Abstract
The digestive tract of five adult specimens of the European hake, Merluccius merluccius L., from Eastern Adriatic, were analysed histologically in 2011, using haematoxylin–eosin and Alcian blue/PAS (Periodic Acid Schiff) methods. The paraffin sections of oesophagus, stomach, intestines, liver, gall bladder and pancreas have been stained with a hematoxylin–eosin technique in order to elucidate the main histological features of the digestive tract. The wall of oesophagus and stomach is formed by four distinctive layers, while those of the intestines and gall bladder consist of three layers: the mucosa, the muscular and the outer layer. The mucosa is the innermost layer, which usually consisted of three different layers: epithelium, lamina propria and muscularis mucosae. The submucosa is a layer of connective tissue and it was not seen in the intestines and gall blader. In the oesophagus, stomach and intestines, as well as in the gall bladder, the muscular layer consists of two layers of muscle fibres: circular and longitudinal. The outermost layer of the upper parts of the visceral organs is adventitia consisting of connective tissue. In the caudal parts of digestive tract, this layer is replaced by serosa. The liver consists of hepatocytes containing a mass of lipid droplets. The pancreatic tissues contain serous acini. The present study suggests that histological features of the digestive tract of the hake Merluccius merluccius L. are mostly similar to those of other carnivorous fish, and according to its feeding habits.
Keywords:
Introduction
The European hake Merluccius merluccius (Linnaeus, 1758) is one of the major commercial and exploited demersal fish throughout the Adriatic Sea (Ungaro et al. Citation2001). It is also distributed in the marine ecosystems throughout the Mediterranean Sea and Atlantic Ocean (FAO Citation1997; Arneri & Morales-Nin Citation2000; Ortiz-Delgado et al. Citation2012). Due to its abundance and commercial value, the hake was an important object of many research studies in the last few decades. The studies mentioned above usually concerned the biology and ecology of the hake (Karlovac Citation1965; Jardas Citation1976; Jukic & Piccinetti Citation1987), its distribution and feeding habits (Karlovac Citation1959; Jukic Citation1972, Citation1984; Papaconstantinou & Caragitsou Citation1987; Oliver & Massutí Citation1995; Carpentieri et al. Citation2005) or its reproduction and population dynamics (Zupanovic Citation1968; Zupanovic & Jardas Citation1986; Oliver Citation1991). This species has also been considered a species for diversification in marine aquaculture (Iglesias et al. Citation2010; Sánchez et al. Citation2011) due to its high growing rates and competitive market prices (Arneri & Morales-Nin Citation2000; Palomera et al. Citation2005). Since the histological features of the European hake’s digestive tract had never been studied before, this study represents the first record of the European hake’s digestive system morphology.
The European hake is an important predator of deeper shelf–upper slope in Mediterranean communities (Carpentieri et al. Citation2005). The diet of hake juveniles usually consists of zooplanktonic crustaceans and decapods, while sexually mature specimens are fully piscivorous in diet (Carpentieri et al. Citation2005). In fish, as in other vertebrates, the digestive tract consists of the alimentary canal, a hollow tube of varying diameter which is longitudinally divided into the oesophagus, the stomach, the intestines and the rectum (Treer et al. Citation1995). The digestive tract also includes some associated organs such as the tongue and the teeth in the oral cavity, and the extramural digestive organs, specifically the liver and gall bladder, and the exocrine pancreas. The salivary glands are usually missing in the oral cavity of fish (Treer et al. Citation1995). Histologically, from the cranial end of the oesophagus to the caudal end of the rectum, the wall of the alimentary canal is formed by four distinctive layers. Starting at the lumen, these layers are: the mucosa, the submucosa, the muscularis externa, and the serosa or adventitia (Kierszenbaum Citation2002). The digestive system of fish shows marked diversity in its morphology and function. This is related to both taxonomy and different feeding habits (Al Abdulhadi Citation2005). Although this species is much exploited for research purposes, including feeding habits (Karlovac Citation1959; Jukic Citation1972, Citation1984; Papaconstantinou & Caragitsou Citation1987; Oliver & Massutí Citation1995; Carpentieri et al. Citation2005), knowledge of its digestive tract histology is still scarce. The present study elucidates for the first time the detailed histology of the European hake’s digestive tract.
Materials and methods
A total of five adult European hake Merluccius merluccius L. specimens were sampled to study the histology of the digestive system. The specimens were collected in the Eastern Adriatic in June 2011 by commercial trawl. We chose specimens in a fed state in order to avoid a fasting effect on the morphological characteristics of the intestinal epithelial cells. The organs of the digestive system were fixed in 10% formalin immediately after collecting. Tissue sections were taken from all parts of the digestive tract, starting from the cranial end of the oesophagus to the caudal end of the rectum. After dehydration in an ascending series of ethanol, the sections were cleared with xylene, and then embedded in paraffin. Tissue sections were cut transversally at 6 μm and mounted on glass slides. The sections were then deparaffinised with xylene and stained with haematoxylin–eosin staining for presenting basic morphology of the digestive organs. The sections were then observed using a light microscope (Axio Imager M.1, Zeiss).
Alcian blue/PAS staining
The sections through the mucosal layer from the oesophagus, the stomach and the intestines were treated with Alcian blue/PAS (Periodic Acid Schiff) staining in order to elucidate the type of the mucous cells and the nature of the produced mucus. The sections were deparaffinised and stained with Alcian blue for 15 minutes. After washing in distilled water, the sections were treated with periodic acid for 10 minutes and then with Schiff’s reagent for 10 minutes. The sections were then dehydrated, cleared and mounted on the glass slides.
Results
The oesophagus
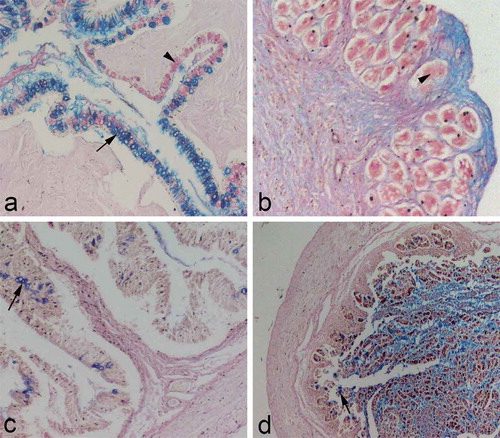
The oesophageal wall of the European hake Merluccius merluccius L. consists of four layers: mucosa (tunica mucosa), submucosa (tunica submucosa), muscular layer (tunica muscularis) and the outer layer (tunica adventitia) (). The mucosa forms high folds, deeply protruding into the oesophageal lumen. The oesophageal mucosa consists of mucosal epithelium at the boundary of the lumen, and lamina propria, a loose areolar connective tissue located just beneath the epithelial basement membrane. Mucosal epithelium is nonkeratinised stratified squamous epithelium. The cells located on the epithelial surface are typical squamous, while those placed at the boundary of the lamina propria are more cuboidal in shape. The nuclei of the squamous cells are mostly flattened and centrally positioned. The basal layer of the epithelial cells is organised as a single layer of cuboidal cells lying on the basement membrane, thus forming the boundary between epithelium and lamina propria. The nuclei of these cells are also centrally positioned but round in shape (). The main feature of the oesophageal epithelium is the presence of the numerous goblet cells scattered among squamous epithelial cells. The goblet cells are large and bubble-like, with nuclei flattened at the basal side of the cell (). When stained with Alcian blue/PAS, the goblet cells mainly stained blue, indicating the predominance of the acidic mucins in the goblet cells (). Lamina propria is a connective tissue layer containing blood vessels. The third mucosal layer, lamina muscularis mucosae, has not been seen. The submucosa is a connective tissue layer adjacent to the mucosa. It also consists of loose connective tissue, but contains more blood vessels. The muscular layer (tunica muscularis) of the European hake’s oesophagus consists of two layers: an inner longitudinal layer and an outer circular layer of muscular fibres. Both layers are made of skeletal muscle fibres. The connective tissue separates the bundles of muscle fibres, bringing them the blood vessels. The connective tissue is even more abundant in the inner muscular layer (). The outer layer of the oesophageal wall is adventitia, a thin layer of connective tissue containing blood vessels.
Figure 1. (a) Transverse section through the oesophageal wall; a: mucosa; b: submucosa; c: inner and outer muscular layer; hematoxyiln and eosin staining, ×100. (b) Tunica mucosa of the oesophagus; a: stratified squamous epithelium; b: lamina propria; (arrow): goblet cell; hematoxyiln and eosin staining, ×400. (c) The muscular layer of the oesophagus; a: inner longitudinal layer; b: outer circular layer; hematoxyiln and eosin staining, ×400. (d) Transverse section through the stomach mucosa; a: simple columnar epithelium; b: lamina propria with gastric glands; hematoxyiln and eosin staining, ×200. (e) The pyloric mucosa; a: simple columnar epithelium; b: lamina propria with no gastric glands; hematoxyiln and eosin staining, ×200. (f) Transverse section through the anterior intestines; a: simple columnar epithelium; b: lamina propria; c: inner and outer muscular layer; hematoxyiln and eosin staining, ×100.
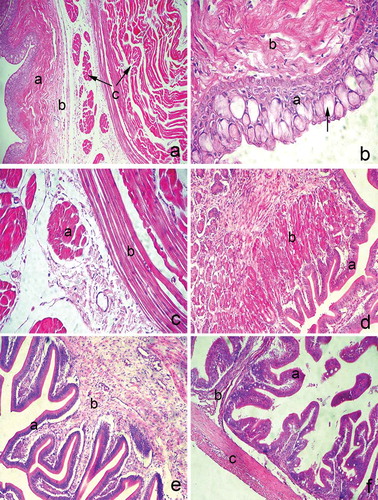
Figure 2. (a) Transverse section through the oesophageal mucosa; goblet cells containing acidic mucins (arrow); goblet cells containing neutral mucins (arrowhead); Alcian blue/PAS, ×100. (b) Transverse section through the stomach mucosa: glands in the lamina propria containing neutral glycosaminoglycans; note the surrounding tissue stained blue (positively to acidic glycosaminoglycans); Alcian blue/PAS, ×100. (c) Transverse section through the mucosa of the anterior intestine: sporadic goblet cells stained positively to acid mucins (arrow); Alcian blue/PAS, ×100. (d) Transverse section through the posterior intestine: sporadic goblet cells stained positively to acidic mucins (arrow); Alcian blue/PAS, ×40.
The stomach
The stomach is a sac-like organ placed between the oesophagus and intestines. European hake’s stomach has a thick wall containing four layers: mucosa (tunica mucosa), submucosa (tunica submucosa), muscular layer (tunica muscularis) and outer layer (tunica serosa). The mucosa consists of epithelium (lamina epithelialis), lamina propria and lamina muscularis mucosae. The epithelium and lamina propria form longitudinal mucosal folds into the stomach lumen, while lamina muscularis mucosae underlays lamina propria separating the mucosa from submucosa. The mucosal surface of the stomach is lined by a simple columnar epithelium containing microvilli on the apical domain of the cell. Numerous large nuclei could be seen on the basal part of the epithelium. Lamina propria contains simple tubular gastric glands (). The gastric glands consist mostly of large cells with basal placed nuclei and cytoplasm filled with granules. When treated with Alcian blue/PAS staining, these cells stained slightly red, probably because they contained neutral glycosaminoglycans (). The submucosa is a thick layer of loose connective tissue containing numerous blood vessels, particularly at the boundary of the muscular layer. Numerous nuclei of fibroblasts could be seen in the submucosal connective tissue. The muscular layer (tunica muscularis) of the stomach consists of two muscle layers separated by a narrow layer of connective tissue containing numerous blood vessels. The inner muscular layer is extremely thick compared to the outer layer and it consists of longitudinal bundles of muscular fibres. The muscular fibres in the outer layer are more obliquely arranged. The serosa is the outermost layer of the stomach, consisting of a thin layer of connective tissue cells and fibres. The pyloric region of the European hake’s stomach contains no pyloric caeca. The pyloric wall also consists of mucosa, submucosa, muscular layer and serosa. The finger-like mucosal folds are lined by simple columnar epithelium similar to that in the stomach fundus, but with no microvilli. Lamina propria of the pylorus consists of loose connective tissue containing blood vessels with no gastric glands (). The muscular layer of the mucosa consists of thin circular muscle fibres. The submucosa is also made of connective tissue. The main feature of the stomach pylorus is the pyloric sphincter, a strong circular muscle forming the inner muscular layer. The outer muscular layer is made of longitudinal muscular bundles. The pyloric serosa is similar to those in the fundus.
The intestines
According to its histological characteristics, the intestines in the European hake could be distinguished in two parts: the anterior and the posterior intestines. The anterior intestines are larger in diameter than the lower intestines. The wall of the upper intestines is thin, containing three layers: mucosa (tunica mucosa), muscular layer (tunica muscularis) and serosa (tunica serosa). The mucosa forms mucosal folds (villi), leaf-like protrusions entering deeply into the lumen. Each fold is lined by simple columnar epithelium. Epithelial cells are elongated with basally placed nuclei and microvilli on their apical domain. The sporadic goblet cells are scattered among the epithelial columnar cells (). When stained with Alcian blue/PAS, these cells stained blue indicating the presence of the acidic mucins (). The central part of the villi is made of lamina propria, a connective tissue layer containing blood vessels. Some smooth muscle cells representing lamina muscularis mucosae could be seen just beneath the lamina propria. Since the submucosa is missing, the muscular layer is directly adjacent to the mucosa. It consists of two muscle layers: the inner circular and the outer longitudinal muscle layer. The outer boundary of the anterior intestines is delimited by tunica serosa.
The mucosa of the posterior intestines differs in structure from those in the anterior intestines. In the posterior intestines, the mucosa forms finger-like folds consisting of epithelium and lamina propria. Each mucosal fold is lined by simple columnar epithelium. Columnar epithelial cells contain microvilli on their apical domain. A few goblet cells could be seen scattered among epithelial cells. The goblet cells stained blue with Alcian blue/PAS, indicating the acidic mucins of their content (). Lamina propria is made of loose areolar tissue containing blood vessels. The two-layered tunica muscularis is adjacent to the mucosa. The muscular layers are similar in structure to those in the anterior intestines ().
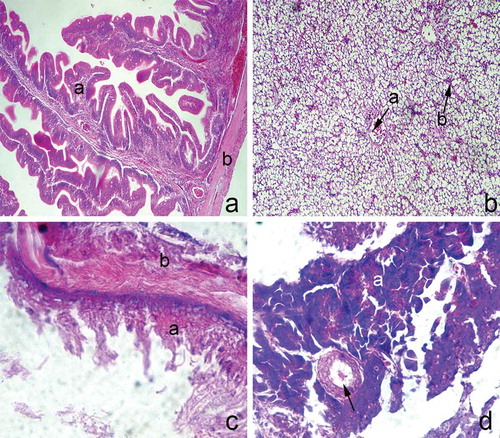
Figure 3. (a) Transverse section through the posterior intestines; a: mucosa; b: muscular layer; hematoxyiln and eosin staining, ×100. (b) The liver parenchyma; a: central vein; b: sinusoids; hematoxyiln and eosin staining, ×100. (c) Transverse section through the gall bladder wall; a: mucosa; b: muscular layer; hematoxyiln and eosin staining, ×400. (d) The pancreas; a: exocrine acini; pancreatic duct (arrow); hematoxyiln and eosin staining, ×200.
The liver
The liver parenchyma of the European hake consists of three lobes. On the microscopic level, it appears continuous, with no distinct boundaries. The liver parenchymal cells (hepatocytes) are arranged in narrow irregular plates which radiate from the central vein and alternate with numerous sinusoids. Hepatocytes contain numerous lipid droplets in their cytoplasm ().
The gall bladder
The wall of the European hake gall bladder consists of a narrow mucosal layer and a muscular layer covered by serosa. The mucosa is lined by simple columnar epithelium. Lamina propria is a connective tissue layer adjacent to the epithelium, thus separating the mucosa from the muscularis externa. The muscularis externa consists of two muscular layers: the inner circularly and outer longitudinally arranged muscles ().
The pancreas
Some pancreatic tissues can be seen near the gall bladder. It consists of round acini containing tightly joined acinar cells. The acinar cells are pyramidal, containing numerous granules on their apical domain. Pancreatic acini are functional units of exocrine pancreas. Endocrine units were not seen in Europen hake pancreas tissues. Some pancreatic ducts could be seen throughout the sections ().
Discussion
The European hake Merluccius merluccius L. is a major demersal fish in marine ecosystems throughout the Atlantic and Mediterranean, and a top predator that occupies different trophic levels during its ontogenetic development (Arneri & Morales-Nin Citation2000; Ungaro et al. Citation2001; Carpentieri et al. Citation2005; Ortiz-Delgado et al. Citation2012). Hake diet shifts from euphauiids (Euphausia couchii, Euphausia krohnii) and decapods (Processa sp., Philoderas sp., Solenocea membranacea), consumed by the smaller hakes, to teleost fish (Engraulis encrasicolus, Cepola macrophthalma, Gaidropsarus biscayensis) consumed by larger hakes (Ungaro et al. Citation1993; Carpentieri et al. Citation2005; Stagioni et al. Citation2011). Usually, prey kind and size are related to the morphology and the size of the fish (Ross Citation1978; Stagioni et al. Citation2011). In particular, the jaw size, intestinal length, reproduction and distribution are in relationship to trophic changes and to intraspecific resource partitioning (Ross Citation1978). Since the hake is a typical carnivorous fish, its oesophagus is a short, muscular tube conducting the food to the stomach. The oesophagus of different teleost fish, like those of most vertebrates, functions in transporting food particles, so it is provided with cells that secrete mucus, and stratified squamous epithelium (Al Abdulhadi Citation2005). The oesophagus of the sea bream Sparus aurata shows a multi-layered mucosa in the upper part and single-layered regions in the lower part. The multi-layered regions are formed by epithelial cells, mucus-secreting cells and cells rich with eosinophilic granules (Cataldi et al. Citation1987). The complex morphology and histochemistry of the oesophagus may indicate additional functions, as was shown in bream fish Mylio cuvieri whose oesophagus exhibits a diversity of morphologically and histochemically recognisable types of mucous cells. The superficial mucous cells react positively to PAS, while the other type reacts positively to Alcian blue (Al Abdulhadi Citation2005).
The oesophagus of the European hake, as shown in the present study, is also layered with stratified squamous epithelium, and it is provided with goblet cells containing the acidic mucins. The secretion activity of goblet cells probably facilitates the transport of the food particles through the oesophagus. The mucosa of the oesophagus also contains lamina propria, a connective tissue layer adjacent to the epithelium, while muscularis mucosa has not been seen. Lamina muscularis mucosa is usually missing in fish (Kozarić Citation2001). In the fresh water stingray Himantura signifer, the oesophagus is covered by stratified columnar epithelium (Chatchavalvanich et al. Citation2006), while in the common eel Anguilla anguilla the oesophagus mucosa consisted of stratified epithelium, columnar epithelium and goblet cells, while striated muscle fibres formed the thick muscular coat (Clarke & Witcomb Citation1980). The two-layered muscularis externa of the European hake oesophagus is made of striated muscular tissue. The activity of enzymes such as non-specific esterase, acid and alkaline phosphatase, was found to be weak to moderate in the hake’s oesophagus (Kozarić et al. Citation2004).
The stomach is a sac-like organ placed between the oesophagus and intestines (Kierszenbaum Citation2002). The histology of fish stomach mucosa is generally simpler than that of higher vertebrates (Arellano et al. Citation2001). The lamina propria of the stomach contains tubular gastric glands and also some mucous cells situated on the basal surface of the gastric glands. The gastric glands are the main part of the stomach mucosa in vertebrates (Kierszenbaum Citation2002), and also in fish, both carnivorous and herbivorous (Al Abdulhadi Citation2005). The European hake’s stomach has a thick wall containing four layers: mucosa, submucosa, muscular layer and outer layer. The mucosal surface of the hake stomach is lined by a simple columnar epithelium containing microvilli on the apical domain of the cell. The stomach of the sea bream Sparus aurata has a single-layered columnar epithelium under which, in the cardiac and fundic portion, gastric glands, comprising all similar cells, are present (Cataldi et al. Citation1987). In the stomach of Solea senegalensis, gastric glands are numerous in the fundic and pyloric regions but absent in the cardiac region (Arellano et al. Citation2001). Columnar epithelium and gastric gland cells were also found in the stomach of the common eel Anguilla anguilla (Clarke & Witcomb Citation1980). According to the present results, mucosal lamina propria of the stomach fundus contains simple tubular gastric glands consisting mostly of large cells with basal placed nuclei and cytoplasm filled with granules. The gastric glands were not found in the stomach pyloric region. The same results were also found in the pylorus of carnivorous fish such as common eel Anguilla anguilla (Clarke & Witcomb Citation1980). In humans, for example, pylorus contains pyloric glands that differ from those found in stomach cardia and fundus (Kierszenbaum & Tres Citation2012). In most species, the main feature of the pyloric region is the presence of the pyloric sphincter, a strong circular muscle enabling food particles to pass towards the intestine (Albrecht et al. Citation2001; Chatchavalvanich et al. Citation2006; Kierszenbaum & Tres Citation2012). The same was confirmed in the present study.
According to the present data, fish intestines usually consist of proximal and distal portions (Al Abdulhadi Citation2005; Chatchavalvanich et al. Citation2006; Dai et al. Citation2007). The European hake intestines histologically consist of three layers: mucosa, muscularis externa and serosa. These layers are continuously present in the total length of the intestines, but since they differ in their morphology, two parts of the intestines could be distinguished: the anterior and posterior intestines. The anterior intestines are larger in diameter than the posterior ones. The mucosal folds in the anterior intestines are more leaf-like, while those in the posterior intestines are finger-like. Even the investigation of enzymatic activity in hake intestines has shown that enzyme activity in anterior intestines differs from those in posterior intestines (Kozarić et al. Citation2004). Goblet cells were found in both parts of the intestines. Intestinal goblets cells were found in some other fish such as sea bream Mylio cuvieri (Al Abdulhadi Citation2005). It was found (Al Abdulhadi Citation2005) that intestinal goblet cells in Mylio cuvieri contain acid mucopolysaccharides, while those in Tilapia spilurus contain neutral mucopolysaccharides. When treated with Alcian blue/PAS, it seems that the intestinal goblet cells contain acidic mucins. Kozarić et al. (Citation2004) described that in the European hake intestinal intraepithelial goblet cells showed no enzymatic activity, but dispersed granular enzymatic activity was observed in the connective tissue of lamina propria. The results reported by Ferrando et al. (Citation2006) showed that the morphology of the mucosal folds of the Liza aurata intestine, as well as the quality and quantity of the mucous from the intestinal goblet cells, bear a relationship with environmental pollution. The environmental pollution also influences the enzymatic activity of the liver, as was described in Coris julis (Fasulo et al. Citation2010). Digestive glands such as the liver and the pancreas have digestive and absorptive functions, and both can be described as exocrine and endocrine glands (Kierszenbaum & Tres Citation2012). The liver parenchyma of the hake contains hepatocytes, numerous sinusoids and a central vein, which have also been described in humans (Kierszenbaum & Tres Citation2012). The pancreas has its exocrine and endocrine parts (Kierszenbaum & Tres Citation2012). In hake pancreatic tissues, only pancreatic acini, which are functional units of exocrine pancreas, were seen. Since the data on liver and pancreas histology in fish are usually scarce, it is difficult to discuss the present results on hake liver and pancreas histology. In conclusion, histology investigations on adult hake digestive tract suggest that histological features of the digestive tract of the hake Merluccius merluccius L. are mostly similar to those of other carnivorous fish, and according to its feeding habits.
Acknowledgements
We thank Mrs. Marica Maretić for her skillful technical assistance.
References
- Al Abdulhadi HA. 2005. Some comparative histological studies on alimentary tract of tilapia fish (Tilapia spilurus) and sea bream (Mylio cuvieri). Egyptian Journal of Aquatic Research 31:387–397.
- Albrecht MP, Ferreira MFN, Caramaschi EP. 2001. Anatomical features and histology of the digestive tract of two related neotropical omnivorous fishes (Characiformes; Anostomidae). Journal of Fish Biology 58:419–430. doi:10.1111/jfb.2001.58.issue-2.
- Arellano JM, Storch V, Sarasquete C. 2001. Histological and histochemical observations in the stomach of the Senegal sole, Solea senegalensis. Histology and Histopathology 16:511–521.
- Arneri E, Morales-Nin B. 2000. Aspects of the early life history of European hake from the central Adriatic. Journal of Fish Biology 56:1368–1380. doi:10.1111/jfb.2000.56.issue-6.
- Carpentieri P, Colloca F, Cardinale M, Belluscio A, Ardizzone GD. 2005. Feeding habits of European hake (Merluccius merluccius) in the central Mediterranean Sea. Fishery Bulletin 103:411–416.
- Cataldi E, Cataudella S, Monaco G, Rossi A, Tancioni L. 1987. A study of the histology and morphology of the digestive tract of the sea-bream, Sparus aurata. Journal of Fish Biology 30:135–145. doi:10.1111/jfb.1987.30.issue-2.
- Chatchavalvanich K, Marcos R, Poonpirom J, Thongpan A, Rocha E. 2006. Histology of the digestive tract of the freshwater stingray Himantura signifer Compagno and Roberts, 1982 (Elasmobranchii, Dasyatidae). Anatomy and Embryology 211:507–518. doi:10.1007/s00429-006-0103-3.
- Clarke AJ, Witcomb DM. 1980. A study of the histology and morphology of the digestive tract of the common eel (Anguilla anguilla). Journal of Fish Biology 16:159–170. doi:10.1111/jfb.1980.16.issue-2.
- Dai X, Shu M, Fang W. 2007. Histological and ultrastructural study of the digestive tract of rice field eel, Monopterus albus. Journal of Applied Ichthyology 23:177–183. doi:10.1111/jai.2007.23.issue-2.
- FAO. 1997. Fishery statistics. Catches and landings 1995. Rome: FAO.
- Fasulo S, Marino S, Mauceri A, Maisano M, Giannetto A, D’Agata A, Parrino V, Minutoli R, De Domenico E. 2010. A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicology and Environmental Safety 73:1565–1573. doi:10.1016/j.ecoenv.2010.01.008.
- Ferrando S, Maisano M, Parrinoa V, Ferrando T, Girosi L, Tagliafierro G. 2006. Gut morphology and metallothionein immunoreactivity in Liza aurata from different heavy metal polluted environments. Italian Journal of Zoology 73:7–14. doi:10.1080/11250000500502228.
- Iglesias JB, Lago MJ, Sánchez FJ, Cal R. 2010. Capture, transport and acclimatization to captivity of European hake, Merluccius merluccius L: Preliminary data on feeding and growth. Aquaculture Research 41:607–609. doi:10.1111/are.2010.41.issue-4.
- Jardas I. 1976. Contribution to the knowledge of the biology of hake in the Adriatic sea. Revue des Travaux de l’institut des Peches Maritimes 40:615–618.
- Jukic S. 1972. Ishrana oslica (Merluccius merluccius L.), bukve (Boops boops), trlje (Mullus barbatus) i arbuna (Pagellus erythrynus L.) u Kastelanskom zaljevu. Acta Adriatica 14:1–40.
- Jukic S 1984. Distribution of hake (Merluccius merluccius, L.) striped mullet (Mullus barbatus, L.) and pandora (Pagellus erythrinus, L.) in the Adriatic Sea. FAO Fisheries Report 290:85–91.
- Jukic S, Piccinetti C. 1987. Biological and economic aspects of mesh size regulation in the multispecies demersal fishery of the Adriatic Sea. Acta Adriatica 28:199–219.
- Karlovac J. 1959. On the feeding on the hake (Merluccius merluccius L.) of the Adriatic Sea. Proceedings General Fisheries Council for the Mediterranean 18:461–464.
- Karlovac J. 1965. Contibution a la connaissance de l`ecologie du merlu Merluccius merluccius L. dans le stade planctonique de sa vie en Adriatique. Rapports et Procès Verbaux Commission Internationale Exploration Scientifique Mer Méditerranée 18:333–339.
- Kierszenbaum AL. 2002. Histology and Cell Biology. New York: Mosby.
- Kierszenbaum AL, Tres LL. 2012. Histology and cell biology. An introduction to pathology. Philadelphia: Elsevier. 701 pp.
- Kozarić Z. 2001. Morfologija riba. Zagreb: Školska knjiga.
- Kozarić Z, Kužir S, Nejedil S, Petrinec Z, Srebočan E. 2004. Histochemical distribution of digestive enzymes in hake, Merluccius merluccius L. 1758. Veterinarski arhiv 74:299–308.
- Oliver P. 1991. Dinamica de la poblacion de Merluza (Merluccius merluccius L.) de Mallorca. Reclutamiento, crescimiento y mortalidad. Illes Balears, Spain: Universitat de les Illes Baleares. 392 pp.
- Oliver P, Massutí E. 1995. Biology and fisheries of western Mediterranean hake. In: Alheit J, Pitcher TJ, editors. Hake: Biology, fisheries, and markets. London: Chapman & Hall. pp. 181–202.
- Ortiz-Delgado JB, Iglesias JB, Sánchez FJ, Cal R, Lago MJ, Otero JJ, Sarasquete C. 2012. A morphohistological and histochemical study of hatchery-reared European hake, Merluccius merluccius (Linnaeus, 1758), during the lecitho-exotrophic larval phase. Scientia Marina 76:259–271. doi:10.3989/scimar.2012.76n2.
- Palomera I, Olivar MP, Morales-Nin B. 2005. Larval development and growth of the Europian hake in the NW Mediterranean. Scientia Marina 69:221–258.
- Papaconstantinou C, Caragitsou E. 1987. A preliminary study of the feeding habits of red mullet Mullus barbatus L. in three marine regions off the western coast of Hellas. Proceedings of the Second Hellenic Symposium on Oceanography and Fisheries 577–583.
- Ross ST. 1978. Trophic ontogeny of the leopard searobin, Prionotus scitulus (Pisces: Triglidae). Fishery Bulletin 76:225–234.
- Sánchez JF, Otero JJ, Cal R, Lago MJ, Gomez C, Iglesias JB. 2011. The first spontaneous spawning of Europian hake, Merluccius merkluccius L: Characteristics of eggs and early larval stages. Aquaculture Research. doi:10.1111/j.1365-2109.2011.02966.x.
- Stagioni M, Montanini S, Vallisneri M. 2011. Feeding habits of European hake, Merluccius merluccius (Actinopterygii: Gadiformes: Merlucciidae), from the Northeastern Mediterranean Sea. Acta Ichthyologica et Piscatoria 41:277–284. doi:10.3750/AIP2011.41.4.03.
- Treer T, Safner R, Aničić I, Lovrinov M. 1995. Ribarstvo. Zagreb: Nakladni zavod Globus. 463 pp.
- Ungaro N, Rizzi E, Marano G. 1993. Note sulla biologia e pesca di Merluccius merluccius (L.) nell’Adriatico pugliese. Biologia Marina 1:329–334.
- Ungaro N, Vrgoc N, Mannini P 2001. The biology and stock assessment of Merluccius merluccius (L.) in the Adriatic Sea: An historical review by geographical management units. GFCM-SAC Working Group on Demersal Species:13–16.
- Zupanovic S. 1968. Study of hake (Merluccius merluccius L.): Biology, and population dynamics in the central Adriatic. Studies and reviews/General Fisheries Council for the Mediterranean. Fao 32:24 pp.
- Zupanovic S, Jardas I. 1986. A contribution to the study of biology and population dynamics of the Adriatic hake, Merluccius merluccius (L). Acta Adriatica 27:97–146.
