Abstract
In 2013–2014, the soil mite communities from six overgrazed grassland ecosystems located in the Trascău Mountains, Romania, were investigated. Forty-six species were identified, with 645 individuals. Some abiotic factors from soil were measured (soil temperature – T, soil water content – H, soil acidity – pH, carbon content – C, total nitrogen – Nt, and C/Nt ratio). Significant statistical differences were obtained between environmental factors. Using a canonical correspondence analysis of mite species abundance, environmental variables and habitats, strong relationships between investigated factors were established.
Introduction
Soil is an important habitat for different groups of mesofauna (i.e. nematodes, mites, springtails, proturans). Soil invertebrates have an important regulatory control over the soil food web and have substantial effects on soil pedogenesis [the distribution of soil particles, the soil’s water-holding capacity and water infiltration rate, the lability of organic compounds, mineralisation, immobilisation, the availability of nitrogen (N) and other nutrients, the transport of compounds and the composition, abundance, dispersal and activity of bacteria and fungi; Hassink et al. Citation1993; Klironomos & Kendrik Citation1995; Wolters Citation2000; Cole et al. Citation2004; Lützow et al. Citation2006; Gutiérrez et al. Citation2010].
Mites (Acari) are one of the most abundant invertebrate groups within soil. Many studies have demonstrated that they are sensitive to modifications of the different physical (soil water content, temperature) and chemical [soil nutrients, organic matter, pH, N and carbon (C)] environmental factors (Ruf & Beck Citation2005; Chikoski et al. Citation2006; Gulvik Citation2007; Nielsen et al. Citation2010, Citation2012; Kardol et al. Citation2011).
In Europe, studies concerning the influence of such abiotic factors on predator soil mite communities (Acari: Mesostigmata) have been conducted mainly in forest ecosystems, revealing that external factors, such as those caused by climate change, can have a direct effect on below-ground communities (Salmane Citation2000; Hasegawa Citation2001; Huhta & Hänninen Citation2001; Huhta & Räty Citation2005; Ruf & Beck Citation2005; Malmström Citation2006; Nielsen et al. Citation2010, Citation2012; Kardol et al. Citation2011; Xu et al. Citation2012). Studies that highlight the correlations between abiotic factors and soil mite communities from other types of terrestrial ecosystems, such as grassland, are few (Cole et al. Citation2005, Citation2006, Citation2008; Briones et al. Citation2009; Wissuwa et al. Citation2012).
In Romania, the situation is similar. Studies that demonstrated the influence of abiotic (soil temperature, soil water content and pH) and biotic (type of habitat vegetation) factors on soil mite communities were made only in forest, cliff and shrub ecosystems (Manu Citation2008, Citation2011a, Citation2011b; Manu et al. Citation2013). Ecological investigations on the edaphic mite communities of Romanian grassland ecosystems have highlighted only the quantitative and qualitative modifications of their population in correlation with vegetation type (Huţu et al. Citation1991; Călugăr Citation2006a, Citation2006b).
In this context, the aim of the present investigation was to determine whether soil environmental variables (temperature, soil water content, pH, C, Nt (total nitrogen), C/Nt (carbon content/total nitrogen)) influence the structure of predator soil mite communities (Acari: Mesostigmata) within different types of overgrazed grasslands, a less studied ecosystem in Romania, and indeed more widely in Europe.
Materials and methods
The mite fauna was sampled in July and September 2013, in six grassland ecosystems. Fifty cores were sampled per ecosystem, to 10 cm depth, with a MacFadyen 5-cm corer. The 50 cores were located within an area of 2500 m2. A total of 300 samples were taken from all investigated grasslands. The samples were taken randomly. The mites were extracted with a modified Berlese–Tullgren funnel, in ethyl alcohol, clarified in lactic acid and identified to species level, using identification keys (Ghiliarov & Bregetova Citation1977; Karg Citation1993; Masan Citation2003, Citation2007; Masan & Fenda Citation2004; Masan & Halliday Citation2010, Citation2014). A total of 645 mites from 49 species were extracted from the 300 cores.
The main abiotic factors (soil temperature – T, soil water content – H%, pH, carbon content – C, total nitrogen – Nt, and C/Nt ratio) were quantified. Twenty-five soil samples from each ecosystem were investigated. The pH was measured with a C532 Jasco Consort pH meter. Soil water content and temperature were measured with a mobile soil hygrometer MST 3000+ and a bimetallic soil thermometer, respectively. Organic carbon and total nitrogen determinations were made by the dry combustion method, using a LECO Truspec CN automatic analyser.
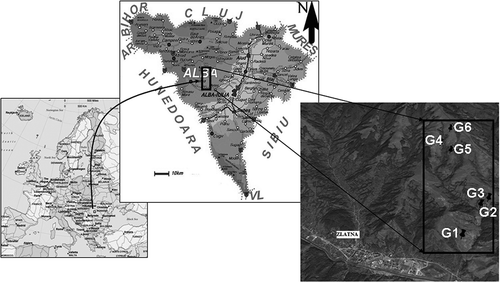
The study was made in six grassland ecosystems within the Trascău Mountains, situated in the south-eastern part of the Apuseni Mountains. Administratively, the Trascău Mountains are allocated to the counties of Cluj (only their northern part, including the Turda and Tureni Gorges, and a small part of the Arieş River valley) and Alba, representing at the same time the mountain unit with the largest area in Alba County (Lazăr Citation2011). The location and characteristics of the six overgrazed ecosystems/grasslands (G1–G6) are as follows:
Ecosystem G1 is situated at 45°06ʹ28.79”N and 23°15ʹ60.82”E, at an altitude of 530 m, with a northern exposure and a slope of 20°. The soil is sandy, with accelerated erosion phenomena. The vegetation is dominated by the following species: Agrostis capillaris, Cynodon dactylon, Juncus inflexus, Rumex acetosella and Thymus sp., with a combined coverage of 75%;
Ecosystem G2 is situated at 45°06ʹ54.35”N and 23°15ʹ51.62”E, at an altitude of 608 m, with a south-west exposure and a slope of 20°. The soil is sandy, while the vegetation is dominated by Agrostis capillaris, Pilosella officinarum and Rumex acetosella, with a combined coverage of 80%;
Ecosystem G3 is situated at 45°06ʹ51.53”N and 23°15ʹ36.49”E, at an altitude of 519 m, with a south-west exposure and a slope of 20°. The soil is sandy. The vegetation is dominated by Achillea millefolium, Frangula alnus, Rubus caesius and Rumex acetosella, with combined coverage of 100%;
Ecosystem G4 is situated at 45°07ʹ55.89”N and 23°15ʹ13.68”E, at an altitude of 875 m, with a south-east exposure and a slope of 24.5°. The soil is sandy, on the calcareous substrate. The vegetation is dominated by the following species: Centaurea triumfetti, Plantago lanceolata, Minuartia verna, Nardus stricta and Potentilla erecta, with a combined coverage of 85%;
Ecosystem G5 is situated at 45°07ʹ47.77”N and 23°15ʹ24.63”E, at an altitude of 808 m, with a southern exposure and a slope of 24°. The soil is sandy, and the vegetation is dominated by Agrostis capillaris, Achillea millefolium, Pilosella officinarum, Nardus stricta, Potentilla erecta, Sanguisorba minor, Thymus sp. and Trifolium arvense, with a combined coverage of 90%;
Ecosystem G6 is situated at 45°08ʹ90.65”N and 23°15ʹ30.99”E, at 958 m altitude, with a northern exposure and a slope of 20°. The soil is sandy, and the vegetation is dominated by Agrostis capillaris, Achillea millefolium, Pilosella officinarum, Lotus corniculatus, Medicago lupulina, Nardus stricta, Thymus sp. and Trifolium pratense, with a combined coverage of 95% ().
Prior to analysis, to fit normality and homoscedasticity assumptions, we used the log(x+1) transformation where x is the total abundance and species richness, respectively. To test whether the total abundance and the species richness are related with the environmental variables we used generalised linear mixed models (GLMM) with a Gaussian distribution of error terms and an identity link function. GLMMs allow taking into account the correlated data among the grasslands. In these models, the environmental variables were introduced as fixed factors and the grasslands were used as random factors. The best-supported models were selected using the Akaike information criterion corrected for small sample sizes (AICc) and the corresponding AICc weights (Johnson & Omland Citation2004). GLMMs were conducted in R with additional functions provided by the R packages lme4 (“lme”, Bates & Maechler Citation2009) and MuMIn (“dredge”, Barton Citation2009).
A canonical correspondence analysis (CCA) was used to study mite community responses to the environmental variables. The permutation procedure (based on 9999 cycles) was used to test the significance of constraints (environmental variables) in CCA for all eigenvalues (Johnson & Omland Citation2004). Prior to analysis, the environmental variables were Z-transformed. CCA was performed using the vegan package (Johnson & Omland Citation2004).
Results
The soil temperature varied between 8.41°C in G4 and 32.58°C in G2; soil water content varied between 48.44% in G2 and 85.48% in G4. The maximum value of the soil pH was obtained in G5 (5.77) and the minimum in G2 (4.51; ).
Table I. Abiotic factors from the grassland ecosystems (G1–G6) from Trascău Mountains (± standard deviation). T, Temperature; H, Soil water content; pH, Soil acidity; C, Carbon content; Nt, Total nitrogen; C/Nt, Carbon content/total nitrogen.
The soil carbon content was highest in G6 (4.62%) and lowest in G1 (1.75%). In the other ecosystems, the values oscillated between these two limits. The nitrogen from soil varied from 0.14% in G1 to 5.66% in G5. If we take into consideration the C/N ratio, the highest value of this variable was recorded in G5 (17.15) and the lowest in G3 (12.82).
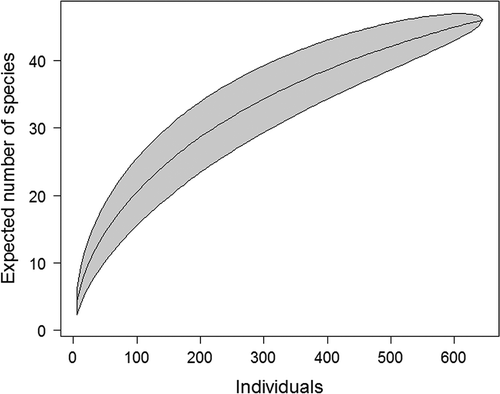
In the six grassland ecosystems, 46 species of Mesostigmata mites were identified, with 645 individuals. However, the number of species was heavily correlated with sampling effort as suggested by the individual-based accumulation curve ().
Figure 2. Individual-based accumulation curve for species richness of mite community. The shaded area represents the 95% confidence intervals.
Taking into account the numerical abundances, the highest value of this structural parameter was recorded in G6 (267 individuals), in comparison with the other ecosystems, such as G2, where the lowest value was obtained (28 individuals; ). Dominant species were: Hypoapis oblonga, Asca bicornis, Hypoaspis vacua, Hypoaspis aculeifer, Hypoaspis praesternalis, Rhodacarellus silesiacus and Rhodacarus denticulatus. Mesostigmata mites with lower numerical abundances (Zercon berlesei, Zercon hungaricus, Trachytes irenae, Prozercon kochi and Parazercon radiatus) are species which prefer natural ecosystems, with habitats rich in organic matter (litter, humus, etc.; Masan & Fenda Citation2004; Manu et al. Citation2013).
Table II. Numerical abundance of the mesostigmatid mites identified from investigated grassland ecosystems (G1–G6).
The best-supported models by the data for both abundance and species richness included Nt and H (). For both abundance and species richness the best model included Nt, and for species richness it was followed closely by the second-best-supported model that included Nt and H.
Table III. Model selection results. Models are ranked in a decreasing Akaike weight order. For clarity, models with Akaike weight < 0.04 are not shown. Statistics include the log likelihood (LL), the number of estimated parameters (K), the second-order Akaike information criterion corrected for small sample sizes (AICc), AIC difference (ΔAICi) and Akaike weights (wi). Nt = Total nitrogen; Nt + H = Total nitrogen + Soil water content; Nt + T = Total nitrogen + Temperature; Nt + H + C = Total nitrogen + Soil water content + Carbon content; Nt + C = Total nitrogen + Carbon content; Nt + H + T = Total nitrogen + Soil water content + Temperature; Nt + pH = Total nitrogen + Soil acidity.
The CCA of the association between mite abundance and the environmental variables and habitat types are shown in and . The first two canonical axes accounted for 42.23% (CCA1 = 21.53%; CCA2 = 20.69%) of the total variation in the original matrix. The first canonical axis was highly correlated with pH (−0.62) whereas the second canonical axis was correlated with Nt (−0.72), temperature (0.62), C (−0.69) and soil water content (−0.554). Species from the upper left quadrant were associated with temperature. The right lower quadrant contains species associated with Nt, C and soil water content. Species associated with pH in particular but also with C/N are placed in the lower left quadrant.
Figure 3. Canonical correspondence analysis bi-plot of abundance mite species and environmental variables. Length and direction of arrows indicate the relative importance and direction of change in the environmental variables (Ar. se. – Arctoseius semiscissus; As.bi. – Asca bicornis; Am.sp. – Amblyseius sp.; Ch. br. – Cheroseius bryophilus; Ga.bi. – Gamasellodes bicolor; Hy.ac. – Hypoaspis aculeifer; Hy.as. – Hypoaspis astronomica; Hy.ka. – Hypoaspis karawaiewi; Hy.ob. – Hypoapis oblonga; Hy.pr. – Hypoaspis preasternalis; Hy.va. – Hypoaspis vacua; Ev.os. – Eviphis ostrinus; Ip.pu. – Iphidonopsis pulvisculus; Ol.se. – Ololaelaps sellnicki; Pa.pe. – Pachylaelaps pectinifer; Pr. ko. – Prozercon kochi; Pr. py. – Protogamasellus pygmaeus; Pr.mi. – Protogamasellus mica; Rh.de. – Rhodacarus denticulatus; Rh.si. – Rhodacarellus silesiacus; Tr.ir. – Trachytes irenae; Tr. pa. – Trachytes pauperior; Ze.re. – Zerconopsis remiger).
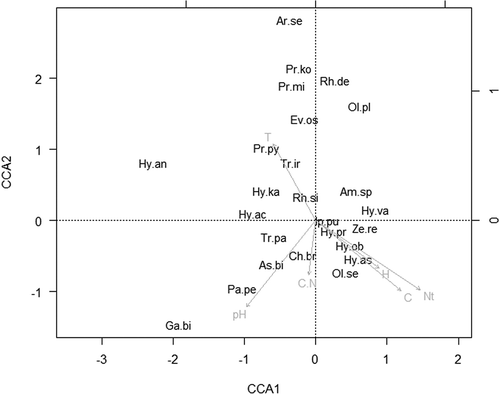
Figure 4. Canonical correspondence analysis bi-plot of abundance of mite species and habitat types (Am.sp. – Amblyseius sp.; Ch. br. – Cheroseius bryphilus; Ga.bi. – Gamasellodes bicolor; Hy.ac. – Hypoaspis aculeifer; Hy.ka. – Hypoaspis karawaiewi; Hy.pr. – Hypoaspis preasternalis; Hy.sp.1 – Hypoaspis sp.; Hy.va. – Hypoaspis vacua; La. sp. – Laioseius sp.; Le.in. – Leioseius insignitus; Ol. pl. – Ololaelaps placentulus; Pr.mi. – Protogamasellus mica; Rh de – Rhodacarus denticulatus; Rh.pe. – Rhodacarellus perspicuus; Rh.si. – Rhodacarellus silesiacus).
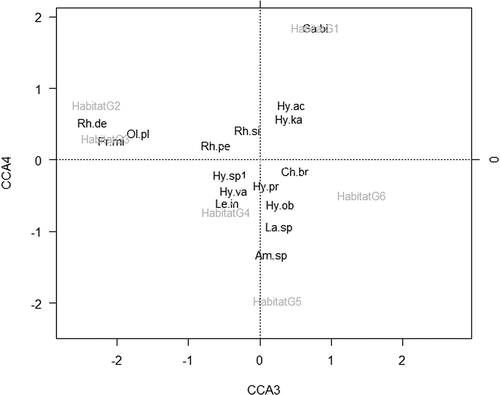
The third canonical axis explained 12.98% of the variability and the fourth canonical axis 10.60% of the variability, and the axes were associated with habitat types. Species of habitats G2 and G3 were separated from species of habitats G5 and G6, whereas species of habitats G4 and G1 were separated from all the others.
Discussion
In the investigated grassland ecosystems 46 species were identified, with 645 individuals. The majority of the identified species prefer meadow-grasslands, the most dominant ones being: Asca bicornis, Hypoaspis vacua, Hypoaspis praesternalis, Hypoapis aculeifer, Lasioseius berlesei, Rhodacarellus silesiacus, Lysigamasus misselus and Protogamasellus mica (Călugăr Citation2006a; Gwiazdowicz Citation2007; Salmane & Brumelis Citation2010; Wissuwa et al. Citation2012).
In terms of species diversity, as well as the abundance of the mite fauna, the best conditions for the development of a rich soil Mesostigmata fauna appear to be in ecosystems G4 (34.69% of the total number of observed species), G5 (30.61%) and G6 (57.14%). These ecosystems are characterised by the highest values of soil water content (over 70%), C (over 4.3%) and Nt (over 0.33%).
Considering the CCA, the length of the vectors of the given variable indicates the importance of the variable on the CCA plot. Species plotted close to the vectors have a strong relationship with them. Species located near the origin do not show a strong relationship with any of the variables. Taking account of these relationships, species as Arctoseius semiscissus, Hypoaspis karawaiewi, Trachytes irenae, Hypospis aculeifer and Protogamasellus mica are influenced by the soil temperature. Studies have revealed that temperatures between 30 and 32°C were the maximum tolerated before significant decreases in numbers were detected in Mesostigmata mites (Huhta & Hänninen Citation2001; Malmström Citation2006, Citation2008; Manu Citation2008, Citation2011a, Citation2011b).
Species such as Trachytes pauperior, Pachylaelaps pectinifer, Asca bicornis, Gamasellodes bicolor are closed linked to the pH and C/N ratio. Similar results have been reported by other authors, concluding that a pH close to neutral is optimal for mesostigmatids (Bedano et al. Citation2005). In contrast, some other research has found that these invertebrates prefer a lower pH, which is favourable for the development of fungi, the food source for other soil invertebrates which in turn form the prey for mesostigmatids (Chikoski et al. Citation2006; Manu Citation2011a; Birkhofer et al. Citation2012). Acidity and dominance of sandy soils (such as those described from the six grassland ecosystems in the present study) are known to contribute to low actinomycete biomass (Kooijman et al. Citation2009; Birkhofer et al. Citation2012).
The soil water content, C and Nt are in strong relationship with species such as Ololalepas sellnicki, Hypoapsis astronomica, Hypoaspis preasternalis, Zerconopsis remiger and Iphidonopsis pulvisculus. Previous studies from Europe have highlighted that these species are characteristic of grasslands or meadows (Salmane Citation2000; Călugăr Citation2006b; Salmane & Brumelis Citation2010).
Huhta and Hänninen (Citation2001) found that the numerical abundance of mesostigmatids is constant at varying soil moisture; other researchers have contradicted this statement (Bedano et al. Citation2005; Kamczyc Citation2006; Manu Citation2008, Citation2011a, Citation2011b). Salmane (Citation2000) stated that the soil relative humidity must be recognised as a limiting factor and even where soil moisture is sufficient, the decisive limiting factor is soil temperature.
Studies on predator mite communities from natural grassland ecosystems revealed that mites have been reported as most abundant in soils with low pH values and nitrate concentrations (Hasegawa Citation2001; Bedano et al. Citation2005; Birkhofer et al. Citation2012; Miller et al. Citation2014). Different studies have shown the relationship between soil mesofauna and nitrate content in grasslands to be very variable, with no, weak negative, strong negative or even positive correlations, depending on land-use type (Jandl et al. Citation2003; Lindberg & Persson Citation2004; Cole et al. Citation2005; Fountain et al. Citation2008; Birkhofer et al. Citation2012).
A similar trend was described for the C/Nt ratio. Studies have revealed that soil arthropods require more N (due to N mineralisation) than C for their development (Hassink et al. Citation1993; Chikoski et al. Citation2006). Carbon to nitrogen ratios vary with climatic conditions, primarily temperature and humidity. Researches have revealed that N mineralisation decrease with decreasing soil moisture and increasing soil temperature (Persson Citation1989; Hassink et al. Citation1993; Chikoski et al. Citation2006). Therefore, it is not surprising that our results showed a pattern in C/N in overgrazed grasslands, with a lower C/N ratio where soil water content varied between 48.44 and 60.28% and soil temperature fluctuated between 29.24 and 32.58°C.
A CCA bi-plot of abundance of mite species and habitat types revealed that some mesostigmatids such as Protogamasellus mica, Ololaelaps placentulus, Rhodacarellus perspicuus, Rhodacarellus silesiacus and Rhodacarus denticulatus occurred in G2 and G3, ecosystems characterised by close average values of soil acidity, C and Nt content. Other species such as Hypoaspis vacua, Leioseius insignitis occurred in G4, the most humid ecosystem, and Hypoaspis aculeifer, Hypoaspis karawaievi, Gamasellodes bicolor in G1, where the soil temperature recorded the highest values and the soil contents of C and N had the lowest percentage.
Hypoaspis oblonga, Cherosieus bryophilus and Hypoaspis praesternalis were found in G5 and G6, ecosystems characterised by close average values, but increased humidity, decreased temperature, more acid soils and higher content of C and Nt, in comparison with the other ecosystems.
Studies from Europe revealed that some species (Rhodacarellus silesiacus, Arctoseius semiscissus, Proctolaelaps pygmaeus and Protogamasellus mica) were characteristic of a range of xerothermophilous habitats (natural or anthropogenic in origin) such as meadows, dunes, urban parks, arable fields, and derelict industrial and mining areas. Other species (e.g. Hypoaspis aculeifer, Veigaia nemorensis and Asca bicornis) have a wide ecological plasticity, being found in different forest habitats and in meadows, as well as in polluted areas (Salmane Citation2000; Gwiazdowicz Citation2007; Salmane & Brumelis Citation2010; Manu Citation2011a, Citation2011b; van Leeuwen et al. Citation2015).
The soil mite communities from the investigated grassland ecosystems, in the present study, are characterised mainly by typical meadow species. Ecosystems defined by the highest values of soil water content, carbon and Nt are characterised by the highest number of mite species and overall numerical abundance. In contrast, those grassland ecosystems with the highest soil temperature and most decreased pH have the lowest species diversity and numerical abundance.
The statistical analysis applied in this study revealed that the environmental variables influence the soil mite communities, and also that the occurrence of certain sensitive species depends on these abiotic factors.
Acknowledgements
This work was supported by the Institute of Biology, Romanian Academy, under Grant RO1567-IBB01/2015, and by the Executive Agency for Higher Education, Research, Development and Innovation Funding, Romania (UE-FISCDI), under Grant 50/2012 ASPABIR.
The authors wish to thank Dr. Peter Mašán, Institute of Zoology, Slovak Academy of Sciences, for confirming the identification of some of the Mesostigmata mites, and Owen Mountford (NERC (National Environment Research Council) Centre for Ecology and Hydrology, UK) for advice on the English text. We thank Prof. Radu Lăcătuşu, from Institute of Soil Science and Agrochemistry, Bucharest, for kindly providing the general soil characterisation in the sampling plots.
We also thank Simona Plumb and Rodica Iosif for their assistance in the laboratory and the field.
References
- Barton K. 2009. MuMIn: multi-model inference. R package,version 0.12.2. Available: http://r-forge.rproject.org/projects/mumin. Accessed Apr 2015 27.
- Bates D, Maechler M. 2009. lme4: Linear mixed-effects models using S4 classes. R package, version 0.999375-31. Available: http://CRAN.Rproject.org/package=lme4. Accessed Apr 2015 15.
- Bedano JC, Cantu MP, Doucet ME. 2005. Abundance of soil mites (Arachnida: Acari) in a natural soil of central Argentina. Zoological Studies 44:505–512.
- Birkhofer K, Schoning I, Alt F, Herold N, Klarner B, Maraun M, Marhan S, Oelmann Y, Wubet T, Yurkov A, Begerow D, Berner D, Buscot F, Daniel R, Diekötter T, Ehnes RB, Erdmann G, Fischer C, Foesel B, Groh J, Gutknecht J, Kandeler E, Lang C, Lohaus G, Meyer A, Nacke H, Näther A, Overmann J, Polle A, Pollierer MM, Scheu S, Schloter M, Schulze ED, Schulze W, Weinert J, Weisser WW, Wolters V, Schrumpf M. 2012. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS ONE 7:1–8.
- Briones MJI, Ostle NJ, McNamara NP, Poskitt J. 2009. Functional shifts of grassland soil communities in response to soil warming. Soil Biology and Biochemistry 41:315–322. doi:10.1016/j.soilbio.2008.11.003.
- Călugăr A. 2006a. Qualitative and quantitative studies upon the edaphic microarthropods fauna in some grassland ecosystems from Moldavian Plain (Romania). Complexul muzeal de ştiinţele naturii „Ion Borcea” Bacău. Studii şi Comunicări 21:230–231.
- Călugăr A. 2006b. On the gamasid fauna (Acari: Gamasina) from the grassland ecosystems of the Moldavian Plain (Romania). Complexul muzeal de ştiinţele naturii „Ion Borcea” Bacău. Studii şi Comunicări 21:232–235.
- Chikoski JM, Ferguson SH, Meyer L. 2006. Effects of water addition on soil arthropods and soil characteristics in a precipitation-limited environment. Acta Oecologica 30:203–211. doi:10.1016/j.actao.2006.04.005.
- Cole L, Bradford MA, Shaw PJA, Bardgett RD. 2006. The abundance, richness and functional role of soil meso- and macrofauna in temperate grassland—A case study. Soil Biology and Biochemistry 33:186–198.
- Cole L, Buckland SM, Bardgett RD. 2005. Relating microarthropod community structure and diversity to soil fertility manipulations in temperate grassland. Soil Biology and Biochemistry 37:1707–1717. doi:10.1016/j.soilbio.2005.02.005.
- Cole L, Buckland SM, Bardgett RD. 2008. Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biology and Biochemistry 40:505–514. doi:10.1016/j.soilbio.2007.09.018.
- Cole L, Dromph KM, Boaglio V, Bardgett RD. 2004. Effect of density and species richness of soil mesofauna on nutrient mineralisation and plant growth. Biology and Fertility of Soils 39:337–343.
- Fountain MT, Brown VK, Gange AC, Symondson WOC, Murray PJ. 2008. Multitrophic effects of nutrient addition in upland grassland. Bulletin of Entomological Research 98:283–292. doi:10.1017/S000748530700555X.
- Ghiliarov MS, Bregetova NG. 1977. Opredelitelii obiataiosik v. Pocive Klesei Mesostigmata. [Handbook for the identification of soil-inhabiting mites (Mesostigmata)]. Leningrad. Russian: Akademia Nauk USSR, Zoologhiceskii Innstitut Evoliocionoi Morphologhii I Ecologhii Jivotnik im A.H. Savertova, Izdaatelistvo Nauka. pp. 1–717.
- Gulvik ME. 2007. Mites (Acari) as indicators of soil biodiversity and land use monitoring: A review. Polish Journal of Ecology 55:415–450.
- Gutiérrez M, Jesús JB, Trigo D, Fernández R, Novo M, Díaz Cosín DJ. 2010. Relationships among spatial distribution of soil microarthropods, earthworm species and soil properties. Pedobiologia 53:381–389. doi:10.1016/j.pedobi.2010.07.003.
- Gwiazdowicz D. 2007. Ascid mites (Acari, Gamasina) from selected forest ecosystems and microhabitats in Poland. Poznan: University Augusta Cieszkowskiego. pp. 1–248.
- Hasegawa M. 2001. The relationship between the organic matter composition of a forest floor and the structure of a soil arthropod community. European Journal of Soil Biology 37:281–284. doi:10.1016/S1164-5563(01)01099-8.
- Hassink J, Bouwman LA, Zwart KB, Bloem J, Brussaard L. 1993. Relationships between soil texture, physical protection of organic matter, soil biota, and C and N mineralization in grassland soils. Geoderma 57:105–128. doi:10.1016/0016-7061(93)90150-J.
- Huhta V, Hänninen SM. 2001. Effects of temperature and moisture fluctuations on an experimental soil microarthropod community. Pedobiologia 45:279–286. doi:10.1078/0031-4056-00085.
- Huhta V, Räty M. 2005. Soil animal communities of planted birch stands in central Finland. Silva Fennica 39:5–19. doi:10.14214/sf.392.
- Huţu M, Bulimar F, Călugăr M. 1991. Efectul amendamentelor calcice asupra microartropodelor edafice dintr-o pajişte fertilizată cu îngrăşăminte azotoase [Effect of calcium treatments on edaphic microarthropods from a fertilized meadow with nitrogen fertilizers]. Analele Ştiinţifice ale Universităţii “Al. I. Cuza” Iaşi 37:235–240. [Romanian].
- Jandl R, Kopeszki H, Bruckner A, Hager H. 2003. Forest soil chemistry and mesofauna 20 years after an amelioration fertilization. Restoration Ecology 11:239–246. doi:10.1046/j.1526-100X.2003.00179.x.
- Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends in Ecology and Evolution 19:101–108. doi:10.1016/j.tree.2003.10.013.
- Kamczyc J. 2006. Microhabitat preferences of Veigaia mollis Karg, 1971 in the mountain reserve “Szczeliniec Wielki”. Biology Letters 43:193–195.
- Kardol P, Reynolds WN, Norby NJ, Classen AT. 2011. Climate change effects on soil microarthropod abundance and community structure. Applied Soil Ecology 47:37–44. doi:10.1016/j.apsoil.2010.11.001.
- Karg W. 1993. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach. [Acari (Acarina) supraorder Parasitiformes (Anactinochaeta) Cohors Gamasina Leach – predatory mites]. Die Tierwelt Deutschlands 59:1–513. [German].
- Klironomos JN, Kendrik B. 1995. Relationships among microarthropods, fungi, and their environment. Plant and Soil 170:183–197. doi:10.1007/BF02183066.
- Kooijman AM, van Mourik JM, Schilder ML. 2009. The relationship between N mineralization or microbial biomass N with micromorphological properties in beech forest soils with different texture and pH. Biology and Fertility of Soils 45:449–459. doi:10.1007/s00374-009-0354-2.
- Lazăr GA. 2011. Trascău Mountains – Geoecological Study. PhD thesis, University Babeş-Bolyai, Cluj- Napoca, Romania.
- Lindberg N, Persson T. 2004. Effects of long-term nutrient fertilisation and irrigation on the microarthropod community in a boreal Norway spruce stand. Forest Ecology and Management 188:125–135. doi:10.1016/j.foreco.2003.07.012.
- Lützow MV, Kögel-Knabner AI, Ekschmitt AK, Matzner BE, Guggenberger CG, Marschner DB, Flessa EH. 2006. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions – A review. European Journal of Soil Science 57:426–445. doi:10.1111/j.1365-2389.2006.00809.x.
- Malmström A. 2006. Effects of wildfire and prescribed burning on soil fauna in boreal coniferous forests. Dissertation (sammanfattning/summary). Uppsala: Sveriges lantbruksuniv. Acta Universitatis Agriculturae Sueciae. pp. 1652–6880.
- Malmström A. 2008. Temperature tolerance in soil microarthropods: Simulation of forest-fire heating in the laboratory. Pedobiologia 51:419–426. doi:10.1016/j.pedobi.2008.01.001.
- Manu M. 2008. The influence of some abiotical factors on the structural dynamics of the predatory mite populations (Acari: Mesostigmata) from an ecosystem with Myricaria germanica from Doftana Valley (Romania). Travaux du Museum D’Histoire Naturelle”Grigore Antipa” 51:463–471.
- Manu M. 2011a. The influence of some environmental factors on the species diversity of the predator mites (Acari: Gamasina) from natural forest ecosystems in Bucegi massif. Travaux du Museum D’Histoire Naturelle”Grigore Antipa” 54:9–20.
- Manu M 2011b. Influence of the cliff microclimate on the population ecology of soil predatory mites (Acari: Mesostigmata - Gamasina) from Romania. In: Bulgarian National Multidisciplinary Scientific Network of the Professional Society for Research Work editor. Proceedings of the Third International Conference “Research People and Actual Tasks on Multidisciplinary Sciences”, Lozenec: Bulgary. p 1.
- Manu M, Băncilă RI, Onete M. 2013. Soil mite communities (Acari: Gamasina) from different ecosystem types from Romania. Belgian Journal of Zoology 143:30–41.
- Masan P. 2003. Macrochelid mites of Slovakia (Acari, Mesostigmata, Macrochelidae). Bratislava: Institute of Zoology, Slovak Academy of Science. pp. 1–149.
- Masan P. 2007. A reviews of the family Pachylaelapidae in Slovakia with systematic and ecology of European species (Acari: Mesostigmata: Eviphidoidea). Bratislava: Institute of Zoology, Slovak Academy of Science. pp. 1–247.
- Masan P, Fenda P. 2004. Zerconid mites of Slovakia (Acari, Mesostigmata, Zerconidae). Bratislava: Institute of Zoology, Slovakia Academy of Science. pp. 1–238.
- Masan P, Halliday B. 2010. Review of the European genera of Eviphididae (Acari: Mesostigmata) and the species occurring in Slovakia. Zootaxa 2585:1–122.
- Masan P, Halliday B. 2014. Review of the mite family Pachylaelapidae (Acari: Mesostigmata). Zootaxa 3776:1–66. doi:10.11646/zootaxa.3776.1.1.
- Miller J, Battigelli JP, Willms WD. 2014. Grazing protection influences soil mesofauna in ungrazed and grazed riparian and upland pastures. Rangeland Ecology and Management 67:429–434. doi:10.2111/REM-D-14-00004.1.
- Nielsen UN, Osler GHR, Campbell CD, Burslem FRP, Van der Wal R. 2010. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. Journal of Biogeography 37:1317–1328. doi:10.1111/j.1365-2699.2010.02281.x.
- Nielsen UN, Osler GHR, Campbell DC, Burslem DFRP, Van der Wal R. 2012. Predictors of fine-scale spatial variation in soil mite and microbe community composition differ between biotic groups and habitats. Pedobiologia 55:83–91. doi:10.1016/j.pedobi.2011.11.002.
- Persson T. 1989. Role of soil animals in C and N mineralisation. Plant and Soil 115:241–245. doi:10.1007/BF02202592.
- Ruf A, Beck L. 2005. The use of predatory soil mites in ecological soil classification and assessment concepts with perspectives for oribatid mites. Ecotoxicology and Environmental Safety 62:290–299. doi:10.1016/j.ecoenv.2005.03.029.
- Salmane I. 2000. Investigation of the seasonal dynamics of soil Gamasina mites (Acari: Mesostigmata) in Pinaceum myrtilosum, Latvia. Ekologia 19:245–252.
- Salmane I, Brumelis G. 2010. Species list and habitat preference of mesostigmata mites (Acari, Parasitiformes) in Latvia. Acarologia 50:373–394. doi:10.1051/acarologia/20101978.
- van Leeuwen JP, Lehtinen T, Lair GL, Bloem J, Hemerik L, Ragnarsdóttir KV, Gísladóttir G, Newton JS, de Ruiter PC. 2015. An ecosystem approach to assess soil quality in organically and conventionally managed farms in Iceland and Austria. Soil 1:83–101. doi:10.5194/soil-1-83-2015.
- Wissuwa J, Salamon JA, Frank T. 2012. Effects of habitat age and plant species on predatory mites (Acari, Mesostigmata) in grassy arable fallows in Eastern Austria. Soil Biology and Biochemistry 50:96–107. doi:10.1016/j.soilbio.2012.02.025.
- Wolters V. 2000. Invertebrate control of soil organic matter stability. Biology and Fertility of Soils 31:1–19. doi:10.1007/s003740050618.
- Xu GL, Kuster TM, Günthardt-Goerg MS, Dobbertin M, Li MH. 2012. Seasonal exposure to drought and air warming affects soil Collembola and mites. PLoS ONE 7:e43102. doi:10.1371/journal.pone.0043102.