Abstract
The functional response of a predator is a key factor regulating the population dynamics of predator-prey systems. This aspect of predatory behaviour must be assessed together with the effects of satiation by overabundant prey when evaluating the potential of biocontrol. The goals of this study were: (1) to identify the consumption rates of juvenile stages of the invasive zebra mussel, Dreissena polymorpha, by the invasive crayfish Procambarus clarkii; (2) to determine the predator functional response; (3) to evaluate the effects of predator satiation on the intensity of predation; and (4) to model the potential impacts of predation by this crayfish on zebra mussel populations. When presented with a range of mussel abundances, P. clarkii showed a functional response of type II, where the mortality of prey increases with decreasing prey abundance. P. clarkii also exhibited a satiation effect, diminishing its consumption rate from 33 mussels/day to 6 mussels/day over a 6-day period. By combining the effects of the functional response and of predator satiation we produced a model that predicts the complete consumption of local populations of up to 150/160 juveniles by one single crayfish over the period of 1 month. This impact may be important in low-density populations or in populations at equilibrium of D. polymorpha, and will be greater the higher the synchronization between the prey reproductive cycle and the activity period of the predator.
Introduction
Predation can exert a powerful influence in the abundance and population structure of prey (Sih et al. Citation1985; Hastings Citation1997). Prey is frequently more vulnerable during specific life stages (e.g., the juvenile or larval period), and the ability of a predator to effectively control their populations is dependent on the predation efficacy upon this stages.
The functional response of a predator is a key factor regulating the population dynamics of predator-prey systems. It describes the rate at which a predator kills its prey at different prey abundances, and its estimation can thus help to determine the predator efficiency in regulating prey populations (Murdoch & Oaten Citation1975). The functional response is an essential component of predator-prey models (Jeschke et al. Citation2002), but its estimation is rarely considered in an invasion context (Hooff & Bollens Citation2004; Radford et al. Citation2007), which is surprising, given the known impacts of introduced predators (Wanless et al. Citation2007). For instance, during the establishment stage of non-native invasive prey, the functional response of a predator can determine whether these prey are excluded from establishing, become established but do not proliferate and spread, or become overabundant and spread to new locations (Murdoch & Oaten Citation1975; Gascoigne & Lipcius Citation2004).
The shape of the functional response curve is dependent on several characteristics such as the encounter rate between prey and predator, capture efficiency and handling time; these characteristics may differ within a species as a consequence of predator or prey size, interference among predators or presence of alternative prey (Safina Citation1990; Tripet & Perrin Citation1994; Elliott Citation2003).
In a basic type I functional response, prey consumption linearly increases with increasing prey abundance. In the type II response, the risk of mortality increases with decreasing prey abundance, whereas the type III response results from decreased predator efficiency in the capture of prey as prey abundance decreases. Consequently, while a type II response can be destabilizing, a type III response stabilizes predator-prey dynamics (Lipcius & Hines Citation1986; Eggleston et al. Citation1992). The distinction between type II and type III functional responses, and hence whether predators will likely de-stabilize or stabilize prey populations, relies on examining prey consumption rates at low prey abundances (Juliano Citation2001).
Limitation of predation rate at high prey abundances has usually been attributed either to the time it takes to handle each prey or to satiation (Jeschke et al. Citation2002), two important factors to take into account when determining the functional response type (Gotelli Citation1995). The exact nature of handling time, satiation and their relationship have been modeled in a variety of different ways, and this has led to considerable confusion. Handling prey is an active process, whereas digestion is a background process. As a consequence, in contrast to handling prey, digestion does not directly prevent the predator from further searching or handling. Rather, digestion influences the predator’s hunger level, which in turn influences the probability that the predator searches for new prey. It is thus necessary to discriminate satiation from handling in a functional response model (Jeschke et al. Citation2002).
A small number of authors have investigated the predatory functional response as a potential predictor of the predatory effects of an invader (Radford et al. Citation2007; Bollache et al. Citation2008). This novel use for functional responses has important implications for invasion ecology: it helps to predict the predatory impacts of an invader, and conversely illuminates the role of native predators in controlling invasions by exotic prey. Furthermore, by quantifying invasion thresholds it may help managers predict the outcomes of species introductions and biocontrol efforts (Twardochleb et al. Citation2012).
The success of introduced species like the zebra mussel, Dreissena polymorpha, is due in part to ineffective predators in the newly colonized systems (Boles & Lipcius Citation1994). The magnitude of the impacts of zebra mussels, like those of other invading species, is strongly related to population size. Thus, identification of the factors that influence zebra mussel abundance can provide a basis for understanding the capacity of its populations to expand and to affect the ecology and economy of invaded aquatic ecosystems.
Zebra mussel densities commonly range from 2000 to 100,000 ind. m−2 in their native range (Nalepa & Schloesser Citation1993). This invasive species has colonized some Italian aquatic ecosystems (Lancione & Gaino Citation2006; Lori & Cianfanelli Citation2006; Gaino et al. Citation2009; Ciutti et al. Citation2011) and was found in highest values (maximum density of ~200,000 ind. m−2) at Lake Trasimeno (Lancione & Gaino Citation2006); and 756 ind. m−2 at Sirmione and 24,756 ind. m−2 at Bardolino, two Lake Garda localities, six years after invasion (Ciutti et al. Citation2011).
McMahon (Citation1996) proposed fishes as the most active predators of settled zebra mussels, but only a handful of studies have indicated the potential of fish predation to control the invasion success and density of exotic mussels (Eggleton et al. Citation2004; Bartsch et al. Citation2005; Watzin et al. Citation2008). The results of Magoulick and Lewis (Citation2002) indicate that predation could potentially suppress initial zebra mussel colonization and recolonization of adult zebra mussels following temperature-dependent mortality, and the relative susceptibility of zebra mussels to predation can be influenced for several biological and ecological factors (Thorp et al. Citation1998). Perry et al. (Citation2000) and Naddaffi et al. (Citation2010) also suggested the potential impacts of predation on recruitment or on the early life history stages of zebra mussels, because during the early stages of an invasion the average zebra mussel size is small and these individuals are therefore more vulnerable.
Crayfish are potentially significant predators of zebra mussels in inland waters and may have the potential to limit zebra mussel populations. Crayfish populations can attain high densities (≥ 18 adults m−2) and biomass (≤ 680 g m−2) (Lorman Citation1980; Mather & Stein Citation1993), and can control the structure and function of benthic communities (Lodge et al. Citation1994; Charlebois & Lamberti Citation1996).
Several studies have investigated crayfish predation on zebra mussels (Piesik Citation1974; Love & Savino Citation1993; MacIsaac Citation1994; Martin & Corkum Citation1994; Perry et al. Citation1997; Schreiber et al. Citation1998; Reynolds & Donohoe Citation2001), including by Procambarus clarkii (Gonçalves Citation2013).
The red swamp crayfish Procambarus clarkii, native to the south-central United States (Louisiana) and north-eastern Mexico (Gherardi & Holdich Citation1999), is a highly diffused alien species with many established populations in Italy (Aquiloni et al. Citation2010), and is spreading, namely in Lake Trasimeno, an optimal habitat for this species (Dörr et al. Citation2006). The highly aggressive behavior, potential for rapid population increase and omnivorous feeding habits of P. clarkii have resulted in numerous ecological impacts manifested across entire lake food webs (Gherardi Citation2006; Gherardi & Acquistapace Citation2007).
In a first analysis of the interaction between P. clarkii and D. polymorpha, experiments showed that P. clarkii predation upon D. polymorpha is size-selective, predation being mainly concentrated on the juvenile stage (Gonçalves Citation2013). To be able to forecast the impact that this crayfish may have on zebra mussels, it is necessary to know the maximum prey consumption as a function of prey abundance (i.e., the predator functional response), as well as the number of mussels that will lead to predator satiation. Martin and Corkum (Citation1994) showed that crayfish did not feed continually, and would stop feeding when satiated. Furthermore, crayfishes were not limited by the handling time associated with consuming small mussels, and individual crayfish showed a functional response type II (Holling Citation1959) when presented with a range of mussel densities.
The goals of this work are: (1) to identify the consumption rates of the D. polymorpha juvenile stage by P. clarkii; (2) to determine the predatory functional response; (3) to evaluate the effects of predator satiation on the intensity of predation; and (4) to model the predatory impact of the crayfish. Our final purpose is to assess whether this species can control, and in what conditions, the zebra mussel populations.
Material and methods
Dreissena polymorpha life history traits
Zebra mussels usually have one annual reproductive cycle (Juhel et al. Citation2003; Lancione & Gaino Citation2006) and the life cycle includes a free-swimming larval stage (veliger) that, after spending several days in the water column, attaches to substratum by byssal threads and metamorphoses into a sessile juvenile. Sexual maturity and first spawning seem to be correlated with a shell length of about 7–9 mm, independent of mussel age (Jantz & Neumann Citation1998). Both juveniles and adults can form compact masses of several hundred individuals – druses – that can range in size from 2 cm to more than 10 cm in diameter.
Zebra mussel lifespan, of 2 to 8 years (Karatayev et al. Citation2006), is dependent on local conditions, being generally shorter in warmer waters (Stanczykowska Citation1977). Four size/age classes for the D. polymorpha specimens were proposed for the Lake Garda population according to the growth rings of the shell (0–10 mm: 1 year; 11–19 mm: 2 years; 17–23 mm: 3 years; 21–26 mm: 4 years) (Annoni et al. Citation1978).
Experimental procedure
Field collection and laboratory maintenance
Individuals of D. polymorpha were hand collected from Lake Bilancino (43°58ʹ41ʹʹN, 11°16ʹ54ʹʹE; Tuscany, Italy); P. clarkii is also present at Bilancino, but for this experiment the crayfishes were collected, using baited traps, in Lake Trasimeno (43°08′N 12°06′E; Umbria, Italy).
Upon their arrival in the laboratory, crayfish were measured (CL – cephalothorax length without rostrum) using a vernier calliper. Mature males were identified by the presence of prominent copulatory hooks and cornified gonopodia (Huner Citation2002), and possible mutilations were recorded. Only intermolt animals with intact chelae and walking legs were used in the experiments. Crayfish were maintained in individual plastic tanks (26.5 × 16.2 × 15 cm) containing 1 L of well water at a temperature ranging from 20 to 28°C (25 ± 1.49 standard deviation, SD), under natural light/dark cycle regime, and fed ad libitum with larvae of Calliphora sp. Water was changed once per week. To avoid pseudoreplication, each crayfish was used only once.
All laboratory experiments were run in plastic aquaria (31 cm diameter; area ~750 cm2) without sediment or gravel at the bottom to prevent any constraints on searching, and containing 2 L of dechlorinated tap water (approximately 5 cm deep). Each crayfish was provided with one shelter (12 cm of gray plastic tubing with a 5 cm diameter) in order to minimize stress. These experiments were run between June and July of two years (2012 and 2013) in the laboratory of the Department of Biology (University of Florence).
Quantifying the functional response
Individual crayfish (both sexes, since our previous work showed that there were no sexual differences in D. polymorpha size preference) were supplied with D. polymorpha ranging in size from 5 to 11 mm shell length. These are the preferred sizes (Gonçalves Citation2013) and correspond to the juvenile stage (Annoni et al. Citation1978 refer to it as the n1 stage). Mussels were supplied at four different quantities (50, 100, 150, 200 individuals) under each of two crayfish starvation treatments (crayfish starvation – 24 hours and 1 week). To properly assess the feeding rates of a predator, particularly crayfish, it is necessary to take into account the previous starvation time. To calculate a “normal” feeding rate, Covich et al. (Citation1981) considered the data obtained after the first 2 days of feeding, because the crayfish, in their experiment, had been starved for ca. 1 week. In various studies, the starvation time considered varies between 24 hours and 9 days (Martin & Corkum Citation1994; Reynolds & Donohoe Citation2001; Mistri Citation2004).
The number of replicates of each combination varied between six and seven. The number of mussels remaining after 24 hours was then counted. A few replicates were discarded from the analyses, since the crayfish moulted and stopped feeding.
Crayfish sizes were chosen in order to homogenize the size distributions under each treatment: starved for 1 week (36.23 ± 0.64 mm; n = 28) and starved for 24 hours (35.31 ± 0.92 mm; n = 15); there were non-significant differences for all comparisons.
Predator satiation experiment
Satiation was determined by placing an individual crayfish (24 males/26 females; CL males = 34.05 ± 3.62 mm/CL females = 34.71 ± 6.73 mm) that had been previously starved for 1 week in the aquarium with 50 zebra mussels ranging in size from 5 to 11 mm shell length (corresponding to the n1 stage), and scattered over the bottom of the aquarium. Each crayfish was allowed to feed at will and the number of mussels remaining after 24 hours was then removed and counted. The 50 zebra mussels were then replaced in each aquarium and the same procedure was repeated for 6 days. The crayfishes that did not consume zebra mussels or that stopped feeding during the 6 days were removed from the experiment, not considered for the analysis and replaced by similar-sized individuals, with which the experiment restarted from day one.
Statistical methods
To estimate the functional response of crayfish, we first calculated the proportional mortality rates (no. of mussels eaten per mussel abundance per 24 hours) per abundance treatment (Mistri Citation2004).
The differences between the number of zebra mussel consumed by crayfish subjected to the two starvation levels were assessed using a general linear model (GLM) that tested for the existence of interactions between prey density and the starvation level of the crayfish.
We used non-parametric Mann-Whitney U tests for the comparisons of maximum and minimum daily consumption between crayfish sexes, and tested for correlations between the crayfish length and these parameters.
For the satiation experiment we fitted the most significant curve to the average consumption of zebra mussel over the 6 days, and calculated the mean of the proportional decreases in the daily consumptions.
All statistical analyses were performed with SPSS Statistics v. 20.
Modelling
The functional response equation was associated with the satiation effect of the predator, displayed over time. Considering 1 month of predator activity, we then evaluated graphically the effect of the predator at different initial abundances of the D. polymorpha n1 (juvenile) stage, and modelled its effect on the next stage (n2; Annoni et al. Citation1978) in order to estimate the potential impacts of predation in the population structure of D. polymorpha. We used the values of n1 resulting from the effect of predation (see results) and the survival estimated for this life stage by Annoni et al. (Citation1978) – σ1 = 0.88 – to obtain the values of n2 (t + 1) .
Results
Quantifying the functional response
The results of the GLM indicated that the number of zebra mussels consumed by crayfish depended only on the initial abundance (F = 4.314; p = 0.011) and not on the starvation level of the predator (F = 1.216; p = 0.278), with no significant interaction between these two variables (F = 0.475; p = 0.702).
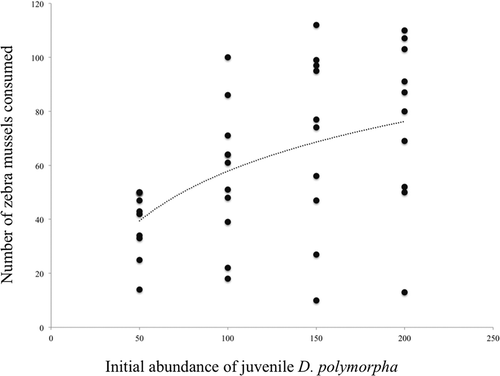
Grouping the results of both starvation treatments, we calculated the curve that best describes the behavior of crayfish: y = 25.55 ln (x) − 64.46 (R2 = 0.235; F = 12.283; p = 0.001; n = 42; standard error of estimate = 25.65; ). We found that P. clarkii is a predator with a functional response of type II, characterized by significantly higher proportional mortality at low prey abundance than at high prey abundance.
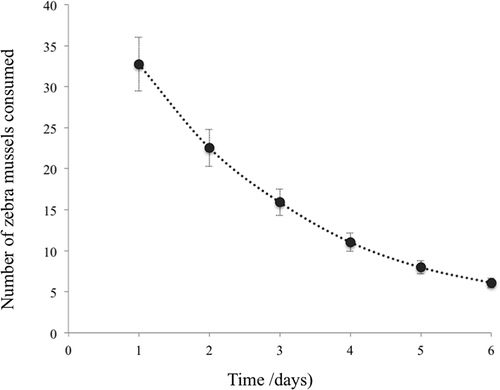
Figure 1. Number of juvenile zebra mussels (Dreissena polymorpha) consumed, in 24 hours, according to their availability. The dotted line shows the function that best describes the predatory behaviour of crayfish faced with increasing numbers of prey (see text for the mathematical expression of this function).
Predator satiation experiment
There were no sexual differences in the maximum daily consumption (U = 93.5; p = 0.854, as well as in the minimum daily consumption (U = 75; p = 0.448) of D. polymorpha. Grouping the results of both sexes, there were positive correlations between crayfish size and both maximum (Spearman’s rho = 0.511; p < 0.001; n = 28) and minimum (Spearman’s rho = 0.644; p < 0.001; n = 28) daily consumption.
Over the 6 days of observation there was a significant decrease in the consumption of D. polymorpha (F = 882,123; p < 0.001; n = 28; ). The quadratic function that best describes the results is Equation 1 [y = no. of D. polymorpha consumed; x = time (days)].
In these experimental conditions, when crayfish were offered a constant prey abundance – n1 = 50 – the amount of prey consumed decreased over time at a rate of 0.71/day (), with a tendency for stabilization after 6 days at around 6 mussels/day ().
Modelling
Considering the equation of Holling (Citation1959) to a functional response of type II, the behavior of this predator could be represented as in Equation 2 (Nn1 is the number of prey consumed of the age class n1; N0n1 the number of initial prey; a the attack success and h the handling time):
Our results () show a functional response of type II, where the dependent variable (number of D. polymorpha consumed in one day) already includes the components “attack success” and “handling time”.
According to our results, the mortality (M) of the n1 stage caused by 1 day of activity of one crayfish is as shown in Equation 3:
Combining the functional response with predator satiation, in order to approximate this to a more realistic situation, we obtain Equation 4:
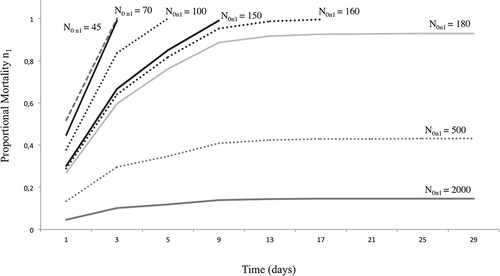
Over time (in the example, 1 month of activity of one crayfish) and according to different initial abundances of D. polymorpha stage n1, the proportional mortality caused by an individual crayfish in a D. polymorpha population can be plotted as in .
Figure 3. Variation of proportional mortality, caused by one crayfish over one month, with different initial zebra mussel abundances. N0n1 refers to the number of juveniles (n1 stage) provided. Up to 150–60 individuals, crayfish predation will eventually consume the entire population (proportional mortality reaches “1”). When more than 500 zebra mussels are present, proportional mortality will be below 0.1.
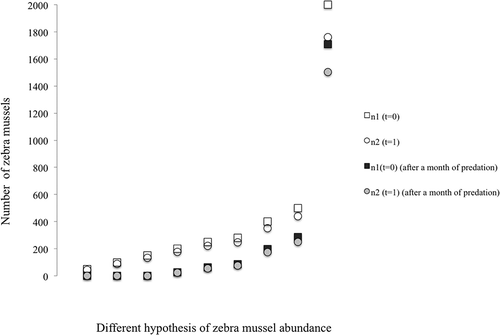
The difference between the number of zebra mussels that survive and pass to the stage n2, when stage n1 does not suffer the effect of 1 month of predation or when predation occurs, is obvious for all initial densities of n1, especially for low abundances, up to 150 mussels (). For increasing numbers of D. polymorpha provided, this effect becomes less and less important. For instance, for initial n1 abundances over 500 individuals, 1 month of predation by one crayfish will decrease the abundance of n2 in the following year at about 43% ().
Discussion
With this study we estimated the maximum daily consumption of one crayfish on D. polymorpha juvenile stages, and verified that this consumption has the potential to cause a strong decrease in the population of zebra mussels when the prey numbers are relatively low.
We also found that this maximum daily consumption depends on the abundance of available prey, according to a functional response type II (Holling Citation1959), which is characterized by a decrease in proportional prey mortality as prey abundance increases.
This type of predator functional response indicates that the real impact in established populations (usually with a high abundance in the case of zebra mussels) can be relatively low. Furthermore, the degree of satiation of the crayfish must also be taken into account. Our results show that the daily consumption of one crayfish after being satiated can be only about 1/6 of the daily consumption of a starved individual.
Since this is a predator that mainly consumes the juvenile stage, the mortality effect in this stage will be transmitted to the other stages of the D. polymorpha populations in the following years. Thus, even a relatively small predation impact can bear relatively important consequences if it is all directed to one specific, more vulnerable stage.
The feeding rates of crayfish on small clams reported by Piesik (Citation1974) were substantially higher than those we found. Mean consumption by Orconectes limosus ranging from 47 to 90 mm (~23–45 mm carapace length) was ca. 36 small (4–5 mm) Dreissena per day; one large female consumed an average of 110 clams per day for 5 days. Procambarus clarkii of similar size consumed on the average only about 10 small (4–6 mm) Corbicula per day, and maximally only 19 (Covich et al. Citation1981).
The functional response of P. clarkii to D. polymorpha was similar to that of other aquatic invertebrate predators given a single type of food (Walde & Davies Citation1984; Martin & Corkum Citation1994). The form of the functional response is dependent not only on prey density, but also on the relative distribution of predator and prey in time (Collins et al. Citation1981) and space (Kaiser Citation1983; de Lafontaine & Leggett Citation1987). It should also be noted that zebra mussels at high density in the wild often form druses, and their formation may impact crayfish size selectivity and feeding rate, as mussels are strongly attached within the three-dimensional structure of a druse and potentially less accessible (Ermgassen & Aldridge Citation2011). Furthermore, small zebra mussels tend to occupy the outermost positions on a druse (Gonçalves, pers. obs.), being more vulnerable to predation, and diverting the attention of crayfish from the inner, larger individuals, which are already able to reproduce.
In agreement with our results Casagrandi et al. (Citation2007) predicted that if the mortality of the juvenile stage is too high, it will have a significant impact on the population structure of D. polymorpha. Thus, to estimate the real impact that P. clarkii may have on D. polymorpha, it is necessary to take into account the season when the juvenile stage is more abundant, as well as the seasonal activity of the predator. A coincidence of the phenology of both species will increase the potential impacts of the predator, while a desynchronization of predator activity and prey reproduction will render the impacts of predation almost null.
Unlike many other species, zebra mussels can have clearly different population behaviors based on the location of the colony, which has caused difficulty in modeling their local dynamics. This variance originates mostly from environmental conditions, such as salinity, pH, temperature fluctuations and levels of different chemicals present in the water (McMahon Citation1996; Abaurre Citation2008). Considering Davis (Citation2010), in a population with a chaotic cycle the predator effect will be stronger during periods in which all stages (including the juvenile stage) are present in very low densities. In cyclically fluctuating populations, the effect of the crayfish can be maximized if it is in accordance with the cyclical synchronization of the population. But where this predator can have a greater impact in a zebra mussel population is in equilibrium situations, which are characterized by relatively low densities of all life-cycle stages, including the juvenile, with about 42% of a population in equilibrium composed of juveniles (Davis Citation2010). This stage is also found in lower densities when the population is characterized by a larger density of the oldest stage (n4), because when the adult population is large there is a higher cannibalization (by filtering) of larvae.
The abundance of juveniles is lower when the veliger larvae begin to fix to the substrate, i.e., after the intense reproduction period of warmer season (spring-summer) (Annoni et al. Citation1978; Lancione & Gaino Citation2006). In Italian aquatic ecosystems, the reproduction period of D. polymorpha occurs between the months of June to August, and the first colonization of substrate will be between August and November (Annoni et al. Citation1978). In a review of population structure of mussels in another Italian ecosystem, Sebino (Lombardia), there was a similar population increase between the months of June and September, at different stations of the same lake (Roncaglio & Borsani Citation2005).
The period when P. clarkii is more active may or may not coincide with the time when the juveniles of D. polymorpha are more abundant. Some studies have documented that P. clarkii activity levels peak during warmer months (Gherardi et al. Citation2000), so crayfish forage less actively during the winter. However, there is evidence that on Lake Trasimeno (Umbria, Italy) crayfish molted from the end of fall to the beginning of summer, with males being more active in winter than females (Dörr & Scalici Citation2013).
Considering this synchronization factor, predation by crayfish can be assumed to be probable after the zebra mussel reproduction period and the first colonization of the substrate, therefore coinciding partially (but not totally, as the colonization of the substrate by the veliger larvae may continue during the fall and winter) with the life-cycle of the mussel. We can thus predict that the impact of P. clarkii on D. polymorpha juvenile stages will be cyclical and seasonal, associated with the zebra mussel reproduction season.
One of the main objectives of the study of the interactions between these two invasive species is to understand whether predation by crayfish can somehow hinder colonization by D. polymorpha. Based on the results presented, it will be important to assess how different densities of crayfish may affect the populations of D. polymorpha, taking into account the possibility of interference competition, as well as the effects of the presence of alternative prey, or even alternative food items, like detritus or macrophytes (Carreira et al. Citation2014). The effects of satiation should also be studied further, as it is still unknown whether crayfish may further reduce their consumption of zebra mussels after a long period.
Acknowledgements
We thank Alberto F. Inghilesi, Gabriele Orioli, Daniele Spigoli and Filipe Barroso for assistance with field collections, and Carolina Laurentino for help with mathematical questions.
Vera Gonçalves’s participation in the PhD program at the University of Florence is funded by the “Fundação para a Ciência e a Tecnologia” (FCT-Portugal) – SFRH/BD/43963/2008.
References
- Abaurre IC. 2008. Ecología del mejillón cebra (Dreissena polymorpha) en el tramo inferior del rio Ebro: problemática y posibilidades de control. Naturaleza y parques nacionales. Serie técnica. Bilbao: Organismo Autónomo parques Nacionales. 288.
- Annoni D, Bianchi I, Girod A, Mariani M. 1978. Inserimento di Dreissena polymorpha (Pallas) (Mollusca Bivalvia) nelle malacocenosi costiere del Lago di Garda (Nord Italia). Quaderni della Civica Stazione Idrobiologica di Milano 6:5–84.
- Aquilone L, Tricarico E, Gherardi F. 2010. Crayfish in Italy: Distribution, threats and management. Invited Review. International Aquatic Research 2:1–14.
- Bartsch MR, Bartsch LA, Gutreuter S. 2005. Strong effects of predation by fishes on an invasive macroinvertebrate in a large floodplain river. Journal of the North American Benthological Society 24:168–177. DOI: 10.1899/0887-3593(2005)024<0168:SEOPBF>2.0.CO;2.
- Boles LC, Lipcius RN. 1994. Potential for predator-mediated biological control of the zebra mussel in the Hudson River estuary. Proceedings of the Fourth International Zebra Mussel Conference. Madison, Wisconsin. pp. 489–500.
- Bollache L, Dick JTA, Farnsworth KD, Montgomery WI. 2008. Comparison of the functional responses of invasive and native amphipods. Biology Letters 4:166–169. DOI: 10.1098/rsbl.2007.0554.
- Carreira BM, Dias MP, Rebelo R. 2014. How consumption and fragmentation of macrophytes by the invasive crayfish Procambarus clarkii shape the macrophyte communities of temporary ponds. Hydrobiologia 721:89–98. DOI: 10.1007/s10750-013-1651-1.
- Casagrandi R, Mari L, Gatto M. 2007. Modelling the local dynamics of the zebra mussel (Dreissena polymorpha). Freshwater Biology 52:1223–1238. DOI: 10.1111/fwb.2007.52.issue-7.
- Charlebois PM, Lamberti GA. 1996. Invading crayfish in a Michigan stream: Direct and indirect effects on periphyton and macroinvertebrates. Journal of the North American Benthological Society 15:551–563. DOI: 10.2307/1467806.
- Ciutti F, Beltrami ME, Confortini I, Cianfanelli S, Cappelletti C. 2011. Non-indigenous invertebrates, fish and macrophytes in Lake Garda (Italy). Journal of Limnology 70:315–320. DOI: 10.4081/jlimnol.2011.315.
- Collins MD, Ward SA, Dixon AFG. 1981. Handling time and the functional response of Aphelinus thomsoni, a predator and parasite of the aphid Drepanosiphumpla tanoidis. The Journal of Animal Ecology 50:479–487. DOI: 10.2307/4069.
- Covich AP, Dye LL, Mattice JS. 1981. Crayfish predation on Corbicula under laboratory conditions. American Midland Naturalist 105:181–188. DOI: 10.2307/2425023.
- Davis PT. 2010. Modeling the effects of cannibalistic behavior in zebra mussel (Dreissena polymorpha) populations. Texas A&M University. 21 pp.
- de Lafontaine Y, Leggett WC. 1987. Effect of container size on estimates of mortality and predation rates in experiments with macrozooplankton and larval fish. Canadian Journal of Fisheries and Aquatic Sciences 44:1534–1543. DOI: 10.1139/f87-185.
- Dörr AJM, La Porta G, Pedicillo G, Lorenzoni M. 2006. Biology of Procambarus clarkii (Girard 1852) in Lake Trasimeno. Bulletin Français De La Pêche Et De La Pisciculture 380:1155–1168. DOI: 10.1051/kmae:2006018.
- Dörr AJM, Scalici M. 2013. Revisiting reproduction and population structure and dynamics of Procambarus clarkii eight years after its introduction into lake Trasimeno (Central Italy). Knowledge and Management of Aquatic Ecosystems 408:1–16.
- Eggleston DB, Lipcius RN, Hines AH. 1992. Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Marine Ecology Progress Series 85:55–68. DOI: 10.3354/meps085055.
- Eggleton MA, Miranda LE, Kirk JP. 2004. Assessing the potential for fish predation to impact zebra mussels (Dreissena polymorpha): Insight from bioenergetics models. Ecology of Freshwater Fish 13:85–95. DOI: 10.1111/eff.2004.13.issue-2.
- Elliott JM. 2003. A comparative study of the functional response of four species of carnivorous stone flies. Freshwater Biology 48:191–202. DOI: 10.1046/j.1365-2427.2003.00982.x.
- Ermgassen PSE, Aldridge DC. 2011. Predation by the invasive american signal crayfish, Pacifastacus leniusculus Dana, on the invasive zebra mussel, Dreissena polymorpha Pallas: The potential for control and facilitation. Hydrobiologia 658:303–315. DOI: 10.1007/s10750-010-0500-8.
- Gaino E, Scoccia F, Lancione T, Ludovisi A. 2009. Invasive molluscs in Umbria inland waters: Ecological and reproductive aspects. Studi Trentino di Scienze Naturali 86:99–104.
- Gascoigne JC, Lipcius RN. 2004. Allee effects driven by predation. Journal of Applied Ecology 41:801–810. DOI: 10.1111/jpe.2004.41.issue-5.
- Gherardi F. 2006. Crayfish invading Europe: The case study of Procambarus clarkii. Marine and Freshwater Behaviour and Physiology 39:175–191. DOI: 10.1080/10236240600869702.
- Gherardi F, Acquistapace P. 2007. Invasive crayfish in Europe: The impact of Procambarus clarkii on the littoral community of a Mediterranean lake. Freshwater Biology 52:1249–1259. DOI: 10.1111/fwb.2007.52.issue-7.
- Gherardi F, Barbaresi S, Salvi G. 2000. Spatial and temporal patterns in the movement of the red swamp crayfish, Procambarus clarkii, an invasive crayfish. Aquatic Sciences 62:179–193.
- Gherardi F, Holdich DM, editors. 1999. Crayfish in Europe as alien species. How to make the best of a bad situation? Rotterdam: AA. Balkema.
- Gonçalves VLR. 2013. Interactions between two invasive alien species, Procambarus clarkii and Dreissena polymorpha, in the aquatic ecosystems of central Italy. PhD Thesis. Università degli Studi di Firenze. 144 pp.
- Gotelli NJ. 1995. A Primer of Ecology. Sunderland, MA: Sinauer Associates.
- Hastings A. 1997. Population biology. Concepts and models. New York: Springer.
- Holling CS. 1959. Some characteristics of simple types of predation and parasitism. The Canadian Entomologist 91:385–398. DOI: 10.4039/Ent91385-7.
- Hooff RC, Bollens SM. 2004. Functional response and potential predatory impact of Tortanus dextrilobatus, a carnivorous copepod recently introduced to the San Francisco Estuary. Marine Ecology Progress Series 277:167–179. DOI: 10.3354/meps277167.
- Huner JV. 2002. Procambarus. In: Holdich DM, editor. Biology of freshwater crayfish. Oxford: Blackwell Scientific Press. pp. 541–574.
- Jantz B, Neumann D. 1998. Growth and reproductive cycle of the zebra mussel in the River Rhine as studied in a river bypass. Oecologia 114:213–225. DOI: 10.1007/s004420050439.
- Jeschke JM, Kopp M, Tollrian R. 2002. Predator functional responses: Discriminating between handling and digesting prey. Ecological Monographs 72:95–112. DOI: 10.1890/0012-9615(2002)072[0095:PFRDBH]2.0.CO;2.
- Juhel G, Culloty SC, O’Riordan RM, O’Connor J, de Faoite L, McNamara R. 2003. A histological study of the gametogenic cycle of the freshwater mussel Dreissena polymorpha (Pallas, 1771) in Lough Derg, Ireland. Journal of Molluscan Studies 69:365–374. DOI: 10.1093/mollus/69.4.365.
- Juliano SA. 2001. Non-linear curve fitting: Predation and functional response curves. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York: Chapman and Hall. pp. 178–196.
- Kaiser H. 1983. Small-scale spatial heterogeneity influences predation success in an unexpected way: Model experiments on the functional response of predatory mites (Acarina). Oecologia 56:249–256. DOI: 10.1007/BF00379698.
- Karatayev AY, Burlakova LE, Padilla DK. 2006. Growth rate and longevity of Dreissena polymorpha (Pallas): A review and recommendations for future study. Journal of Shellfish Research 25:23–32. DOI: 10.2983/0730-8000(2006)25[23:GRALOD]2.0.CO;2.
- Lancione T, Gaino E. 2006. The invasive zebra mussel Dreissena polymorpha in Lake Trasimeno (Central Italy): Distribution and reproduction. Italian Journal of Zoology 73:335–346. DOI: 10.1080/11250000600918001.
- Lipcius RN, Hines AH. 1986. Variable functional responses of a marine predator in dissimilar homogeneous microhabitats. Ecology 67:1361–1371. DOI: 10.2307/1938692.
- Lodge DM, Kershner MW, Aloi JE, Covich AP. 1994. Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food web. Ecology 75:1265–1281. DOI: 10.2307/1937452.
- Lori E, Cianfanelli S. 2006. New records of Dreissena polymorpha (Pallas, 1771) (Mollusca: Bivalvia: Dreissenidae) from Central Italy. Aquatic Invasions 4:281–283. DOI: 10.3391/ai.2006.1.4.11.
- Lorman JG. 1980. Ecology of the crayfish Orconectes rusticus in northern Wisconsin. PhD Thesis, University of Wisconsin, Madison. 252 pp.
- Love J, Savino J. 1993. Crayfish (Orconectes virilis) predation on zebra mussels (Dreissena polymorpha). Journal of Freshwater Ecology 8:253–259. DOI: 10.1080/02705060.1993.9664861.
- MacIsaac HJ. 1994. Size-selective predation on zebra mussels (Dreissena polymorpha) by crayfish (Orconectes propinquus). Journal of the North American Benthological Society 13:206–216. DOI: 10.2307/1467239.
- Magoulick DD, Lewis LC. 2002. Predation on exotic zebra mussels by native fishes: Effects on predator and prey. Freshwater Biology 47:1908–1918. DOI: 10.1046/j.1365-2427.2002.00940.x.
- Martin GW, Corkum LD. 1994. Predation of zebra mussels by crayfish. Canadian Journal of Zoology 72:1867–1871. DOI: 10.1139/z94-254.
- Mather M, Stein RA. 1993. Direct and indirect effects of fish predation on the replacement of a native crayfish by an invading congener. Canadian Journal of Zoology 50:1279–1288.
- McMahon R. 1996. The physiological ecology of the zebra mussel, Dreissena polymorpha, in North America and Europe. American Zoologist 36:339–363. DOI: 10.1093/icb/36.3.339.
- Mistri M. 2004. Predatory behavior and preference of a successful invader, the mud crab Dyspanopeus sayi (Panopeidae), on its bivalve prey. Journal of Experimental Marine Biology and Ecology 312:385–398. DOI: 10.1016/j.jembe.2004.07.012.
- Murdoch WW, Oaten A. 1975. Predation and population stability. Advances in Ecological Research 9:1–131.
- Naddaffi R, Petterson K, Eklöv. 2010. Predation and physical environment structure the density and population size structure of zebra mussels. Journal of the North American Benthological Society 29:444–453. DOI: 10.1899/09-071.1.
- Nalepa TF, Schloesser DW. 1993. Zebra Mussels: Biology, impacts, and control. Boca Raton, FL: Lewis Publishers/CRC Press.
- Perry WL, Lodge DM, Lamberti GA. 1997. Impact of crayfish predation on exotic zebra mussels and native invertebrates in a lake-outlet stream. Canadian Journal of Fisheries and Aquatic Sciences 54:120–125.
- Perry WL, Lodge DM, Lamberti GA. 2000. Crayfish (Orconectes rusticus) impacts on zebra mussel (Dreissena polymorpha) recruitment, other macroinvertebrates and algal biomass in a Lake-outlet stream. The American Midland Naturalist 144:308–316. DOI: 10.1674/0003-0031(2000)144[0308:CORIOZ]2.0.CO;2.
- Piesik Z. 1974. The role of the crayfish Orconectes limosus (Raf.) in extinction of Dreissena polymorpha (Pall.) subsisting on steelon-net. Polskie Archiwum Hydrobiologh 21:401–410.
- Radford IJ, Dickinson KJM, Lord JM. 2007. Functional and performance comparisons of invasive Hieracium lepidulum and co-occurring species in New Zealand. Austral Ecology 32:338–354. DOI: 10.1111/j.1442-9993.2007.01700.x.
- Reynolds JD, Donohoe R. 2001. Crayfish predation experiments on the introduced zebra mussel, Dreissena polymorpha, in Ireland, and their potential for biocontrol. Bulletin Français De La Pêche Et De La Pisciculture 361:669–681. DOI: 10.1051/kmae:2001012.
- Roncaglio P, Borsani G. 2005. Analisi della strutura di popolazione del mollusco bivalve Dreissena polymorpha (Pallas, 1771) nel Sebino (Lombardia, Italia Settentrionale). “Natura Brescian” Annu. Museu Civico di Scienze Naturali di Brescia 34:49–53.
- Safina C. 1990. Bluefish mediation of foraging competition between roseate and common terns. Ecology 71:1804–1809. DOI: 10.2307/1937588.
- Schreiber S, Odelstrom T, Pettersson K, Eichelberg D. 1998. The zebra mussel Dreissena polymorpha as a food source for the signal crayfish Pacifastacus leniusculus in Lake Erken – laboratory experiments. Ergebnisse Der Limnologie 51:169–176.
- Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K. 1985. Predation, competition, and prey communities: A review of field experiments. Annual Review of Ecology and Systematics 16:269–311. DOI: 10.1146/annurev.es.16.110185.001413.
- Stanczykowska A. 1977. Ecology of Dreissena polymorpha (Pall.) (Bivalvia) in lakes. Polskie Archiwum Hydrobiologii 24:461–530.
- Thorp JH, Delong MD, Casper AF. 1998. In situ experiments on predatory regulation of a bivalve mollusk (Dreissena polymorpha) in the Mississippi and Ohio Rivers. Freshwater Biology 39:649–661. DOI: 10.1046/j.1365-2427.1998.00313.x.
- Tripet F, Perrin N. 1994. Size-dependent predation by Dugesia lugubris (Turbellaria) on Physa acuta (Gastropoda): Experiments and model. Functional Ecology 8:458–463. DOI: 10.2307/2390069.
- Twardochleb LA, Novak M, Moore JW. 2012. Using the functional response of a consumer to predict biotic resistance to invasive prey. Ecological Applications 22:1162–1171. DOI: 10.1890/11-0871.1.
- Walde SJ, Davies RW. 1984. Invertebrate predation and lotic prey communities: Evaluation of in situ enclosure/exclosure experiments. Ecology 65:1206–1213. DOI: 10.2307/1938328.
- Wanless RM, Angel A, Cuthbert RJ, Hilton GM, Ryan PG. 2007. Can predation by invasive mice drive seabird extinctions? Biology Letters 3:241–244. DOI: 10.1098/rsbl.2007.0120.
- Watzin MC, Joppe-Mercure K, Rowder J, Lancaster B, Bronson L. 2008. Significant fish predation on zebra mussels Dreissena polymorpha in Lake Champlain, U.S.A.. Journal of Fish Biology 73:1585–1599. DOI: 10.1111/jfb.2008.73.issue-7.