Abstract
The present paper investigates the diet and the food composition of Trachinotus ovatus in the central Mediterranean Sea (Strait of Messina). Moreover, the first documented data on plastic ingestion by T. ovatus are also reported. Samples ranging between 16.5 and 28.0 cm fork length were collected between May and November 2012 in the Strait of Messina (central Mediterranean Sea) by trolling lines. T. ovatus fed mainly on pelagic crustaceans and fishes, although the contribution of mollusks was also important. The absence of dominant prey indicated a generalist feeding behavior. The plastic debris was found in the stomach content of T. ovatus with a high percentage of occurrence (%O = 24.3%). Considering the commercial interest that T. ovatus has in some small-scale fishery markets, the potential impact of plastics on the trophic web and human consumption should be investigated.
Introduction
During recent years, climate change has influenced and modified the abundance and distribution of marine species worldwide (Occhipinti-Ambrogi Citation2007) and it is included among the main factors determining the phenomena of tropicalisation and meridionalisation of the Mediterranean Sea (Francour et al. Citation1994; Andaloro & Rinaldi Citation1998). As regards Mediterranean fish species, changes concern distribution (a spread of indigenous thermophilic species towards northern areas), species composition, competition and quantitative and qualitative variation in fishery catches (Andaloro & Rinaldi Citation1998; Bianchi Citation2007; Azzurro et al. Citation2011).
Trachinotus ovatus (L., 1758), Carangidae, is a thermophilic species (Bianchi et al. Citation2014), which has extended during the last decade its distribution in some northeastern Mediterranean areas (Lipej & Dulčić Citation2004; Raya & Sabatés Citation2015) and has been recorded as increasing in catches of artisanal fishery in the southern Tyrrhenian (Azzurro et al. Citation2011). The positive trend in catches of this carangid in part compensates for a decrease of other commercial fish and represents a valid alternative to common target species for maintaining the economic sustainability of small-scale fishing activities. Despite the fact that this species is becoming more important for local fisheries (AA.VV Citation2014), few data are still available on its biology and ecology, part of which is focused on farming studies (Tutman et al. Citation2004; Assem et al. Citation2005), aimed to check the potential value of T. ovatus as an aquaculture product, also thanks to its quick adaptation to confinement and tolerance of captivity conditions (Tutman et al. Citation2004). The analysis of age and growth of T. ovatus from southern Mediterranean Sea shows that this fish can reach 4 years of life (within the investigated size range of about 7–26 cm total length; Mourad Citation1999), whereas Abdallah (Citation2002) calculated the length–weight relationship (a = 0.022; b = 2.73; total length (TL) range = 3.4–23.3 cm). Recently, T. ovatus was also found among prey of swordfish in the central Mediterranean (Romeo et al. Citation2009). The few data on the feeding activity of T. ovatus concern only juvenile specimens (less than 100 mm TL) from southern Adriatic Sea (Batistic et al. Citation2005), coast of Israel (Chervinski & Zorn Citation1977) and Canary Islands (Moreno & Castro Citation1995). Dietary data on adult T. ovatus are needed, as well as on those fishes which are extending their distribution areas, in order to evaluate their role in the Mediterranean trophic web and to understand the interactions with other species, feeding relationships and competition, and their impact on the food chain.
The present paper investigates the diet and the food composition of adult T. ovatus in the central Mediterranean Sea (Strait of Messina). Moreover, the first documented data on plastic ingestion by T. ovatus are also reported. This finding allows us to also discuss in this paper the growing problem of plastic presence in Mediterranean waters and the introduction of dangerous chemicals into the marine food web. The extent of plastic ingestion by Mediterranean fishes is still largely unknown, but some authors (e.g. Deudero & Alomar 2014; Romeo et al. Citation2015) have already underlined the warning of this phenomenon also in relation to human consumption and health.
Materials and methods
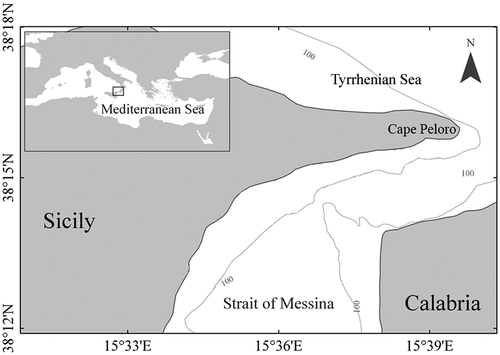
Specimens of T. ovatus were collected between May and November 2012 by trolling lines and artificial bait in the Strait of Messina (central Mediterranean Sea), close to Cape Peloro ().
The fork length (FL) was measured to the nearest 0.1 cm, and total weight (TW) was recorded to the nearest 0.01 g. The stomachs were dissected out and their contents examined by stereomicroscope. Prey remains were identified to the lowest possible taxon, using taxonomic keys (Whitehead et al. Citation1984–1986; Riedl Citation1991; Falciai & Minervini Citation1992; Avancini et al. Citation2006). Individuals of each prey taxon were counted, weighed (blotted dry for measurement of wet weight) and preserved in 70% ethanol.
The feeding incidence (%FI = individuals with identifiable prey remains/total number of fishes × 100) was used to evaluate the rate of feeding activity, whereas the degree of stomach fullness was estimated by the stomach content index (%SCI = wet weight of stomach content/body wet weight × 100).
To assess the importance of prey items in the diet of T. ovatus, the following dietary indices were calculated: abundance percentage (%N = number of individuals of prey i/total number of prey × 100), weight percentage (%W = weight of prey i/total weight of all prey × 100) and frequency of occurrence (%F = number of stomachs containing prey i/total number of stomachs containing prey × 100). Moreover, the percentage index of relative importance (%IRI) was estimated (Hyslop Citation1980) as follows: IRI = (%N + %W)*(%F) and %IRIi = (IRIi ⁄ ∑IRI) × 100.
The feeding behaviour of T. ovatus was assessed using the Costello graphical method (Costello Citation1990) modified by Amundsen et al. (Citation1996), plotting the prey-specific abundance against the frequency of occurrence in a two-dimensional graph. The prey-specific abundance is calculated as follows:
where Pi is the prey-specific abundance of prey i, Si is the total abundance (as weight or number) of prey i, and Sti is the total stomach contents in only those specimens with prey i in their stomachs. According to Amundsen et al. (Citation1996), information on prey importance, feeding strategy and niche width contribution can be inferred through the position of prey types in the two-dimensional plot.
The trophic level of T. ovatus was estimated according to Pauly and Christensen (Citation1995), as follows:
where TROPHj is the fractional trophic level of prey j and DCj represents the fraction of j in the diet of the consumer species (here, T. ovatus). The TROPH can vary between 2.0, for herbivorous/detritivorous, and 5.0, for piscivorous/carnivorous organisms (Pauly et al. Citation1998, Citation2000; Pauly & Palomares Citation2000). TROPH value was calculated using the weight contribution and the trophic level of each prey species to the diet. Trophic level values of prey were assigned according to Pauly et al. (Citation2000) and Stergiou and Karpouzi (Citation2002).
To evaluate potential seasonal-related diet variations, a non-parametric multivariate analysis of variance (PERMANOVA) was performed on prey abundance. Data were transformed to square root and analysed on the basis of Gower distance using 4999 permutations. Pair-wise comparisons were computed when significant differences (p < 0.05) among factor levels were detected. Then, a multivariate multiple permutation test (SIMPER) was performed to determine the contribution of each prey category to the average dissimilarity between groups. All of these analyses were conducted using the statistical software PRIMER6 and PERMANOVA+ (Clarke & Warwick Citation2001; Anderson et al. Citation2008).
Finally, the stomach content of each specimen was visually inspected under a stereomicroscope (80× magnification) in order to individuate the potential presence of plastic fragments. When plastic particles were found, they were photographed by stereomicroscope Zeiss Discovery V8 coupled with Axiovision AxioVs40 version 4.8.2.0 digital image processing software. The following data for each plastic fragment were recorded: colour (according to a chromatic scale), shape (assigned following geometrical criteria), length, width and weight. The percentage of occurrence (%O) of plastic debris in the stomach content of T. ovatus was calculated as the proportion of individuals containing plastics on the total samples (including also stomachs without prey items):
The plastics ingested were then categorised as macroparticles (> 25 mm), mesoparticles (5–25 mm) and microparticles (< 5 mm), following Galgani et al. (Citation2013), and the percentage of each category was calculated as the proportion of each plastic category against the total amount of plastic found in the stomachs.
Results
Overall, 115 individuals of T. ovatus ranging between 16.5 and 28.0 cm FL (mean FL = 23.4 cm; standard deviation = 2.5) and 68.2 and 368.84 g (mean TW = 220.3 g; standard deviation = 68.3) were collected. On 115 stomachs analysed, 42 were empty, producing a feeding incidence value %FI = 58.3. The degree of stomach fullness (SCI) for the whole sampling ranged between 0.03 and 2.49%, with a median value of 0.19%. The prey list and dietary index values (%N, %W, %F and %IRI) for each item are reported in together with the information on their developmental life stage (A = adult; J = juvenile; L = various stages of larvae).
Table I. Diet composition of Trachinotus ovatus and percentage values of dietary indexes calculated for each prey item: abundance (%N); weight (%W); frequency of occurrence (%F); index of relative abundance (%IRI). Life stage of prey is also reported (A = adult; J = juvenile; L = various stages of larvae).
Crustacea were the most important prey for T. ovatus, in terms of number and frequency of occurrence, with a dominance of calanoid copepods and euphausiids. This latter group was mainly represented by the species Euphausia krohnii (Brandt, 1851) which had a %IRI value of 24.46. A valuable contribution in weight to the diet of T. ovatus was given by teleosts, in particular Sardinella aurita (Valenciennes, 1847), and Engraulis encrasicolus (Linnaeus, 1758) (%W = 33.70 and 11.90, respectively). The analysis of fish prey highlighted also the ingestion of mesopelagic fishes and a case of cannibalism (a juvenile specimen of T. ovatus preyed by an adult of 26.0 cm FL). T. ovatus fed also on pelagic gastropods, mainly represented by Atlanta sp. (%IRI = 9.88), and occasionally on insects. In some stomachs, the presence of plant seeds and plastic particles was also detected.
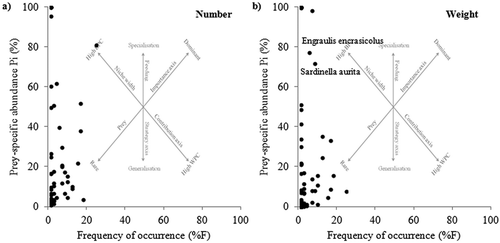
The feeding strategy of T. ovatus is shown in , where the frequency of occurrence (%F) is plotted against prey-specific abundance (Pi), expressed as number () and weight (). Results indicate a generalist feeding behavior of T. ovatus, as also demonstrated by the absence of dominant prey. Most of the food categories are located in the lower left corner of the diagrams or close to the y-axis, in a graph region where prey are considered of low importance, since they were consumed at a low frequency.
Figure 2. Costello graph (modified by Amundsen et al. Citation1996): relationship between frequency of occurrence (%F) of prey items and prey-specific abundance (Pi), expressed as A, number and B, weight, respectively, in the diet of Trachinotus ovatus, collected in the Strait of Sicily. The graph background shows the explanatory Costello diagram and its interpretation of feeding strategy (BPC = between-phenotype component; WPC = within-phenotype component).
The calculated value of trophic level (TROPH) for T. ovatus was 3.90.
PERMANOVA analysis on prey abundance in fish caught during the three investigated seasons (autumn: n = 23, FL range = 22.0–27.8 cm; spring: n = 19, FL range = 16.8–28.0 cm; summer: n = 31, FL range = 16.5–27.8 cm) revealed significant differences in diet composition (F = 2.6853, p < 0.01). Pair-wise comparisons showed summer and spring to be different from autumn (p < 0.01). As demonstrated by the SIMPER test, the main contributors to these differences were E. krohnii, more abundant in autumn (average abundance = 1.27), and calanoid copepods, more abundant in spring (average abundance = 1.82), than in the other two seasons.
Finally, shows data on plastic debris found in the stomach content of 28 T. ovatus (ranging between 20.0 and 27.8 cm FL), with a percentage of occurrence (%O) equal to 24.3%. In two cases, more than one piece of plastic was recorded. Plastic fragments had different shapes and colour ( and ). According to the dimensional categories of Galgani et al. (Citation2013), the plastic debris ingested belong to microparticles (83.3%) and mesoparticles (16.7%).
Table II. Data on colour, shape and size of plastic debris in stomach content of Trachinotus ovatus specimens collected in the Strait of Messina. FL = fork length; TW = total weight.
Discussion
The analysis of the diet of T. ovatus shows that this predator fed mainly on pelagic crustaceans and fishes, although the contribution of mollusks was also important. Cnidarians and annelids were occasional prey, and a small fraction of food was represented by insects. Terrestrial plant seeds were also found in some stomachs, as well as plastic fragments.
The food composition of the diet of T. ovatus in the study area seems to be influenced by hydrodynamic, biological and behavioural factors. The Strait of Messina is characterised by strong upwelling currents, which determine a passive transport and concentration of planktonic species and, in general, slow-swimming organisms (Mazzarelli Citation1909; Berdar et al. Citation1983; Guglielmo et al. Citation1995). Moreover, this hydrodynamism allows the presence in surface waters of vertically migrant mesopelagic fauna (Mazzarelli Citation1909; Genovese et al. Citation1971; Berdar et al. Citation1983; Guglielmo et al. Citation1995), which usually rise at night from deep waters to upper layers to graze or feed on small zooplankton. Nictameral migrations enhance food encounter probabilities, since several prey found in T. ovatus stomachs are usually reported from mesopelagic waters: some copepods (e.g. Pleuromamma gracilis) and decapods (e.g. Primno macropa), euphausiids and mollusks (Atlantidae, Creseidae, Peraclidae), as well as Myctophidae and Sternoptychidae among fish (Berdar et al. Citation1983; Guglielmo et al. Citation1995). The influence of the hydrodynamic system on the trophic web of the Strait of Messina has already been highlighted for large pelagic predators occurring in the area (Romeo et al. Citation2012; Battaglia et al. Citation2013).
A consistent number of prey items can be categorised as larval or juvenile stages and were mainly represented by decapods and fish. Moreover, a case of cannibalism was observed, concerning a young T. ovatus (FL = 1.4 cm) preyed by an adult of 26.0 cm FL. This phenomenon is not frequently encountered in carangids, although it has already been reported in Atropus atropos in the Indian Ocean (Sivakami Citation1997).
The analysis of feeding habits of T. ovatus by the Costello graphical method (Costello Citation1990) modified by Amundsen et al. (Citation1996) did not identify a strict trophic relationship between predator and a specific prey item, but highlighted a broad prey spectrum, made up of non-dominant prey. These results together with the evidence of significant seasonal differences suggest that T. ovatus can be described as an opportunistic feeder, showing a particular voracity, as demonstrated by the ingestion of several plastic particles and some plant seeds, likely confused for planktonic organisms.
While in this paper we examined the diet of adult T. ovatus, some considerations on ontogenic changes could be inferred if our results are compared with those reported by Chervinski and Zorn (Citation1977), Moreno and Castro (Citation1995) and Batistic et al. (Citation2005), who analysed feeding habits of juvenile specimens. Differences among specific prey may vary depending on the study area; however, the food of juvenile T. ovatus seems mainly based on crustaceans and insects (Chervinski & Zorn Citation1977; Moreno & Castro Citation1995; Batistic et al. Citation2005); adults (present paper) rely also on fish prey and pelagic Gastropoda, whereas insects have low importance.
The calculated value of trophic level (TROPH = 3.90) for T. ovatus is higher than 3.73, the value provided by Froese and Pauly (Citation2014), but this may be due to different length ranges of examined samples, and then to dietary changes depending on fish size.
The occurrence of a relatively high amount of plastic debris in the stomach content of T. ovatus may represent a potential risk for this species. However, the impact of plastic ingestion on fish and the contaminant transfer to the trophic web is still poorly known in Mediterranean waters (Deudero & Alomar 2014). Nevertheless, the Mediterranean Sea was recently defined as one of the most impacted areas in the world by plastic pollution (Cozar et al. Citation2014; Fossi et al. Citation2014). The ingestion of plastic particles in this area has been reported in several marine organisms, ranging from zooplankton to top predators (Fossi et al. Citation2012, Citation2014; Cole et al. Citation2013; Collignon et al. Citation2014; Ivar do Sul & Costa Citation2014; Romeo et al. Citation2015). In particular, plastic debris has been already found in the stomachs of some Mediterranean pelagic fish such as bluefin tuna, albacore, swordfish, dolphin fish and bogue (Massutí et al. Citation1998; Deudero Citation2001; Karakulak et al. Citation2009; De la Serna et al. Citation2012; Deudero & Alomar 2014, Citation2015; Romeo et al. Citation2015). As in other pelagic predators, the consumption of plastic debris by T. ovatus may also be in part determined by the predation on gregarious prey. Indeed, according to Romeo et al. (Citation2015), the fish habit of feeding on small prey aggregated in schools may increase the probability of ingesting plastic together with food.
The high percentage of occurrence of plastic fragments in the stomach of T. ovatus may also be related to the morphology and hydrodynamic regime of the study area, characterised by strong currents that may create convergence zones in which floating plastic debris may accumulate. This phenomenon is already known, with higher proportions, in the oceanic gyres (Boerger et al. Citation2010; Davison & Asch Citation2011). The presence of insects and some neustonic organisms (e.g. Porpita porpita) among prey suggests the behaviour of hunting just beyond the surface layer, as observed also during the sampling operations, and this may make T. ovatus more vulnerable to the ingestion of floating plastic debris.
Further studies are needed to investigate the impact of plastic ingestion on the biology of this species, from a toxicological point of view, taking into account also the aspects linked to the transport, accumulation and bioavailability of persistent, bioaccumulative and toxic (PBT) chemicals and other toxic substances (such as phthalates, nonylphenol, bisphenol A and brominated flame retardants) associated with plastics. Considering the commercial interest that T. ovatus has in some small-scale fishery markets, the potential impact on human consumption and health should be investigated more deeply. Moreover, the present data could be useful for the implementation of the Marine Strategy Framework Directive (MSFD; 2008/56/EC) which aims to achieve Good Environmental Status (GES) for European marine waters by 2020, with specific regard to Descriptor 10 on “Marine litter”.
References
- AA.VV. 2014. Identification and characterization of the small-scale driftnet fisheries in the Mediterranean (DRIFTMED). MAREA: Mediterranean Alieutic Resources Evaluation and Advice, specific contract n° 8, final report. 287 pp.
- Abdallah M. 2002. Length-weight relationship of fishes caught by trawl off Alexandria, Egypt. Naga, The ICLARM Quarterly 25:19–20.
- Amundsen PA, Gabler HM, Staldvik FJ. 1996. A new approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. Journal of Fish Biology 48:607–614.
- Andaloro F, Rinaldi A. 1998. Fish biodiversity change in Mediterranean Sea as tropicalisation phenomenon indicator. In: Enne G, D’Angelo M, Zanolla C, editors. Indicators for assessing desertification in the Mediterranean. Rome: ANPA & Osservatorio Nazionale sulla Desertificazione. pp. 201–206.
- Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for PRIMER: Guide to software and statistical methods. Plymouth: PRIMER-E.
- Assem SS, El-Serafy SS, El-Garabawy MM, El-Absawy MEG, Kaldus SK. 2005. Some biochemical aspects of reproduction in female Trachinotus ovatus (Carangidae). Egyptian Journal of Aquatic Research 31:315–327.
- Avancini M, Cicero AM, Di Girolamo I, Innamorati M, Magaletti E, Sertorio Zunini T, editors. 2006. Guida al Riconoscimento del Plancton dei Mari Italiani, Vol. II - Zooplancton Neritico. Roma: Ministero dell’Ambiente e della Tutela del Territorio e del Mare – ICRAM. 232 pp. 134 tavv.
- Azzurro E, Moschella P, Maynou F. 2011. Tracking signals of change in Mediterranean fish diversity based on local ecological knowledge. PLoS ONE 6:e24885. doi:10.1371/journal.pone.0024885.
- Batistic M, Tutman P, Bojanic D, Skaramuca B, Kožul V, Glavic N, Bartulovic V. 2005. Diet and diel feeding activity of juvenile pompano (Trachinotus ovatus) (Teleostei: Carangidae) from the southern Adriatic, Croatia. Journal of the Marine Biological Association of the UK 85:1533–1534. doi:10.1017/S0025315405012749.
- Battaglia P, Andaloro F, Consoli P, Esposito V, Malara D, Musolino S, Pedà C, Romeo T. 2013. Feeding habits of the Atlantic bluefin tuna, Thunnus thynnus (L. 1758), in the central Mediterranean Sea (Strait of Messina). Helgoland Marine Research 67:97–107. doi:10.1007/s10152-012-0307-2.
- Berdar A, Cavaliere A, Cavallaro G, Giuffrè G, Potoschi A. 1983. Lo studio degli organismi spiaggiati nello Stretto di Messina negli ultimi due secoli. Naturalista Siciliano 7:3–17.
- Bianchi CN. 2007. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 580:7–21. doi:10.1007/s10750-006-0469-5.
- Bianchi CN, Corsini-Foka M, Morri C, Zenetos A. 2014. Thirty years after: Dramatic change in the coastal marine ecosystems of Kos Island (Greece), 1981-2013. Mediterranean Marine Science. doi:10.12681/mms.678.
- Boerger CM, Lattin GL, Moore SL, Moore CJ. 2010. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Marine Pollution Bulletin 60:2275–2278. doi:10.1016/j.marpolbul.2010.08.007.
- Chervinski J, Zorn M. 1977. Note on occurrence and the food of juvenile kachlan (Trachinotus ovatus, Linnaeus) (Pisces, Carangidae) from the Mediterranean. Aquaculture 10:179–185. doi:10.1016/0044-8486(77)90020-5.
- Clarke KR, Warwick RM. 2001. Change in marine communities: An approach to statistical analysis and interpretation. 2nd ed. Plymouth: PRIMER-E.
- Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. 2013. Microplastic ingestion by zooplankton. Environmental Science & Technology 47:6646–6655.
- Collignon A, Hecq J-H, Galgani F, Collard F, Goffart A. 2014. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Marine Pollution Bulletin 79:293–298. doi:10.1016/j.marpolbul.2013.11.023.
- Costello MJ. 1990. Predator feeding strategy and prey importance: A new graphical analysis. Journal of Fish Biology 36:261–263. doi:10.1111/jfb.1990.36.issue-2.
- Cozar A, Echevarria F, Gonzalez-Gordillo JI, Irigoien X, Ubeda B, Hernandez-Leon S, Palma AT, Navarro S, Garcia-de-Lomas J, Ruiz A, Fernandez-de-Puelles ML, Duarte CM. 2014. Plastic debris in the open ocean. Proceedings of the National Academy of Sciences of the United States of America 111:10239–10244. doi:10.1073/pnas.1314705111.
- Davison P, Asch RG. 2011. Plastic ingestion by mesopelagic fishes in the North Pacific subtropical gyre. Marine Ecology Progress Series 432:173–180. doi:10.3354/meps09142.
- De la Serna JM, Godoy MD, Olaso I, Zabala J, Majuelos E, Báez JC. 2012. Preliminary study on the feeding of bluefin tuna (Thunnus thynnus) in the Mediterranean and the Strait of Gibraltar area. Collective Volume of Scientific Papers ICCAT 68:115–132.
- Deudero S. 2001. Interspecific trophic relationships among pelagic fish species underneath FADs. Journal of Fish Biology 58:53–67. doi:10.1111/jfb.2001.58.issue-1.
- Deudero S, Alomar C. 2014. Revising interactions of plastics with marine biota: Evidence from the Mediterranean. In: Marine Litter In The Mediterranean And Black Seas Tirana, Albania, 18–21 June 2014. CIESM Workshop Monographs 46. pp. 79–85.
- Deudero S, Alomar C. 2015. Mediterranean marine biodiversity under threat: Reviewing influence of marine litter on species. Marine Pollution Bulletin 98:58–68. doi:10.1016/j.marpolbul.2015.07.012.
- Falciai L, Minervini R. 1992. Guida dei crostacei decapodi d’Europa. Padova, Italy: Franco Muzzio. 283 pp.
- Fossi MC, Coppola D, Baini M, Giannetti M, Guerranti C, Marsili L, Panti C, de Sabata E, Clò S. 2014. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Marine Environmental Research 100:17–24. doi:10.1016/j.marenvres.2014.02.002.
- Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, Minutoli R. 2012. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Marine Pollution Bulletin 64:2374–2379. doi:10.1016/j.marpolbul.2012.08.013.
- Francour P, Boudouresque CF, Harmelin JG, Harmelin-Vivien M, Quignard JP. 1994. Are the Mediterranean waters becoming warmer? Information from biological indicators. Marine Pollution Bulletin 28:523–526. doi:10.1016/0025-326X(94)90071-X.
- Froese R, Pauly D, editors. 2014. FishBase. World Wide Web electronic publication. Available: www.fishbase.org. Accessed Nov 2014
- Galgani F, Hanke G, Werner S, Oosterbaan L, Nilsson P, Fleet D, Kinsey S, Thompson RC, VanFraneker J, Vlachogianni T, Scoullos M, Mira Veiga J, Palatinus A, Matiddi M, Maes T, Korpinen S, Budziak A, Leslie H, Gago J, Liebezeit G. 2013 Monitoring guidance for marine litter in European seas. JRC scientific and policy 223 reports, Report EUR 26113 EN. 120 pp. Available: https://circabc.europa.eu/w/browse/85264644-ef32-224 401b-b9f1-f640a1c459c2. Accessed Feb 2015.
- Genovese S, Berdar A, Guglielmo L. 1971. Spiaggiamenti di fauna abissale nello Stretto di Messina. Atti della Società Peloritana di Scienze Fisiche. Matematiche e Naturali 17:331–370.
- Guglielmo L, Crescenti N, Costanzo G, Zagami G. 1995. Zooplankton and micronekton communities in the straits of Messina. In: Guglielmo L, Manganaro A, De Domenico E, editors. The straits of Messina ecosystem. Proceedings of the symposium, 4–6 April 1991, Messina: University of Messina. pp. 247–269.
- Hyslop EJ. 1980. Stomach content analysis: A review of methods and their application. Journal of Fish Biology 17:411–429. doi:10.1111/jfb.1980.17.issue-4.
- Ivar do Sul JA, Costa MF. 2014. The present and future of microplastic pollution in the marine environment. Environmental Pollution 185:352–364. doi:10.1016/j.envpol.2013.10.036.
- Karakulak S, Salman A, Oray IK. 2009. Diet composition of bluefin tuna (Thunnus thynnus L. 1758) in the Eastern Mediterranean Sea, Turkey. Journal of Applied Ichthyology 25:757–761. doi:10.1111/jai.2009.25.issue-6.
- Lipej L, Dulčić J. 2004. The current status of Adriatic fish biodiversity. In: Griffiths HI, Kryštufek B, Reed JM, editors. Balkan biodiversity: Pattern and process in the European hotspot. Dordrecht, the Netherlands: Kluwer Academic Publishers. pp. 291–306.
- Massutí E, Deudero S, Sánchez P, Morales-Nin B. 1998. Diet and feeding of dolphin (Coryphaena hippurus) in western Mediterranean waters. Bulletin of Marine Science 63:329–341.
- Mazzarelli G. 1909. Gli animali abissali e le correnti sottomarine dello Stretto di Messina. Rivista Mensile di Pesca e Idrobiologia 11:177–218.
- Moreno T, Castro JJ. 1995. Community structure of the juvenile of coastal pelagic fish species in the Canary Islands waters. Scientia Marina 59:405–413.
- Mourad MH. 1999. Age determination of Trachinotus ovatus (L.) based on otolith weight. Journal of King Abdulaziz University: Marine Sciences 10:149–155. doi:10.4197/mar.10-1.10.
- Occhipinti-Ambrogi A. 2007. Global change and marine communities: Alien species and climate change. Marine Pollution Bulletin 55:342–352. doi:10.1016/j.marpolbul.2006.11.014.
- Pauly D, Christensen V. 1995. Primary production required to sustain global fisheries. Nature 374:255–257. doi:10.1038/374255a0.
- Pauly D, Froese R, Sa-a P, Palomares M, Christensen V, Rius J. 2000. TrophLab manual. Manila, Philippines: ICLARM.
- Pauly D, Palomares ML. 2000. Approaches for dealing with three sources of bias when studying the fishing down marine food web phenomenon. CIESM Workshop Series 12:61–66.
- Pauly D, Trites AW, Capuli EV, Christensen V. 1998. Diet composition and trophic levels of marine mammals. ICES Journal of Marine Science 55:467–481. doi:10.1006/jmsc.1997.0280.
- Raya V, Sabatés A. 2015. Diversity and distribution of early life stages of carangid fishes in the northwestern Mediterranean: Responses to environmental drivers. Fisheries Oceanography 24:118–134. doi:10.1111/fog.2015.24.issue-2.
- Riedl R. 1991. Flora e fauna del Mediterraneo. Padova: Muzio F, editor. Hamburg/Berlin: Verlag Paul Parey. 777 pp.
- Romeo T, Battaglia P, Pedà C, Consoli P, Andaloro F, Fossi MC. 2015. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Marine Pollution Bulletin 95:358–361. doi:10.1016/j.marpolbul.2015.04.048.
- Romeo T, Battaglia P, Pedà C, Perzia P, Consoli P, Esposito V, Andaloro F. 2012. Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgoland Marine Research 66:295–306. doi:10.1007/s10152-011-0270-3.
- Romeo T, Consoli P, Castriota L, Andaloro F. 2009. An evaluation of resource partitioning between two billfish, Tetrapturus belone and Xiphias gladius, in the central Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 89:849–857. doi:10.1017/S0025315408002087.
- Sivakami S. 1997. On the food habits of the fishes of the family Carangidae a review. Journal of the Marine Biological Association of India 38:118–123.
- Stergiou KI, Karpouzi VS. 2002. Feeding habits and trophic levels of Mediterranean fish. Reviews in Fish Biology and Fisheries 11:217–254. doi:10.1023/A:1020556722822.
- Tutman P, Glavić N, Kožul V, Skaramuca B, Glamuzina B. 2004. Preliminary information on feeding and growth of pompano, Trachinotus ovatus (Linnaeus, 1758) (Pisces; Carangidae) in captivity. Aquaculture International 12:387–393. doi:10.1023/B:AQUI.0000042135.88381.f4.
- Whitehead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E, editors. 1984–1986. Fishes of the North-eastern Atlantic and Mediterranean. Vols. I, II, III. Paris: UNESCO.