- Xiang ZF, Yang BH, Yu Y, Yao H, Grueter CC, Garber PA, Li M. 2014. Males collectively defend their one-male units against bachelor males in a multi-level primate society. American Journal of Primatology 76:609–617. doi:10.1002/ajp.22254.
- Yan KH, Su YJ, Ren RM. 2006. Social behavioral repertoires and action patterns of Sichuan snub-nosed monkey (Rhinopithecus roxellana). Acta Theriologica Sinica 26:129–135.
- Yu PL, Liao MY, Hu HB, Zhao BY, Yang JY, Bao WD. 2009a. Preliminary observation on affiliative behaviors of Rhinopithecus roxellana in a provisioned group in the Shenonongjia national Nature Reserve. Chinese Journal of Zoology 44:43–48.
- Yu PL, Yang JY, Hu HB, Bao WD, Yu HL, Yao H, Wu F. 2009b. Social behavior spectrum of a provisioned group of Scichuan snub-nosed monkeys at Shennongjia. Journal of Biology 26:8–10.
- Yu Y, Xiang ZF, Yao H, Grueter CC, Li M. 2013. Female snub-nosed monkeys exchange grooming for sex and infant handling. PLoS One 8:e74822. doi:10.1371/journal.pone.0074822.
- Zhang P. 2003. Studies on social structure and allogrooming behaviors in Sichuan Snub Nosed Monkeys (Rhinopithecus roxellana). Xi’an: North-Western University.
- Zhang P, Li BG, Wada K, Tan CL, Watanabe K. 2003. Social structure of a group of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling mountains of China. Acta Zoologica Sinica 49:727–735.
- Zhang P, Watanabe K, Li BG, Tan CL. 2006. Social organization of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, central China. Primates 47:374–382. doi:10.1007/s10329-006-0178-8.
- Ziegler TE, Scheffler G, Snowdon CT. 1995. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior 29:407–424. doi:10.1006/hbeh.1995.1028.
- Ziegler TE, Sousa MBC. 2002. Parent-daughter relationships and social controls on fertility in female common marmosets, Callithrix jacchus. Hormone Behavior 42:356–367. doi:10.1006/hbeh.2002.1828.
- Zumpe D, Michael RP. 2005. Relation between the dominance rank of female rhesus monkeys and their acess to males. American Journal of Primatology 13:155–169. doi:10.1002/ajp.1350130206.