Abstract
Our purpose is to elucidate the roles of some molecules and DNA methylation in cerebellar development. Immunocytochemistry was performed to investigate the expression of activated Notch1 and Sonic Hedgehog (Shh), as well as DNA methylation during cerebellar development at various ages. Notch1 could be expressed in neural stem cells, newborn neurons and Purkinje cells during cerebellar development. However, Shh could be expressed mainly in developing radial glia or Bergmann cells specifically. DNA methylation was present during cerebellar development, mainly in neural progenitor cells and developing granule cells. The formation and maturation of both Purkinje cells and Bergmann cells are required for the differentiation and migration of granule cells which were bound to DNA methylation. DNA methylation and Shh and Notch signaling pathways function cooperatively during cerebellar development. The Notch pathway mainly contributes to the differentiation of Purkinje cells, but Shh is often involved in the development of Bergmann cells. DNA methylation is necessary for the differentiation and migration of granule cells. The expression of Notch1 and Shh in Purkinje cells and Bergmann cells, following the initiation of DNA methylation in granule cells, indicates that DNA methylation is regulated by Notch and Shh pathways during cerebellar development.
Introduction
Epigenetic regulation of gene expression is involved in both muscle development and regeneration (Palacios & Puri Citation2006). As we know, gene transcription is often influenced by histone modifications, methylation, acetylation, SUMOylation (small ubiquitin-like modifier, SUMO), ubiquitination and phosphorylation of DNA (Berger Citation2007; Martelli et al. Citation2009). Recently, DNA methylation has received more and more attention in the neuroscience community due to its essential roles for brain development. During prefrontal cortical development, DNA methylation shows temporospatial patterns, usually fast during the prenatal period and slow after birth (Numata et al. Citation2012). DNA methylation is specifically involved in neuroapoptosis, synaptogenesis and so on. On the other hand, DNA methylation can be regulated by some signaling pathways, such as Notch and Sonic Hedgehog (Shh) pathways. Previous studies showed that during stem cell differentiation, DNA methylation can be controlled by both the Notch pathway and the Shh pathway (Hitoshi et al. Citation2011). Using Sox2 (Sex-determining-region-Y-related high-mobility-group-box protein-2) deleted chick embryos, Hu et al. (Citation2012) demonstrated that the DNA methyltransferase 3A (DNMT3A), which was mediated directly by some genes, like Sox2 and Shh, could promote neural crest development.
Due to its specific physiological functions and pathological roles, cerebellar development has received much focus by neuroscientists; however, the complicated mechanisms of cerebellar development are not well understood (Bosma Citation2010). Moreover, further work needs to be done with regard to DNA methylation and some signaling pathways during cerebellar development specifically. Recently, it has been proposed that some signaling pathways, such as the Shh and Notch pathways, and DNA methylation could provide new insights into understanding brain development (Hatten & Roussel Citation2011; Sun et al. Citation2014). In our study, we investigated DNA methylation during cerebellar development and compared the expression of Shh and Notch. Our data will be useful to understand the role of DNA methylation and its regulative molecules during cerebellar development.
Materials and methods
Animals
All experiments were carried out in accordance with the institutional guidelines of Henan University for animal welfare. Adult male and female C57BL/6 J mice were placed in breeding cages in standard laboratory animal housing with a 12 h:12 h light:dark cycle. Embryonic or postnatal offspring were produced from timed pregnancies (E, day of conception; E0, day of vaginal plug to be found in mated females; P, days postnatal; P0, the first 24 h after birth). Pups were born at E19. A total of 163 embryos and postnatal pups at E8–19 and P0–360 were used in this study. To obtain embryonic mice at specific stages, pregnant dams were anesthetized (sodium pentobarbital, 40 mg/kg, intraperitoneal injection or i.p.) and fetuses at various ages were harvested by Cesarean section. From E8 to 13, whole embryos were fixed with 4% paraformaldehyde (PFA) in 0.01 M phosphate buffer (PB; pH 7.2) for 2–3 days at 4°C. From day E14 onwards, fetal brains were carefully separated and immersion-fixed in 4% PFA for 1–2 days at 4°C. For postnatal mice, pups were anesthetized (sodium pentobarbital, 20 mg/kg, i.p.) and perfused transcardially with 4% PFA. Cerebella were removed, and fixation was continued at 4°C for 1–2 days.
Immunocytochemistry
Paraffin-embedded embryos (< E14) were sectioned horizontally or sagittally (5–7 µm); from E14 onwards, whole brains or separated cerebella were sectioned coronally or sagittally (50 µm) using a vibratome. Sections were rinsed in 0.01 M PB and pre-incubated in blocking solution (5% normal goat serum) for 30 min at room temperature before immunofluorescent labelling. Nestin, glial fibrillary acidic protein (GFAP), S100, Ki67, double-cortin (DCX), neuronal nuclear antigen (NeuN), Calbindin and forkhead box p2 (Foxp2) were respectively used as markers to visualize radial glial cells (Nestin, S100), astrocytes (GFAP), newborn neurons (Ki67, DCX), mature neurons (NeuN) and Purkinje cells (Calbindin). To understand the DNA methylation level in neurons and glia of cerebellum, DNA methyltransferase 3a (DNMT3a), DNA methyltransferase 1 (DNMT1) and 5-methylcytosine (5-mC) were used in our study. In the meantime, Notch1 (one of the Notch receptors), Hes1 (the downstream protein of Notch pathway) and Shh protein were tested with immunofluorescent labeling. Sections were incubated overnight at 4°C with indicated dilutions of the different primary antibodies: mouse monoclonal anti-Nestin antibody (1:200; Santa Cruz, SC33677), rabbit polyclonal anti-activated Notch1 antibody (1:500; Abcam, AB4648), rabbit polyclonal anti-Hes1 antibody (1:500; Abcam, AB71559), rabbit polyclonal anti-Dnmt3a antibody (1:500; Abcam, AB4897), rabbit polyclonal anti-Dnmt1 antibody (1:100; Abcam, AB19905), mouse monoclonal anti-5-mC antibody (1:500; Abcam, AB10805), rabbit polyclonal anti-GFAP (1:500; Beijing Zhong Shan-Gloden Bridge, ZA-0117), rabbit polyclonal anti-DCX (1:500; Cell Signaling, 4604), mouse monoclonal anti-NeuN (1:500; Chemicon, MAB377B), rat monoclonal anti-Shh (1:500; Abcam, AB50515) and mouse monoclonal anti-Calbindin (1:500; Abcam, AB9481). In some sections, double labeling of radial glial cells was performed using rabbit polyclonal anti-Nestin antibody (1:1000, Abcam, AB5968) in addition to mouse anti-Nestin. After multiple washes in 0.01 M PB, appropriate secondary antibodies were added at the indicated dilutions and incubated at room temperature for 3 h. The secondary antibodies were: Alexa Fluro 488 donkey anti-mouse Immunoglobulin G (IgG) (1:500; Invitrogen, A21202), Alexa Fluro 488 donkey anti-rabbit IgG (1:500; Invitrogen, A21206), Alexa Fluro 568 goat anti-mouse IgG (1:600; Invitrogen, A11004) and Alexa Fluro 568 goat anti-rabbit IgG (1:600; Invitrogen, A11011). Sections were counterstained with DAPI (4′,6-diamidino-2-phenylindole) (1:60,000; Santa Cruz, SC3598) for 1–2 min after immunolabeling. Sections were coverslipped under 65% glycerol in 0.01 M PB and imaged using an epifluorescence microscope (BX61, Olympus) with rhodamine, fluorescein isothiocyanate (FITC) or ultraviolet filter sets. High-quality sections were photographed using an Olympus laser confocal microscope (FV1000, Olympus, Japan), using separate scans with 568 nm (red), 488 nm (green) and 350 nm (blue) laser lines.
Results
Differentiation of Purkinje cells and Notch1 expression
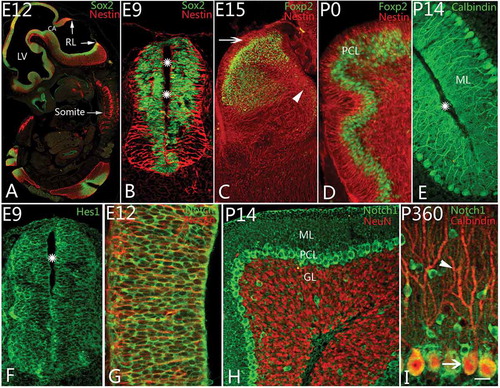
To understand the development of Purkinje cells, an introduction to cerebellar morphogenesis is in order. The development of the neural tube is an anlage of brain development in general (, ). From a histological point of view, the neural tube makes up a pseudostratified neuroepithelium which contains various cells, such as neural progenitor cells and glia. Nestin and Sox2 were used as markers for the radial glial cells and neural progenitor cells in the study. At as early as E9, Nestin-positive radial glial cells, with long projections from lumen to basement membrane, were arranged in the neural tube radially, and numerous Sox2-positive neural stem cells were located in the neural tube, especially on the nearby lumen surface (). The anterior part of the neural tube consisted of the prosencephalon, mesencephalon and rhombencephalon (). The cerebellum is developed from the isthmus, the junction of the mesencephalon and rhombencephalon. Initially, the neural tube in the dorsolateral part of the alar plate in the isthmus thickened to form rhombic lips which continued to develop into the cerebellum (Carletti & Rossi Citation2008; Hoshino Citation2012). In the present study, from a sagittal section view, there were germinative zones of neurogenesis, the ventricular zone (VZ) and rhombic lip (RL) (A). All cerebellar neurons and glia derive from two germinative zones (Carletti & Rossi Citation2008; Hoshino Citation2012); for instance, Bergmann cells and Purkinje cells originate from VZ, and granule cells derive from RL (see also Zhang et al. Citation2010). With cerebellar development, the RL became more and more thick, and there were numerous Foxp2-positive cells that moved along radial glial cells from the neuroepithelium toward the Purkinje cell layer at E15 (). At P0, only 4–5 rows of Foxp2-positive cells were located in the Purkinje cell layer (), and, finally, these newborn Purkinje cells started to differentiate into mature neurons which were arranged in one row (). First upstream proteins (Notch receptor) and last downstream proteins (Hes) were tested in this study. In the early stage, both Notch1 and Hes1 were expressed in the neuroepithelium of the neural tube. These cells appeared round or oval in shape and were located mainly near the lumen of the neural tube (). Notch1- and Hes1-positive cells were similar to each other in their morphology and location during neurulation. Considering that epithelial cells in the neural tube mainly belong to neural stem cells, these Notch1- and Hes1-positive cells can be hypothesized as neural stem cells. In order to demonstrate the conclusion further, Notch1 and Nestin double immunolabeling was carried out. We found that Notch1 was not expressed in Nestin-positive glial cells (), indicating the Notch pathway was correlated to neuron differentiation rather than glial development. With cerebellar development, Notch1 could even be visualized in the matrix of molecular layer. The majority of neurons that expressed Notch1 were migrating Purkinje cells in the subventricular zone, or Purkinje cells in Purkinje cell layer (PCL) () and some migrating postmitotic newborn neurons from external granular layer (EGL) to internal granular layer (IGL) (). Interestingly, Notch1 was mainly expressed in the cell bodies of Purkinje cells rather than in their dendrites (). Moreover, weak signals of Notch1 could be found in granule cells as well ().
Figure 1. Notch1 and Purkinje cell differentiation. A, Neurulation and cerebellar development at embryonic day 12 (E12) (Sox2 (Sex-determining-region-Y-related high-mobility-group-box protein-2) and Nestin immunolabeling). Rhombic lips (RL) are the anlage of cerebellum. Lateral ventricle (LV), cerebral aqueduct (CA) and somites are marked. B–E, Neural progenitor cell and Purkinje cell differentiation. With Nestin (red) and Sox2 (green) immunolabeling, the radial glial cells and neural progenitor cells were found at age E9 (B). The radial glial cells appeared radially around the cavity (stars) of the neural tube, and the neural progenitors were located in the neural epithelium. The radial glia extended apical and basilar projections toward the pia and the lumen. At E15, rhombic lip thickened and became large (C), and newborn Purkinje cells started to migrate from the ventricular zone (arrow head) to the Purkinje cell layer (PCL). With Nestin & Foxp2 (forkhead box p2) double immunolabeling, at this age, numerous Foxp2 positive cells (green) have migrated from ventricle zone (VZ) (arrowhead) to PCL along the processes of radial glial cells (red; C). The external granular layer could be recognized (arrow) in the figure without Foxp2 positive cells inside. At postnatal day (P0), the migrating Foxp2 positive Purkinje cells were mainly concentrated in PCL with 3–4 rows (D). With Calbindin immunolabeling, only one or two rows of Purkinje cells have been located in PCL after P5 onward (E). The molecular layer (ML) and cortical sulcus (star) are marked in the photo. F–G, Notch pathway and neural progenitor cells in neural tube. Hes1 (green), a downstream protein in Notch pathway, could be expressed in neural progenitor cells (green) around the cavity (star) of the neural tube at E9 (F). Notch1 (green) could be also found in cell bodies of neural progenitors among which are the Nestin positive radial glia (red) at E12 (G). H–I, Notch1 expression and Purkinje cell differentiation after postnatal day. With Notch1 and NeuN double immunolabeling, Notch1 is expressed strongly in Purkinje cells (green) above granular layer (GL, red) and interneurons in molecular layer at P14 (H). In adult, using Calbindin (red) and Notch1 (green) immunolabeling, Notch1 is expressed in mainly cell bodies of Purkinje cells, but no expression in the dendrites (arrowhead, red; I). Scale bars: A = 400 μm; B, F = 35 μm; C = 100 μm; D, E, H = 50 μm; G = 25 μm; I = 20 μm.
Development of radial glial cells and Shh expression
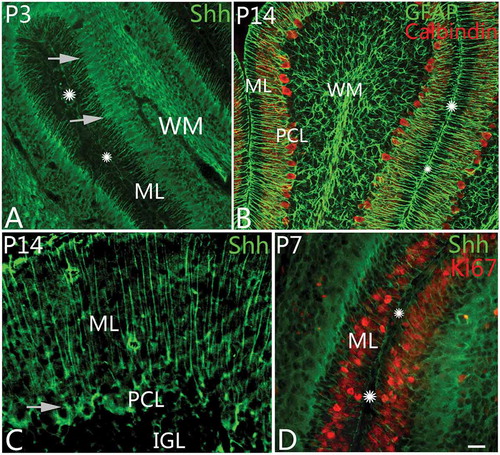
The Shh pathway plays a key role in regulating vertebrate organogenesis, especially for brain development. In our study, we observed the expression of Shh during cerebellar development from embryonic day E9 to adulthood. We found that Shh expression was highly correlated with the differentiation and development of radial glial cells. At as early as E9, the radial glial cells could be labeled in the neural tube with Nestin immunolabeling (). At this time, the processes of radial glial cells spanned over the entire cerebellar parenchyma from neuroepithelium to pia (). In this study, Shh was mainly expressed in radial glia. In embryonic days, Shh expression was weak and located mainly in the matrix of cerebellar cortex. After P0, Shh was recognized in the projections of radial glia. When radial glia developed into Bergmann cells (), the cell bodies moved up and settled down in the Purkinje cell layer with strong expression of Shh (). On P3–5, Shh-positive radial glial cells showed the strongest fluorescence with cell bodies in PCL, which decreased afterwards. In adulthood, Shh was expressed in the projections of Bergmann cells and weakly in the matrix of the molecular layer (). With Ki67 and Shh double immunolabeling, the Ki67-positive newborn neurons appeared in close contact with the projections of Shh-positive Bergmann cells in the molecular layer (). Considering that the newborn neurons in the external granular layer are bound to migrate into the internal granular layer along Bergmann cells, this supports that Shh was involved in neural migration as well (Fleming et al. Citation2013; De Luca et al. Citation2015). Previous studies showed that Purkinje cells could express Shh as well, but we did not find the same phenomenon in our study. Additional investigation of this phenomenon should be done in future.
Figure 2. Shh expression and the differentiation of Bergmann cells. A, At postnatal day 3 (P3), Sonic Hedgehog (Shh) protein (green) could be found in Bergmann cells. In the photo, their cell bodies are arranged with one row (arrows) in the Purkinje cell layer. The molecular layer and cortical sulcus are marked with ML and stars, respectively, as well. B, At P14, with GFAP (glial fibrillary acidic protein) and Calbindin double immunolabeling, radial glia and Purkinje cells can be shown. The GFAP-positive radial glial cells are similar to the Shh-positive cells in their shape or location, suggesting that they belong to same kind of cells. The molecular layer and sulcus are marked with ML and stars as well. C, Shh can be expressed in Bergmann cells and the matrix of molecular layer (ML) at P14. The arrow points to the cell body of an Shh-positive cell. D, With Ki67 and Shh double immunolabeling, the neural progenitor cells (red) in ML were presumed to migrate into IGL along Bergmann cells (green). Scale bars: A, B= 50 μm; C = 20 μm; D = 25 μm.
Differentiation of granule cells and DNA methylation
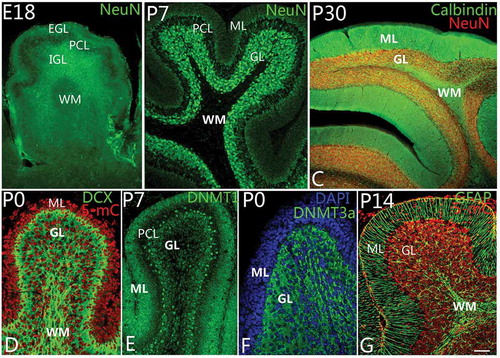
DNA methylation is prevalent during development, and its significance is to ensure cells’ deterministic differentiation and to prevent differentiated cells from reverting back to stem cells. Therefore, DNA methylation is very important to animal development and can determine the direction of cell lineage. In this study, we investigated DNA methylation of developing cerebellum from an early stage. We found that DNA methylation mainly happened in neural progenitor cells and newborn or mature granule cells. In prenatal and early postnatal stages, there were numerous neural stem cells and newborn neurons in EGL, which would migrate toward IGL (Zhang et al. Citation2010). Initially, these neural stem cells and newborn neurons occupied the whole EGL, and at E18, numerous newborn neurons in EGL started to migrate into IGL and differentiated into granule cells (). At P5, only a few cells were located in the superficial zone of the molecular layer (). With continuous migration, the molecular layer became a sparing cell layer, with dense dendrites of Purkinje cells (). We observed DNA methylation during cerebellar development. We analyzed 5-mC DNMT3a and DNMT1, respectively. In our observations, DNA methylation occurred in neural progenitor cells and later in newborn or mature granule cells of EGL and IGL (), harmonizing with the migrating and mature granule cells (). Interestingly, 5-mC and DNMT1 were mainly expressed in the nuclei of neural progenitor cells and putative granule cells in EGL and IGL (), while, on the other hand, DNMT3a was expressed in the cytoplasm of granule cells in IGL (). However, no Purkinje cells were found with DNA methylation during the developing cerebellum. Since DNA-methylated positive cells were mainly neural progenitor cells, putative granule cells (newborn granule cells) and mature granule cells with various degrees of differentiation, we believe that DNA methylation is most likely associated with the differentiation of granule cells.
Figure 3. Differentiation of granule cells and DNA methylation. A–C, Granule cell differentiation and migration. With neuronal nuclei (NeuN) immunolabeling, the newborn neurons in the external granular layer (EGL) could be observed to migrate into the internal granular layer (IGL) as early as at embryonic day 18 (E18) (A). The white matter (WM) located in the deep IGL was marked. At P7, almost all neurons in the molecular layer (ML) had migrated into the granular layer (GL) completely (B). The WM is a cell-free zone (NeuN immunolabeling). At P30, with NeuN and Calbindin double immunolabeling, cerebellar lamination had been formed typically with ML, Purkinje cell layer, granular layer (GL) and WM (C). D–F, DNA methylation during cerebellar development. At P0, with 5-methylcytosine (5-mC) and Doublecortin (DCX, green) immunolabeling, 5-methylcytosine (red) can be expressed in the cell nuclei of both ML and GL (D). The expression of DNA methyltransferase 1 (DNMT1) showed the same pattern as 5-mC (E). It suggests that 5-mC and DNMT1 are mainly involved in cell proliferation in the lineage of granule cells. Interestingly, DNA methyltransferase 3a (DNMT3a) could be mainly expressed in the cytoplasm of granule cells in GL rather than in ML (F), suggesting DNA methylation has a close relationship with the development and differentiation of granule cells. The cells in ML and GL were counterstained with Dapi (blue). In G, Bergmann cells (green) are shown to arrange radially in the ML with Nestin immunolabeling. With cerebellar development, ML become a cell-free zone, due to the migration of neurons (red) into GL (G). Scale bars: A, C = 100 μm; B = 50 μm; D–G = 25 μm.
Discussion
Notch pathway serves as an important mediator for the differentiation of Purkinje cells
As an important pathway for cell-to-cell communication, the Notch signaling pathway mediates the differentiation of Purkinje cells and is involved in neurogenesis and neural differentiation (Lütolf et al. Citation2002; Bolós et al. Citation2007; Aguirre et al. Citation2010). Using a conditional gene ablation approach, Klein et al. (Citation2004) demonstrated that the modulator of the Notch pathway, Numb, was involved in the maturation of cerebellar granule cells. In mutant animals (Numb-deficient mice), the development of granule cells is aberrant, and the migration of postmitotic granule cells to the internal granule cell layer is delayed. Our study also proved that the Notch pathway is probably involved in cell differentiation, especially for Purkinje cells, which harmonized with the studies from Lütolf et al. (Citation2002) and others (Patten et al. Citation2003). They showed that Notch1-deficient cells did not complete differentiation but were eliminated by apoptosis, resulting in a reduced number of neurons in the adult cerebellum. In the present study, during cerebellar development, Notch1 expression could be found in various cells, such as neural stem cells and newborn neurons in the early developmental stage. In addition, with age increase and neural stem cells differentiating into various neurons, Notch1 was mainly expressed in Purkinje cells. This suggests that the Notch pathway mediates the differentiation from stem cells to Purkinje cells. As we know, there are four different Notch receptors, referred to as Notch1, Notch2, Notch3 and Notch4. The Notch pathway is initiated when ligands combine with Notch receptors (Nam et al. Citation2003; Yamamoto et al. Citation2012). Under the participation of γ-secretase, intracellular domain (NICD) and DNA-binding protein RBPJ (recombination signal-binding protein Jκ), the expression of genes Hes1 and Hes5 is induced, which finally leads to the regulation of DNA transcription (Iso et al. Citation2003; Kageyama et al. Citation2007). We observed that Notch1 and Hes1 could be expressed in neural progenitor cells and newborn neurons at early developing age, but Notch1 was mainly found in Purkinje cells after birth, suggesting the Notch pathway could control stem cell differentiation, especially the differentiation of Purkinje cells.
Sonic hedgehog (Shh) serves as a key molecule for the differentiation and development of neural glial cells
Previous studies showed that Shh has played an important role in regulating vertebrate organogenesis, especially for the brain (Palma et al. Citation2005). In the cerebellum, Shh is usually produced by Purkinje cells, and Shh protein can induce the differentiation of Bergmann glia (Dahmane & Ruiz i Altaba Citation1999; Wallace Citation1999). Blocking Shh results in deficient differentiation of granule cells and Bergmann glia (Dahmane & Ruiz i Altaba Citation1999). Shh can directly induce Bergmann glia differentiation with collagen explant assay. When cultured Bergmann cells were treated with Shh 2 for 48 h, the glial cells would proliferate with rapidly growing processes. This suggests that Shh can induce the migration and differentiation of Bergmann cells (Dahmane & Ruiz i Altaba Citation1999). In our study, Shh could be expressed in the developing cerebellum. Before P0, Shh expression was very weak and mainly located in the matrix of cerebellar cortex. After birth, Shh could be found in Bergmann cells. In P3–5, Shh-positive radial glial cells showed the strongest fluorescence. However, in adulthood, Shh could be expressed not only in the projections of Bergmann cells but also in the matrix of the molecular layer. This phenomenon suggests that the Shh pathway is involved in the development of Bergmann cells. Growth differentiation factor 10 (Gdf10) factor is thought to participate in Shh regulation of the development of Bergmann cells (Mecklenburg et al. Citation2014). The blockade of Shh function in vivo resulted in deficient Bergmann glia differentiation as well as abnormal development of Purkinje neurons (Dahmane & Ruiz i Altaba Citation1999). Previous studies also showed that Shh could be expressed in many kinds of cells in the brain (Huang et al. Citation2010), including choroid plexus and Purkinje cells, but in our study, the Shh-positive cells in cerebellum were Bergmann glia. This tiny difference needs to be explained with further investigation.
DNA methylation mediates the differentiation of granule cells
DNA methylation is a biochemical process where a methyl group is added to the cytosine or adenine DNA nucleotides (Bird Citation1986). DNA methylation often occurs at the five positions of cytosine, typically in a cytosine-phosphorothioate-guanine (CpG) dinucleotide context, but non-CpG methylation is also prevalent in embryonic stem cells (Bird Citation2002). Since DNA methylation can alter the expression of genes in cell division and cell differentiation during embryonic stage, DNA methylation therefore plays an important role in the formation of organs (Razin et al. Citation1984). In central nervous system (CNS) development, it is involved in the synaptogenesis and nerve repair as well (Palacios & Puri Citation2006). Our study showed that DNA methylation occurred throughout the whole developmental process from the early embryonic period to adult. For instance, in the neural tube stage, DNA methylation existed in neural progenitor cells. With cerebellar development, the DNA methylation-positive cells were mainly located in the granule cells of EGL and IGL. DNA methylation probably functions to maintain granule cell stability and prevents the dedifferentiation of cells during cerebellar development. As we know, 5-mC and DNMT1 are called maintenance methyltransferase which can keep inherent methylation status during continuous DNA replication in cell proliferation, while, on the other hand, DNMT3a is called de novo DNA methyltransferase which can produce new DNA methylation in DNA nucleotide during cell differentiation. Therefore, 5-mC and DNMT1 are usually expressed in cell nuclei and are mainly correlated with the proliferation of stem cells, and DNMT3 exists in cytoplasm and is correlated with the differentiation of cells (Manoharan et al. Citation2015; Qin et al. Citation2015). In our study, we found that 5-mC and DNMT1 were mainly expressed in the nuclei of neural progenitor cells or newborn granule cells in EGL and IGL, but DNMT3a was expressed in the cytoplasm of mature granule cells in IGL, strongly suggesting that DNA mythylation was involved in the differentiation of granule cells. In this study, Purkinje cells showed negative DNA methylation, suggesting that DNA methylation is mainly associated with the differentiation and maturation of granule cells.
Shh and Notch pathways participate in the regulation of DNA methylation during cerebellar development
In the early stage of limb development, artificial controls of DNA methylation and Shh level can give rise to abnormality in limb development (Stancheva & Meehan Citation2000; Tetsuji & Nakashima Citation2010). In the meantime, Yakushiji et al. (Citation2007) found that in the limb enhancer region, the Shh gene was highly methylated in the frog leg, while, on the other hand, the hypomethylated gene in the Xenopus tadpole had complete limb regeneration ability, suggesting that regeneration of limbs is associated with the methylation status of Shh. In our study, we showed some morphological and developmental evidence that the biological influence of DNA methylation may be mediated through Notch and Shh pathways. We have found that either Shh and Notch pathways or DNA methylation are key chemical processes during cerebellar development. Shh and Notch pathways play important roles in the neural differentiation of neural progenitor cells into Bergmann glia and Purkinje cells respectively. On the other hand, DNA methylation strongly affects cell migration and differentiation of granule cells. It is known that during cerebellar development, Purkinje cells can help Bergmann cell bodies move up from neuroepithelium into PCL. Once Bergmann cells are settled down in PCL, granule cells start to migrate from EGL into IGL properly. The present study showed that Purkinje cell differentiation, Bergmann cell development and granule cell migration seem to be regulated by the Notch pathway, Shh pathway and DNA methylation, respectively. Considering chronological order, in which Purkinje cells and Bergmann cells appear earlier than the migration of granule cells, we have reason to hypothesize that DNA methylation is probably regulated by Notch and Shh pathways.
In summary, the molecules Shh and Notch are important to cerebellar development. Notch1 could be expressed in the neural stem cells, newborn neurons and Purkinje cells, suggesting that the Notch pathway mainly controls the differentiation of Purkinje cells. On the other hand, Shh is uniquely expressed in the developing radial glia or Bergmann cells, suggesting that Shh protein is involved in the development of Bergmann cells. Furthermore, DNA methylation is correlative with the differentiation and migration of granule cells. The chronological order among Purkinje cells, Bergmann cells and granule cells suggests that most likely DNA methylation is regulated by the Notch and Shh pathways.
Additional information
Funding
References
- Aguirre A, Rubio ME, Gallo V 2010. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467:323–327. DOI:10.1038/nature09347.
- Berger SL 2007. The complex language of chromatin regulation during transcription. Nature 447:407–412. DOI:10.1038/nature05915.
- Bird A 2002. DNA methylation patterns and epigenetic memory. Genes & Development 16:6–21. DOI:10.1101/gad.947102.
- Bird AP 1986. CpG-rich islands and the function of DNA methylation. Nature 321:209–213. DOI:10.1038/321209a0.
- Bolós V, Grego-Bessa J, De La Pompa JL 2007. Notch signaling in development and cancer. Endocrine Reviews 28:339–363. DOI:10.1210/er.2006-0046.
- Bosma MM 2010. Timing and mechanism of a window of spontaneous activity in embryonic mouse hindbrain development. Neurons and Networks in the Spinal Cord 1198:182–191.
- Carletti B, Rossi F 2008. Neurogenesis in the cerebellum. The Neuroscientist 14:91–100. DOI:10.1177/1073858407304629.
- Dahmane N, Ruiz i Altaba A 1999. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126:3089–3100.
- De Luca A, Parmigiani E, Tosatto G, Martire S, Hoshino M, Buffo A, Leto K, Rossi F 2015. Exogenous Sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development. Cerebellum 14:72–85. DOI:10.1007/s12311-014-0596-x.
- Fleming JT, He W, Hao C, Ketova T, Pan FC, Wright CV, Litingtung Y, Chiang C 2013. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Developmental Cell 27:278–292. DOI:10.1016/j.devcel.2013.10.008.
- Hatten ME, Roussel MF 2011. Development and cancer of the cerebellum. Trends in Neurosciences 34:134–142. DOI:10.1016/j.tins.2011.01.002.
- Hitoshi S, Ishino Y, Kumar A, Jasmine S, Tanaka KF, Kondo T, Kato S, Hosoya T, Hotta Y, Ikenaka K 2011. Mammalian Gcm genes induce Hes5 expression by active DNA demethylation and induce neural stem cells. Nature Neuroscience 14:957–964. DOI:10.1038/nn.2875.
- Hoshino M 2012. Neuronal subtype specification in the cerebellum and dorsal hindbrain. Development, Growth & Differentiation 54:317–326. DOI:10.1111/j.1440-169X.2012.01330.x.
- Hu N, Strobl-Mazzulla P, Sauka-Spengler T, Me B 2012. DNA methyltransferase3A as a molecular switch mediating the neural tube-to-neural crest fate transition. Genes & Development 26:2380–2385. DOI:10.1101/gad.198747.112.
- Huang CCJ, Miyagawa S, Matsumaru D, Parker KL, Yao HHC 2010. Progenitor cell expansion and organ size of mouse adrenal is regulated by Sonic hedgehog. Endocrinology 151:1119–1128. DOI:10.1210/en.2009-0814.
- Iso T, Kedes L, Hamamori Y 2003. HES and HERP families: Multiple effectors of the Notch signaling pathway. Journal of Cellular Physiology 194:237–255. DOI:10.1002/(ISSN)1097-4652.
- Kageyama R, Ohtsuka T, Kobayashi T 2007. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development 134:1243–1251. DOI:10.1242/dev.000786.
- Klein AL, Zilian O, Suter U, Taylor V 2004. Murine numb regulates granule cell maturation in the cerebellum. Developmental Biology 266:161–177. DOI:10.1016/j.ydbio.2003.10.017.
- Lütolf S, Radtke F, Aguet M, Suter U, Taylor V 2002. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development 129:373–385.
- Manoharan A, Du Roure C, Rolink AG, Burger L, Matthias P 2015. The de novo DNA Methyltransferases Dnmt3a and Dnmt3b regulate the onset of Igkappa light chain rearrangement during early B-cell development. European Journal of Immunology 45:2343–2355. DOI:10.1002/eji.201445035.
- Martelli G, Zaccagnino N, Milella L, Greco I 2009. Characterization of the DNA methylation activity by gene expression analysis in Fragaria vesca. VI International Strawberry Symposium 842:569–572.
- Mecklenburg N, Martinez-Lopez JE, Moreno-Bravo JA, Perez-Balaguer A, Puelles E, Martinez S 2014. Growth and differentiation factor 10 (Gdf10) is involved in Bergmann glial cell development under Shh regulation. Glia 62:1713–1723. DOI:10.1002/glia.v62.10.
- Nam Y, Weng AP, Aster JC, Blacklow SC 2003. Structural requirements for assembly of the CSL.intracellular Notch1.Mastermind-like 1 transcriptional activation complex. Journal of Biological Chemistry 278:21232–21239. DOI:10.1074/jbc.M301567200.
- Numata S, Ye T, Hyde TM, Guitart-Navarro X, Tao R, Wininger M, Colantuoni C, Weinberger DR, Kleinman JE, Lipska BK 2012. DNA methylation signatures in development and aging of the human prefrontal cortex. The American Journal of Human Genetics 90:260–272. DOI:10.1016/j.ajhg.2011.12.020.
- Palacios D, Puri PL 2006. The epigenetic network regulating muscle development and regeneration. Journal of Cellular Physiology 207:1–11. DOI:10.1002/(ISSN)1097-4652.
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Builla A, Ruiz i Altaba A 2005. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132:335–344. DOI:10.1242/dev.01567.
- Patten BA, Peyrin JM, Weinmaster G, Corfas G 2003. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. Journal of Neuroscience 23:6132–6140.
- Qin W, Wolf P, Liu N, Link S, Smets M, Mastra FL, Forné I, Pichler G, Hörl D, Fellinger K, Spada F, Bonapace IM, Imhof A, Harz H, Leonhardt H 2015. DNA methylation requires a DNMT1 ubiquitin interacting motif (UIM) and histone ubiquitination. Cell Research 25:911–929. DOI:10.1038/cr.2015.72.
- Razin A, Webb C, Szyf M 1984. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America 81:2275–2279. DOI:10.1073/pnas.81.8.2275.
- Stancheva I, Meehan RR 2000. Transient depletion of xDnmt1 leads to premature gene activation in Xenopus embryos. Genes & Development 14:313–327.
- Sun W, Zang L, Shu Q, Li X 2014. From development to diseases: The role of 5hmC in brain. Genomics 104:347–351. DOI:10.1016/j.ygeno.2014.08.021.
- Tetsuji M, Nakashima K 2010. Oxygen tension can control the DNA methylation status of GFAP promoter through Notch signaling and allows propagation and maturation of neuronal progenitor. Neuroscience Research 68:E352–E352. DOI:10.1016/j.neures.2010.07.1559.
- Wallace VA 1999. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Current Biology 9:445–448. DOI:10.1016/S0960-9822(99)80195-X.
- Yakushiji N, Suzuki M, Satoh A, Sagai T, Shiroishi T, Kobayashi H, Sasaki H, Ide H, Tamura K 2007. Correlation between Shh expression and DNA methylation status of the limb-specific Shh enhancer region during limb regeneration in amphibians. Developmental Biology 312:171–182. DOI:10.1016/j.ydbio.2007.09.022.
- Yamamoto S, Charng WL, Rana NA, Kakuda S, Jaiswal M, Bayat V, Bellen HJ 2012. A mutation in EGF repeat-8 of Notch discriminates between Serrate/Jagged and Delta family ligands. Science 338:1229–1232. DOI:10.1126/science.1228745.
- Zhang Y, Niu B, Yu D, Cheng X, Liu B, Deng J 2010. Radial glial cells and the lamination of the cerebellar cortex. Brain Structure & Function 215:115–122. DOI:10.1007/s00429-010-0278-5.
