Abstract
Different hypotheses exist to explain the ability of individuals or species to modify their behaviours in response to the urban environment. Our study addresses risk-taking in urban birds as an essential behavioural change in cities allowing the species to manage living in anthropic habitats. Specifically, we tested role of phylogeny and the environment on risk-taking, expressed in lower escape distances. We adopted a comparative approach and compared the flight distance of urban pigeons and urban crows in Paris with rural wild rock doves and crows in Sardinia, thus contrasting environmental conditions (urban or rural), species (columbids vs. corvids) and type (feral or wild). Pigeons had lower flight distance than crows in both rural and urban environments, and rural individuals of both species had higher flight distance than urban individuals. However, this intraspecific difference was higher in pigeons than in crows, and the interspecific difference was higher in urban than in rural areas. Our study shows that risk-taking in birds is the outcome of a complex interplay between several environmental and phylogenetic factors, and confirms the hypothesis of increased risk-taking in urban pigeons as a result of a pre-adaptation due to artificial selection, as often suggested but never scientifically demonstrated.
Introduction
Urban ecosystems are recognised to be used by a wide array of different animal species previously associated only with rural landscapes (Croci et al. Citation2008). Species in the urban areas are exposed to new environmental pressures enhancing modified behaviours (Stephen et al. Citation2006). Behavioural modifications may relate to temporal and spatial activity patterns, foraging or reproductive timing strategies (Francis & Chadwick Citation2012; Jacquin et al. Citation2013). Because human waste sites and city streets provide substantial amounts of food in the forms of trash, urban animal species commonly exhibit a marked increase in their consumption of these items (Kristan et al. Citation2004; Prange et al. Citation2004). When nutrition improves, it is common for reproductive rates to increase, often resulting in greater litter sizes, greater survival of offspring and ultimately greater densities (Robbins Citation1993).
Higher thresholds for fear and risk-taking, expressed in lower escape distances, is an essential behavioural change in urban environments allowing the species to manage living in anthropic habitats, e.g. characterised by human presence (Møller Citation2008; Levey et al. Citation2009). Some studies suggest this ability to beneficially modify behaviours in response to changes in the environment arises from innate behaviours, independently from experience (Wright et al. Citation2010). Under this hypothesis, interspecific variation in risk-taking would be the consequence of intrinsic traits and result from phylogenetic pre-adaptation (Sol & Lefebvre Citation2000). In a comparison of rural bird populations of urbanised and non-urbanised species, Møller (Citation2009) reported that the generally lower flight distance of urban populations, relative to their rural counterparts, already existed in rural populations before their expansion in the urban environments.
From a similar perspective, successful urban species are often suggested to be pre-adapted through previous human selective breeding for animals adaptable to conditions of captivity (Sol et al. Citation2002). The case of urban pigeons (Columba livia Gmelin, 1789) is particular, because urban populations descend mostly from domestic pigeons (Johnston & Janiga Citation1995; Stringham et al. Citation2012); they have been thus presumably selected for tolerance to human proximity (Johnston & Janiga Citation1995). Indeed, they are accounted in two studies with lower neophobia and better innovation rates in urban street experiments than two taxonomically close species, the zenaida dove (Zenaida aurita) and common ground dove (Columbina passerine), coming from wild populations (Seferta et al. Citation2001; Webster & Lefebvre Citation2001). These differences are assumed to be caused by differences in artificial selection (Bouchard et al. Citation2007).
However, urbanisation-related divergence in behavioural phenotypes in this species could also be explained by phenotypic plasticity, i.e. the ability of an organism to express different phenotypes. This ability depends on individuals’ current or recent environmental conditions, and varies with experience or ontogeny (Yeh & Price Citation2004; Slabbekoorn & den Boer-Visser Citation2006; Stephen et al. Citation2006). Studies on neophobia in captive parrots showed, for instance, that populations living in complex habitats were less neophobic than those living in simpler ones (Mettke-Hofmann et al. Citation2002). Similarly, wild-caught immature song-sparrows from anthropic habitats were less neophobic than wild-caught swamp-sparrows from shrub-wetlands, whereas the tendency was not confirmed when wild-caught from both species were hand-raised from the nestling stage, suggesting that lower neophobia resulted from a plastic behaviour favored by a more diverse experience (Greenberg & Mettke-Hofmann Citation2001).
In the present study, we aimed to investigate risk-taking in urban feral pigeons, and tested whether it is the expression of (1) a pre-adaptation resulting from their former domestication, or of (2) living in an urban environment. To do so, we first compared risk-taking – in the form of flight distance – of urban feral pigeons with those of pigeons thought to be wild rock doves, living in their natural habitat (see Material and methods). Then, we made a cross-comparison with urban and rural populations of corvids (urban carrion crows, Corvus corone Linnaeus, 1758, and wild hooded crows, Corvus cornix Linnaeus, 1758). Indeed, feral urban pigeons and crows are the most highly urbanised bird species in the Western Palearctic (Johnston & Janiga Citation1995). Under the pre-adaptation hypothesis, we predict that urban feral pigeons should have lower flight distances than both rural wild rock doves and non-domesticated crows (from both urban and rural environments). Alternatively, under the urban environment hypothesis, we predict that both urban groups would show lower flight distance than rural related species.
Materials and methods
The study took place from April 2012 to May 2013, during the months of April–May and September–November, excluding the winter season. We conducted 60 and 70 flight distance trials for rural and urban groups, respectively. Per trial, the tested birds could be a group of up to 10 individuals. All measurements were conducted by the same experimenter (ZS).
Urban feral pigeons and urban carrion crows were tested in urban places, in seven different sites in Paris (France) located at more than 500 m from each other: Île de la Cité, 1st district, one spot; Île Saint Louis 4th district, two spots; Champ de Mars, 7th district, three spots; Invalides, 7th district, one spot. The testing sites were chosen based on the regular presence of birds in these places. For each group of birds (one pigeon and one crow group per site) we measured flight distance on six successive occasions, and conducted one trial per day per group.
With regard to the rural pigeons we tested, pure wild colonies of rock doves are now very rare all around the Mediterranean countries (if still existing; see Johnston & Janiga Citation1995). In the “Parco Naturale Regionale di Porto Conte” (Nurra di Alghero, Sardinia Island, 5350 ha), a very large colony (about 3000 pigeons) of rock doves was still present in th near past (Baldaccini et al. Citation2000), even if some pigeons bear signs of introgression of feral characters. Now, this colony has almost disappeared and only a few pigeons (maybe less than 200) are present in the area. Their shelters are scattered along the sea cliff, and this fact prevented us from testing along the sea shore itself. Nevertheless, we were able to locate a small colony (no more than 15 pigeons) inland, near the northern part of the protected area, hosted in an abandoned jail (“Le Prigionette”, Torre del Porticciolo). The plumage of these specimens appeared to be of the blue rock type, but we are aware of the fact that some introgression of feral characters may be present also in this colony, and that mongrel progeny of ferals and rock doves always show a tendency to revert to the original plumage of the rock dove. From the genetic point of view, these birds clustered with other wild specimens captured at the site of the old colony mentioned before (unpublished data). As a consequence, we assume that the Le Prigionette specimens can be considered wild rock in a rural state. All tests were performed very near the colony site, where we never observed other groups of feral or domestic pigeons, even if they are present in villages not far from the protected areas.
Though we are aware that the independence of our data cannot be completely ascertained, this is however the only opportunity to conduct a study integrating a wild population of rock doves, a threatened species at European level (IUCN Red List of Threatened Species Citation2014).
We consider that the use of two different species – Corvus corone and Corvus cornix – in the comparison between urban and rural crows does not affect the results, given the very low genetic difference between carrion and hooded crows, so far regarded as subspecies of the same taxon (Saino et al. Citation1992). Rural hooded crows were tested at several locations in proximity to the same site, where we also tested the rural pigeons.
Each trial started with the experimenter putting 100 g of corn seed on the ground and moving 15 m away from the patch. Once a bird began feeding, the experimenter waited for 20 s so as to give other birds the opportunity to approach the feeding site. She then walked towards the food patch and the birds at a constant pace, and stopped and put a mark on the ground when the last bird left the feeding site, either by foot or by flying. The distance between the mark at which the birds stopped their feeding and the food location was then measured with a tape.
We were interested in differences between pigeons and crow groups, and more particularly between urban/rural environments, and between species (pigeons vs. crows). Because the six measurements per tested group could not be considered independent, we considered group as a random effect in a linear mixed model where the log of flight distance was the dependent variable; the species and the environments (plus their interaction) were the fixed factors. If the interaction was significant, we performed post hoc tests within each level of factor. We used two-tailed Type 3 tests for fixed effects with the significance level set at α = 0.05. All statistical analyses were performed using SAS (version 9.4).
Results
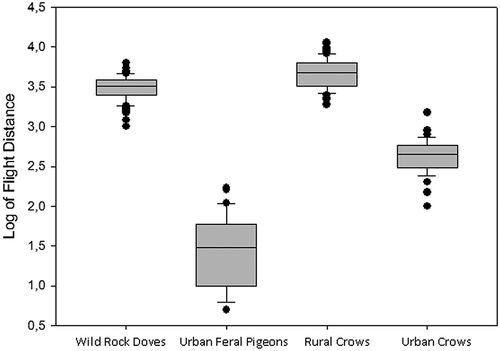
We found significant effects of species, environment and an interaction between species and environment (). Rural pigeons had lower flight distance than rural crows (post hoc tests; F1,18 = 28,35; P < 0,0001), urban pigeons had lower flight distance than urban crows (F1,11 = 224,73; P < 0,0001), and rural individuals of both species had higher flight distance than urban individuals (post hoc tests; crows: F1,14 = 357,74; P < 0,0001; pigeons: F1,15 = 1127,96; P < 0,0001). However, this intraspecific difference was higher in pigeons than in crows, and the interspecific difference was higher in urban than in rural areas (; ).
Table I. Significance of the fixed effects of the environment and taxonomy, and their interaction on the flight distance of birds (LMM).
Table II. Estimates ± standard error (SE) of the mixed model.
Discussion
Our results seem to suggest that risk-taking in birds is the outcome of a complex interplay between several environmental and phylogenetic factors. First, the study confirms the generally admitted evidence that living in an urban environment pushes species to flexibly revise their fear thresholds.
Second, our findings also highlight the role of pre-adaptation in higher risk-taking in urban birds. The fact not only that urban pigeons have lower flight distance than urban crows, but also that wild rock doves are more prone to risk-taking than rural crows prior to any artificial selection, provides, in addition to Møller’s study (Citation2009) in rural birds only, a cross-species and cross-areas support for the pre-adaptation hypothesis, which would need further testing with more species. In fact, the rock dove Columba livia has a long history of proximity to humans, and this species was domesticated as a consequence of its active urbanisation starting with the spread of agriculture and the development of urban settlements at the beginning of the Bronze Age, in the Fertile Crescent area of the Middle East (Bodenheimer Citation1960). Nevertheless, we are aware that some “domesticated traits” may be present in our rock doves as a consequence of putative cross-breeding with feral or domestic pigeons.
This seems consistent also with data on city colonisation by different Columba species (Evans et al. Citation2010) that did not go through human domestication like the urban Columba livia: the woodpigeon C. palumbus, the hill pigeon C. rupestris which had already settled in towns in the early 1900s, the band-tailed pigeon C. fasciata in the United States, the picazuro pigeon C. picazuro (urbanised in south-east Brazil and Argentina) and the spot-winged pigeon C. maculosa (urbanised in Bolivia and Peru; Gibbs et al. Citation2001).
Yet our study simultaneously confirms the hypothesis of increased risk-taking in urban Columba livia as a consequence of their former domestication. Indeed, the lower flight distance in pigeons compared to crows was stronger in urban areas, and the variance in risk-taking between rural and urban individuals as an effect of the environment is, further, significantly higher in pigeons than in crows. Since unlike crows both pigeon groups differ, besides their environment, also in their status of wild vs. feral, these variances can be confidently interpreted as the outcome of pigeons’ transition through domestication. Our findings are thus the first empirical evidence of the often-assumed low flight distance in feral pigeons, as a result of their artificial selection for human tolerance as domestics, prior to their feralisation.
Acknowledgements
We are grateful to Lidia Fleba, Parco Naturale Regionale di Porto Conte, and to Massimo Scandura, University of Sassari, for the precious help given to Z. S. in Sardinia. We wish to thank three anonymous referees for helpful comments on an earlier draft of the manuscript.
References
- Baldaccini NE, Giunchi D, Mongini E, Ragionieri L. 2000. Foraging flights of wild rock doves (Columba l. livia): A spatiotemporal analysis. Italian Journal of Zoology 67:371–377. DOI: 10.1080/11250000009356342.
- Bodenheimer FS. 1960. Animal and man in Bible land. Leiden: Brill.
- Bouchard J, Goodyer W, Lefebvre L. 2007. Social learning and innovation are positively correlated in pigeons (Columba livia). Animal Cognition 10:259–266. DOI: 10.1007/s10071-006-0064-1.
- Croci S, Butet A, Clergeau P. 2008. Does urbanization filter birds on the basis of their biological traits? The Condor 110:223–240. DOI: 10.1525/cond.2008.110.issue-2.
- Evans KL, Hatchwell BJ, Parnell M, Gaston KJ. 2010. A conceptual framework for the colonization of urban areas: The blackbird Turdus merula as a case study. Biological Revues 85:643–667.
- Francis RA, Chadwick MA. 2012. What makes a species synurbic? Applied Geography 32:514–521. DOI: 10.1016/j.apgeog.2011.06.013.
- Gibbs D, Barnes E, Cox J. 2001. Pigeons and doves: A guide to the pigeons and doves of the world. Robertsbridge: Pica Press.
- Greenberg R, Mettke-Hofmann C. 2001. Ecological aspects of neophobia and neophilia in birds. Current Ornithology 16:119–178.
- International Union for Conservation of Nature. 2014. The IUCN Red List of Threatened Species. Available: http://www.iucnredlist.org/. Accessed Jul 2014 10.
- Jacquin L, Récapet C, Prévot-Julliard A-C, Leboucher G, Lenouvel F, Erin T, Frantz A, Corbel H, Gasparini J. 2013. A potential role for parasites in the maintenance of bird color polymorphism in cities. Oecologia 173:1089–1099. DOI: 10.1007/s00442-013-2663-2.
- Johnston RF, Janiga M. 1995. Feral pigeons. New York: Oxford Univ. Press.
- Kristan WB, Boarman WI, Crayon JJ. 2004. Diet composition of common ravens across the urban-wildland interface of the West Mojave Desert. Wildlife Society Bulletin 32:244–253. DOI: 10.2193/0091-7648(2004)32[244:DCOCRA]2.0.CO;2.
- Levey DJ, Londono GA, Ungvari-Martin J, Hiersoux MR, Jankowski JE, Poulsen JR, Stracey CM, Robinson SK. 2009. Urban mockingbirds quickly learn to identify individual humans. Proceedings of the National Academy of Sciences of the United States of America 106:8959–8962. DOI: 10.1073/pnas.0811422106.
- Mettke-Hofmann C, Winkler H, Leisler B. 2002. The significance of ecological factors for exploration and neophobia in parrots. Ethology 108:249–272. DOI: 10.1111/eth.2002.108.issue-3.
- Møller AP. 2008. Flight distance of urban birds, predation, and selection for urban life. Behavioral Ecology and Sociobiology 63:63–75. DOI: 10.1007/s00265-008-0636-y.
- Møller AP. 2009. Successful city dwellers: A comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159:849–858. DOI: 10.1007/s00442-008-1259-8.
- Prange S, Gehrt SD, Wiggers EP. 2004. Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. Journal of Mammalogy 85:483–490. DOI: 10.1644/BOS-121.
- Robbins CT. 1993. Wildlife feeding and nutrition. San Diego, CA: Academic Press.
- Saino N, Lorenzini R, Fusco G, Randi E. 1992. Genetic variability in a hybrid zone between carrion and hooded crows (Corvus corone corone and C. c. cornix, Passeriformes, Aves) in north-western Italy. Biochemical Systematics and Ecology 20:605–613. DOI: 10.1016/0305-1978(92)90018-9.
- Seferta A, Guay P-J, Marzinotto E, Lefebvre L. 2001. Learning differences between feral pigeons and zenaida doves: The role of neophobia and human proximity. Ethology 107:281–293. DOI: 10.1046/j.1439-0310.2001.00658.x.
- Slabbekoorn H, den Boer-Visser A. 2006. Cities change the songs of birds. Current Biology 16:2326–2331. DOI: 10.1016/j.cub.2006.10.008.
- Sol D, Lefebvre L. 2000. Behavioural flexibility predicts invasion success in birds introduced to New Zealand. Oikos 90:599–605. DOI: 10.1034/j.1600-0706.2000.900317.x.
- Sol D, Timmermans S, Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Animal Behaviour 63:495–502. DOI: 10.1006/anbe.2001.1953.
- Stephen S, Ditchkoff S, Saalfeld T, Gibson CJ. 2006. Animal behavior in urban ecosystems: Modifications due to human-induced stress. Urban Ecosystems 9:5–12. DOI: 10.1007/s11252-006-3262-3.
- Stringham SA, Mulroy EE, Xing J, Record D, Guernsey MW, Aldenhoven JT, Osborne EJ, Shapiro MD. 2012. Divergence, convergence, and the ancestry of feral populations in the domestic rock pigeon. Current Biology 22:302–308. DOI: 10.1016/j.cub.2011.12.045.
- Webster SJ, Lefebvre L. 2001. Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Animal Behaviour 62:23–32. DOI: 10.1006/anbe.2000.1725.
- Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA. 2010. Behavioral flexibility and species invasions: The adaptive flexibility hypothesis. Ethology Ecology & Evolution 22:393–404. DOI: 10.1080/03949370.2010.505580.
- Yeh PJ, Price TD. 2004. Adaptive phenotypic plasticity and the successful colonization of a novel environment. The American Naturalist 164:531–542. DOI: 10.1086/an.2004.164.issue-4.