Abstract
The alien polychaete Polydora cornuta was found in Venice, Marinetta and Barbamarco lagoons (north-western Adriatic Sea). Reproductive specimens were collected from all the above-mentioned lagoons at different sampling times, showing the presence of established populations. The highest recorded population density of this species was 2880 ind. m–2. Data on adult morphology, reproductive traits and early stage larval morphology are provided. An examination of old samples revealed the presence of this species in Italian waters at least since 2009, and maybe even as far back as the early 1990s. The identity of P. cornuta may have been concealed for a long time because of taxonomic confusion with P. ciliata. Previous records of this species were recently published for the western Mediterranean Sea, on the Turkish and Greek Aegean coasts. The present paper extends the geographical distribution of P. cornuta to the Adriatic Sea and adds a new record to the list of alien species in the Italian waters.
Introduction
The spionid polychaete Polydora cornuta Bosc, Citation1802 has been reported as an invasive species in many regions worldwide (Blake & Kudenov Citation1978; Tena et al. Citation1991; Radashevsky & Hsieh Citation2000; Radashevsky Citation2005; Radashevsky & Selifonova Citation2013). P. cornuta has been considered able to colonise distant areas by ship transport in ballast waters and among fouling organisms on ship hulls (Radashevsky & Selifonova Citation2013). Due to its ability to spread and colonise new areas, it has been included among the worst invasive species in the Mediterranean region (Zenetos et al. Citation2012). However, considering the taxonomic history of the taxon, the expansion of P. cornuta can be very confusing. The species was first described by Bosc (Citation1802) on the South Carolina coast. Subsequently, the species has been confused with and synonymised by other authors as P. ciliatum Agassiz, 1867, P. ligni Webster, 1879, P. littorea Verrill, 1881 and P. amarincola Hartman, 1936. The identity of P. cornuta remained mainly undefined until its redescription and neotype designation by Blake and Maciolek (Citation1987). The presence of P. cornuta in several areas has been frequently hidden due to taxonomic confusion with other more commonly known species (Çinar et al. Citation2005; Surugiu Citation2012; Radashevsky & Selifonova Citation2013). Moreover, the variability of morphological, reproductive and molecular characters, among different populations, leads some authors to hypothesise that P. cornuta may also be a combination of different cryptic species (Rice & Simon Citation1980; Rice et al. Citation2008).
The first Mediterranean record of this species was from the port of Valencia (Tena et al. Citation1991), followed by two cases from the Aegean Sea, off the Turkish (Çinar et al. Citation2005) and Greek coasts (Simboura et al. Citation2008). Surugiu (Citation2012) and Radashevsky and Selifonova (Citation2013) highlighted in their studies that P. cornuta was colonising large areas of the northern Black Sea. However, increasing records from the steno-Mediterranean area point out the possibility of more areas being colonised by this species.
Northern Adriatic lagoons, and in particular Venice lagoon, are some of the main hotspots in the Mediterranean for the introduction of exotic species (Occhipinti-Ambrogi et al. Citation2011). In recent decades a combination of factors, including the construction of commercial and tourist ports, as well as aquaculture activities, has led to significant environmental changes. These environmental conditions and other sources of disturbance, including high levels of pollution in the sediments, can easily turn these ecosystems into suitable environments for the ecological characteristics of many invasive species. In the Venice area, these changes probably enhanced the introduction of at least 30% of the exotic species present in Italian marine and brackish waters (Occhipinti-Ambrogi et al. Citation2011).
P. cornuta is an opportunistic species, tolerant to a wide range of salinity and temperature fluctuations, and characterised by early maturation, high larval production and the ability to colonise disturbed and polluted substrata and establish high-density populations in a short time-frame (Dauer et al. Citation1981; Zajac Citation1991a, Citation1991b). Additionally, it is often considered to be an organic pollution indicator (Grassle & Grassle Citation1974; Rice & Simon Citation1980). P. cornuta may become invasive and cause changes in the composition and abundance of native species in hard- and soft-bottom benthic communities (Çinar et al. Citation2005; Dagli et al. Citation2011). In some cases, P. cornuta may also interfere directly with human activities. Although rare, mass invasions of this spionid may occur in mud-filled crevices of the shells in oyster beds, resulting in the accumulation of sediment, faeces and rejected material that eventually could cause oyster infections and death (Loosanoff & Engle Citation1941).
In the Venice lagoon, P. cornuta was first reported in the early 1990s as P. ligni (Casellato & Ragazzo Citation1997; Tagliapietra et al. Citation1998). Other papers mentioned the potential presence of this species in Italian lagoons, but did not provide information on its abundance or distribution (Basset et al. Citation2007; Mistri & Munari Citation2008; Tagliapietra & Minelli Citation2009). In spite of these indications, the presence of this polychaete in these waters is still not fully acknowledged (Castelli et al. Citation2008; Mikac Citation2015), probably due to the lack of taxonomic works ascertaining the identity of this species.
The aim of this study was to verify the taxonomic identity of the polychaetes collected in three North Adriatic lagoons, as well as to investigate the distribution of P. cornuta in these environments.
Material and methods
Study area
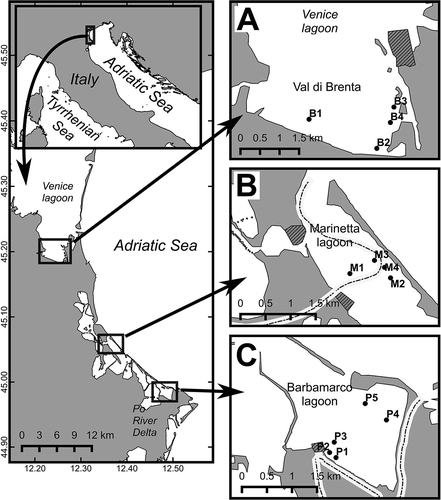
The three studied lagoons are on the north-west coast of the North Adriatic Sea, on a coastal stretch about 30 km long (). Val di Brenta is a polyhaline microtidal lagoon, with a surface area of about 9.6 km2, located in the southern part of the Venice Lagoon (). Fresh water inputs are present in both the western and eastern parts of the lagoon. In the central area of the lagoon, there are facilities for harvesting clams, and other local fishing activities. Marinetta lagoon (surface area about 2.5 km2) is a smaller portion on the sea mouth of the wider Vallona lagoon (B). On the southern side, the lagoon hosts the terminal stream of Po di Levante River. The central area of the lagoon is exploited mainly for clam farming. Barbamarco lagoon has a surface area of about 6.7 km2, and is located in the northern part of the Po River delta (). The main fresh water inputs are located in the southern part, close to a small port, mainly devoted to local fishing activities. Clam farms are located in the south-eastern area, close to the sea connection.
Figure 1. Location of the study area: A, Southern part of the Venice lagoon (Val di Brenta); B, Marinetta lagoon; C, Barbamarco lagoon. Dots represent the location of the sampling stations. Dotted lines are the Po riverbed branches. Shaded areas represent the location of ports, harbours and marinas.
Sampling procedure
The following study is based on a total of 87 soft-bottom sediment samples collected from the three lagoons described above. Routine monitoring activities were planned according to independent projects for each lagoon, and therefore the sampling dates followed a different time schedule. Four stations B1, B2, B3 and B4 in Val di Brenta were sampled on February 2014, July 2014 and March 2015; four stations M1, M2, M3 and M4, in Marinetta lagoon, were sampled on March 2014, June 2014 and March 2015; five stations P1, P2, P3, P4 and P5, in the southern portion of Barbamarco lagoon, were sampled on February 2015 (; ). Complete information regarding sampling stations is reported in .
Table I. Characterisation of sampling sites where specimens of Polydora cornuta were collected in the three North Adriatic lagoons. The number of individuals is the sum of three replicates. The number of mature females includes the females hatching egg capsules.
In each station, at each sampling time, three sediment samples were collected with a hand-driven Ekman grab (sampling area 0.022 m2), washed through a 0.5-mm mesh sieve, and the retained fraction fixed in 70% ethanol solution. Sediment samples were also taken with a 3-cm-diameter plastic corer and stored in plastic jars for grain size analyses. All organisms were sorted, counted and identified at the lowest possible taxonomic level (i.e. species) under a stereomicroscope and then preserved in 70% ethanol for long-term storage. Sediment grain-size was analysed according to Dean (Citation1974).
All specimens of Polydora cornuta were examined for diagnostic characters under a Nikon SMZ-B2 stereoscope 50X. An aqueous solution of Shirlastain A was used to stain specimens and enhance the contrast of the external morphological features (Petersen Citation1998). Due to the lack of the posterior end in most sampled individuals, the width of specimens at chaetiger 5 (excluding chaetae) was measured and used as a parameter related to the body size. For each sample, the number of mature females and the number of egg capsules found in tubes were counted. A random subsample of egg capsules was taken as well. The eggs or larvae were extracted from capsules with a needle and counted. Egg diameter was measured by image analysis. A Panasonic Lumix DMC-LX3 mounted on a Zeiss Jena Amplival microscope was used for digital photographs, and ImageJ 1.46 was used for morphometric measures. Photographs of relevant morphological features were taken to illustrate the descriptions.
The specimens of P. cornuta from the Adriatic Sea were sent to the Senckenberg Museum Frankfurt (SMF) and the Muséum National d’Histoire Naturelle, Paris (MNHN), and were also deposited in the Laboratory of Benthos Ecology collection of the Institute for Environmental Protection and Research, Rome (ISPRA).
Results
Polydora cornuta Bosc, Citation1802
Polydora cornuta: Blake & Maciolek Citation1987: 12–14, ;
Radashevsky & Hsieh Citation2000: 205–208, ;
Radashevsky Citation2005: 6–10, , –F (adult); 10–12, –C (larvae);
Surugiu Citation2012: 47–50, ;
Radashevsky & Selifonova Citation2013: 263–264.
Polydora ligni: Blake, Citation1969: 4–6, , (larval development);
Light, Citation1978: 175–178, fig. 176A–I.
Figure 2. Polydora cornuta. A, anterior end, dorsal view; a = occipital antenna; B, chaetiger 5 from right side, dorsal view; sp = modified spines; cc = companion chaetae strictly adhering to convex side of the spine; C, chaetae from chaetiger 5: from left to right, unworn posterior spine; unworn posterior companion chaeta; worn anterior companion chaeta on convex (left) and concave (right) side; D, notopodial chaetae from chaetiger 20: s = short capillaries, w = winged capillaries, lc = long capillaries, the square shows the detail of the winged terminal part of the chaeta; E, hooded hook from posterior chaetigers; F, posterior end and pygidium. Scale bars: A = 1.5 mm; B, D = 100 mm; C = 50 mm; E = 30 mm; F = 1 mm.
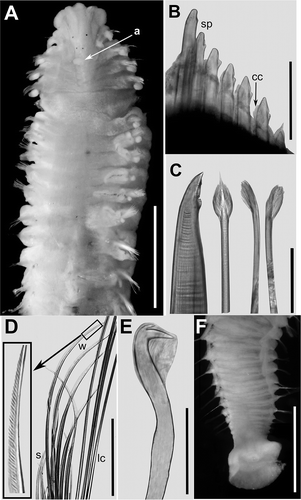
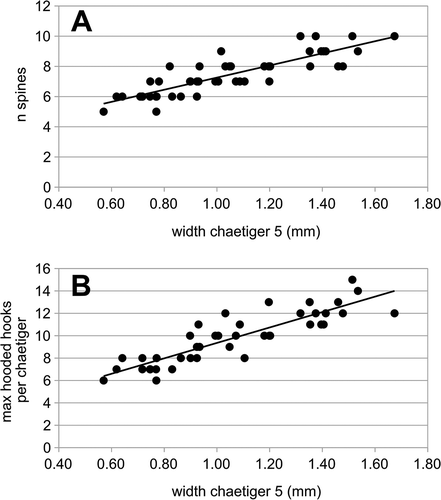
Figure 3. Polydora cornuta. A, relationship between size of individuals (width of chaetiger 5) and number of spines in the same chaetiger (linear model: y = 3.24 + x·4.01; r = 0.853; P < 0.0001; N = 45); B, relationship between size of individuals (width of chaetiger 5) and maximum number of hooded hooks in posterior segments (linear model: y = 2.46 + x·6.88; r = 0.875; P < 0.0001; N = 41).
Material examined
Venice lagoon (Val di Brenta): St. B1, 8 July 2014, two specimens (stored: one, MNHN PNT 53; one, ISPRA-14), 21 March 2015, two specimens (ISPRA-15); St. B4, 8 July 2014, 36 specimens (10, SMF 24093; 26, ISPRA-16). Marinetta lagoon: St. M1, 20 March 2015, 59 specimens (five, MNHN PNT 54; seven, SMF 24094; 47, ISPRA-17); St. M2, 23 June 2014, two specimens (one, SMF 24095; one, ISPRA-18), 20 March 2015, one specimen; St. M3, 23 June 2014, two specimens (two, ISPRA-19), 20 March 2015, 21 specimens (four, MNHN PNT 55; 17, ISPRA-20); St M4, 23 June 2014, four specimens (four, ISPRA-21), 20 March 2015, two specimens (two, ISPRA-22). Barbamarco lagoon: St. P1, 16 February 2015, 35 specimens (25, MNHN PNT 56; 10, ISPRA-23); St. P2, 16 February 2015, 125 specimens (25, SMF 24095; 50, ISPRA-24); St. P3, 16 February 2015, one specimen; St. P4, 16 February 2015, nine specimens (five, ISPRA-25); St. P5, 16 February 2015, five specimens.
Additional material
Adriatic Sea specimens reported as Polydora ciliata (Johnston, 1838) in unpublished data sets, previously collected during routine monitoring activities along the coast up to 1 km offshore, in the mixing area between the estuarine and marine waters: St. CC009, 45°02ʹ23.0“N, 12°24ʹ43.8”E, August 2009, fine sand mixed with mud, 3 m depth: 21 specimens. St. CE013, 45°02ʹ34.5“N, 12°24ʹ57.2”E, May 2014, fine sand, 6 m depth: four specimens.
Morphology
Specimens ranged from 1.7 to 18.9 mm long, and from 0.6 to 1.7 mm wide (chaetiger 5). Only one complete individual found: 55 chaetigers, 12.6 mm long and 0.9 mm wide. Prostomium distally expanded, incised on frontal edge (); two pairs of small eyes, absent in some specimens; low narrow caruncle extending to the end of chaetiger 3. Caruncle bearing an antenna (). Chaetiger 1 without notochaetae, only short winged neurochaetae; notopodial lamella digitiform, shifted dorsally with respect to the subsequent segments; neuropodial lamella similar in shape and aligned with the following notopodial lamellae.
Chaetigers 2 to 4 with finely granulated, limbate notochaetae; notopodial postchaetal lamella well developed, cirriform; neuropodia with finely granulated, limbate chaetae; ventral to notochaetae a rounded lamella on chaetigers 2–3, less developed on chaetiger 4.
Chaetiger 5 dorsally enlarged (), with a row of 6–10 falcate spines (); anterior spines extending well beyond body wall, posterior spines nearly embedded in body wall. Spines with an accessory tooth on the concave side, most easily discernible on unworn posterior spines, and a slender subdistal longitudinal flange (). Tip of the spines, flange and secondary tooth often worn and rounded on anterior spines. Spines flanked by feather-like companion chaetae, closely adhering to convex side of the spine (). Newly developed posterior companion chaetae with pointed, brush-like distal tip, covering major spine as a cup or hood (Figure 2B, C). No capillary chaetae present above, nor below the spines of chaetiger 5.
Chaetiger 6 with three rows of finely granulated, limbate notochaetae; in the subsequent chaetigers notochaetae gradually become smooth, unwinged capillaries, with the exception of the middle row made of thin, finely striated, limbate chaetae (); notopodial postchaetal lamellae well developed, gradually smaller in size from about chaetiger 15. Chaetiger 6 with finely granulated, limbate neurochaetae; neuropodial lamella absent.
Branchiae from chaetiger 7 almost to the end of the body; length of the branchiae barely exceeds the dorsal midline. Neuropodia of chaetiger 7 with a single row of bidentate hooded hooks, with constriction and manubrium on shaft (); hooded hooks increasing in number up to 15 in middle parapodia; companion capillary chaetae absent. Pygidium with a ventral, disk-like, flat membrane and dorsal anal aperture ().
The body colour in alcohol varied from white to pale pink salmon; black pigmentation absent.
Remarks
Polydora cornuta specimens from the North Adriatic Sea agree with descriptions provided by Blake and Maciolek (Citation1987) and Radashevsky (Citation2005).
In the sampled populations the number of eyes varied from zero (1.3%) to two (0.6%), three (0.6%) and four (97.5% of specimens). Lack of eye spots was more frequent in mature specimens than in small ones. The number of eyes usually reported for P. cornuta is four (Blake Citation1971; Blake & Maciolek Citation1987), but several studies found a number of eyes that varied from zero to four (Rasmussen Citation1973; Radashevsky & Hsieh Citation2000), and up to six in some cases (Rice & Simon Citation1980). Blind specimens could constitute up to 7% of the P. cornuta population (Rice & Simon Citation1980).
Out of 306 specimens, 11 were without occipital antenna. A closer inspection showed that the antenna was damaged and lost in six specimens, and completely lacking in five individuals. However, the absence of the antenna in some of the smaller specimens is in line with expected intraspecific variation (Rice & Simon Citation1980; Mustaquim Citation1986).
The number of spines of chaetiger 5 changed as a function of the size of the specimen (). The morphology of spines was variable among and within specimens (see ). A small longitudinal flange between the main tooth and the secondary tooth was observed in most cases, but in some the flange appeared to be missing. The spines and the companion chaetae are variable in shape, because they are used in tube construction and maintenance, and thus are subject to wear (Rice et al. Citation2008). Wear patterns of the spines make the chaetae more blunt distally and may reduce the size or the shape of the subdistal tooth.
None of the specimens found in this study had bundles of capillary setae below the spines of chaetiger 5, agreeing with the descriptions of P. cornuta from the Western Mediterranean and the Black Sea (Çinar et al. Citation2005; Radashevsky Citation2005; Simboura et al. Citation2008; Surugiu Citation2012; Radashevsky & Selifonova Citation2013). Bundles of capillary setae on chaetiger 5 have been reported in populations from Victoria, Australia (Blake & Kudenov Citation1978) and Florida (Rice Citation1980; Rice & Simon Citation1980), and in a Mediterranean population in the port of Valencia (Tena et al. Citation1991). This morphological feature has been considered to be the distinguishing characteristic between some reproductively isolated North American populations found by Rice (Citation1991) and Rice et al. (Citation2008).
The number of hooded hooks was related to the size of specimens (), ranging from six to 15 per ramus (mean = 10.02; standard error, SE = 0.36; n = 41). Up to 15 hooks per ramus were reported to be found in Atlantic specimens (Blake & Maciolek Citation1987), while Tena et al. (Citation1991) reported more than 15 hooks per ramus in specimens from the port of Valencia, and nine hooks reported for eastern Mediterranean specimens (Çinar et al. Citation2005).
The presence of a middle row of limbate, finely striated capillary chaetae in the middle and posterior notopodia was observed in all P. cornuta specimens from the North Adriatic Sea. These notochaetae differ from those of the chaetigers 2–4 and 6 in the finely striated pattern of the limbate part. This feature is more evident in larger specimens, but is detectable with light microscopy in small specimens as well. Previous studies did not describe the above-mentioned differences in capillaries of other P. cornuta populations (Blake & Kudenov Citation1978; Blake & Maciolek Citation1987; Çinar et al. Citation2005; Radashevsky Citation2005; Surugiu Citation2012).
P. cornuta is very similar to P. ciliata; therefore, the two species are frequently confused with each other. Nevertheless, P. cornuta can be readily distinguished from P. ciliata by the presence of the occipital antenna. In addition, P. cornuta has a feather-shaped companion chaetae strictly adhering to the main spine on chaetiger 5, while P. ciliata has broom-like pointed or pennoned companion chaetae close to the main spines but not adhering to them (Radashevsky Citation2005). P. ciliata also has postero-ventral capillary chaetae on chaetiger 5, which is usually absent in P. cornuta, though this character appears to be unstable in both species (Blake & Kudenov Citation1978; Rice & Simon Citation1980; Mustaquim Citation1986).
Reproduction
Brooding females were found in the three lagoons, with higher numbers in February and March of 2015 in Marinetta and Barbamarco (). In Val di Brenta, reproductive individuals were mostly found in the summer of 2014. Gametes are developed in the middle chaetigers, starting from chaetigers 13–15, where large number of oocytes (in females) and dense sperm patches (in males) are present in the coelomic cavity.
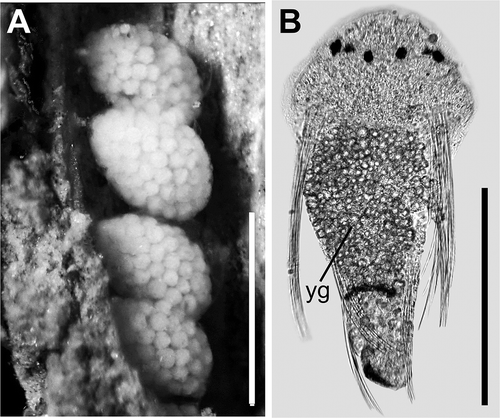
Females deposit eggs in capsules which lie together inside the tube in a string, attached to the tube by two thin thread-like filaments (). Overall, 22 tubes with capsules were found. A maximum of 24 egg capsules per tube were counted (mean = 15.2; SE = 1.62; n = 10). A single capsule contained from 75 to 205 eggs/larvae (mean = 124.1; SE = 9.3; n = 20). The eggs were spherical, filled with yolky globules, with a diameter ranging from 76 to 130 mm (mean = 100.8; SE = 0.8; n = 125). No unfertilised eggs were observed inside capsules containing larvae.
Larval morphology
Early three-chaetiger larvae were found in 50 egg capsules from four hatching females. Three-chaetiger larvae were each about 250 mm long, with three pairs of black eyes, comprising two pairs of lateral eyes and one pair of median eyes, with lateral eyes on either side situated close to one another and therefore appearing to be a single pair of eyes (). The gut was filled with yolky granules. There was one transverse bar of melanophores on the dorsal side of chaetiger 3, and black pigment diffused on the distal end of the pygidium. The chaetae of chaetiger l were the longest, extending posteriorly up to the bar of melanophores on chaetiger 3; slightly curved, with fine serrations on the convex side.
Density
In Val di Brenta, P. cornuta was absent in February 2014, but was found with low frequency and average low abundance in the two subsequent sampling times (). Relatively high abundance was found on one occasion at B4 station in July 2014, where P. cornuta represented 8.3% of the total macrofauna.
Figure 5. Polydora cornuta mean densities measured at each sampling station at each sampling time. A, Venice lagoon (Val di Brenta); B, Marinetta lagoon; C, Barbamarco lagoon. Error bars are standard errors (n = 3).
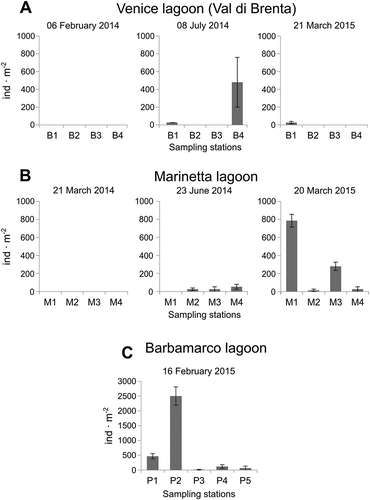
In Marinetta lagoon P. cornuta was absent in March 2014, but was detected at three stations in June 2014, and in all sampling stations in March 2015 (). The highest abundance was detected at M1 station in March 2015, with an average density of 786.7 (± 70.5 SE) ind. m–2, accounting for 3.6% of the total macrofauna.
The highest abundance of P. cornuta registered in this study was found in Barbamarco lagoon, where at station P2 it reached an average density of 2500 (± 310.2 SE) ind. m–2 representing 3.8% of the macrofauna (). On 16 February 2015, P. cornuta were recorded at all of the sampling stations, with densities ranging from 40 to 2880 ind. m–2.
Moreover, in a revision of benthic material taken in August 2009 and May 2014, 1 km offshore of the Marinetta lagoon, up to 300 ind. m–2 were found (all individuals previously reported as P. ciliata; unpublished data).
Habitat and ecology
Polydora cornuta is a tube-dwelling worm, living in soft mud, sandy mud, sand and shells in which mud tubes are found. The tubes are fragile, made of silt particles, fine sand grains and detritus, brownish-yellowish, up to 20 mm long and up to 3 mm in diameter.
Two specimens of P. cornuta were found in their mud tubes within a living Manila clam (Ruditapes philippinarum (Adams & Reeve, 1850), shell length 5.5 cm), in a nacre pocket along the postero-ventral edge of the shell. The shape of the pocket means that the worms could not have perforated the shell. It seems most likely that worms entered between the mantle and the valve, and subsequently the clam kept the worms isolated in this pocket by secreting a layer of nacre on them. One of these specimens was a female incubating 10 egg capsules containing larvae at a three-chaetiger early stage.
P. cornuta was found in brackish-water environments, in sites characterised by still water and proximity to fresh water sources (channels or river mouths), at a salinity regime ranging between 10 and 36 PSU and a depth ranging between 0.5 and 1.5 m. In the sampling sites the sediments were mainly composed of mud and fine sandy silt, with little shell debris and high organic content, with macrofaunal communities dominated by Streblospio shrubsolii (Buchanan, 1890), Oligochaeta Tubificidae and Chironomus Kieffer, 1915 larvae.
Several specimens were collected 1 km offshore of the Marinetta lagoon, in a mixing area up to 6 m deep between the estuarine and marine waters, on fine sand mixed with mud, in a dense Chamelea gallina (Linnaeus, 1758) and Lentidium mediterraneum (O. G. Costa, 1830) community, along with Owenia fusiformis Delle Chiaje, 1844 and Prionospio caspersi Laubier, 1962.
Distribution
Polydora cornuta is distributed in temperate and subtropical zones worldwide (Radashevsky Citation2005). This species has been reported in the east, west and gulf coasts of North America, the Caribbean Sea, Argentina, Brazil, Australia, China, Korea, Japan, India, Baltic Sea, North Sea, Kattegat and Öresund, Baltic Sea, western and eastern Mediterranean Sea (Tena et al. Citation1991; Çinar et al. Citation2005; Simboura et al. Citation2008) and the Black Sea (Surugiu Citation2005, Citation2012; Radashevsky & Selifonova Citation2013).
Discussion
Taxonomic analyses demonstrated the presence of Polydora cornuta in all of the sampled lagoons, as well as in the offshore samples. Information regarding morphology, distribution and reproductive activity is provided. P. cornuta has been considered one of the most invasive species to spread across the Mediterranean Sea (Zenetos et al. Citation2005), with records extending from the western (Tena et al. Citation1991) to eastern Mediterranean basin (Çinar et al. Citation2005; Simboura et al. Citation2008), the Bosphorus Strait (Karhan et al. Citation2008) and the Black Sea (Radashevsky Citation2005; Surugiu Citation2005). The occurrence of established populations of P. cornuta in North Adriatic brackish waters seems to confirm that this species has a strong ability to colonise new areas. This species is often introduced by transport in ballast waters into new areas connected to large seaports (Çinar et al. Citation2005); thus, the first introduction in the Adriatic waters may have been in the Venice lagoon. The port of Venice is the main port in the Adriatic, in terms of passenger transport and occurrence of shipping lines, and the second most important in terms of goods (Eurostat Citation2015). Its location and the fact that it is a semi-closed lagoon with localised high levels of pollution (also caused by industrial settlements) have been proven to be two of the main points for the introduction of alien species to Italian coasts (Occhipinti-Ambrogi et al. Citation2011).
The occurrence of P. cornuta has been previously recorded in the literature of the benthic ecology of the Venice lagoon. Tagliapietra et al. (Citation1998) reported this species as P. ligni in the northern part of the lagoon (Palude della Rosa) during a field study carried out in 1991, describing it as a “general opportunist early colonizer of any available substratum”. Another study carried out 3 years later seemed to confirm the presence of P. ligni in macrofaunal assemblages of the central part of the lagoon and in Val di Brenta (Casellato & Ragazzo Citation1997). Unfortunately, specimens reported by the above-mentioned authors were not available for a re-examination (D. Tagliapietra pers. comm.); therefore, their identification could not be confirmed.
The presence of P. cornuta has probably been hidden by a lack of up-to-date identification keys and by the taxonomic confusion with the congener P. ciliata (Çinar et al. Citation2005; Simboura et al. Citation2008). In this study, taxonomic analyses carried out on soft sediment grab samples from three lagoons at several sampling times did not bring to light any specimens of P. ciliata. All 306 Polydora individuals found in the three lagoons belonged to P. cornuta. Moreover, further benthic material collected offshore of the Marinetta lagoon in 2009 and 2014 was checked, revealing the specimens previously identified as P. ciliata to be P. cornuta. This is quite surprising since P. ciliata has been widely reported along the Adriatic coast (see references in Mikac Citation2015), and it is also the polychaete with the highest frequency of records across the Italian lagoons (Basset et al. Citation2007). Çinar et al. (Citation2005) re-identified P. ciliata specimens from old benthic material on the Turkish coast of the Aegean Sea in 1986 as P. cornuta, thus indicating that the species had established itself earlier than originally thought in the Izmir Bay. Similarly, Surugiu (Citation2012) re-examined a collection of specimens of P. ciliata from the Black Sea, revealing a misidentification of P. cornuta and thus raising doubts about the presence of P. ciliata in that area. All other records of P. ciliata from the Northern Adriatic should be verified as well.
Additionally, aquaculture activities have been hypothesised to play a role in the spreading dynamics of P. cornuta (Simboura et al. Citation2008). In the three studied environments, as well as in most Adriatic lagoons, Manila clams (Ruditapes philippinarum) have been farmed on a wide scale since the late 1980s (Solidoro et al. Citation2000; Pranovi et al. Citation2008). The presence of clam fields in several areas close to sampling stations seems to suggest that there may be a relationship between aquaculture activities and the occurrence of P. cornuta, though there are currently no studies providing proof of this connection.
Abundances reached up to 2880 ind. m–2 in Barbamarco lagoon, on silty-clay sediment close to a marina and in proximity to a fresh water source from the Po river. Similar densities have been found for this species in Izmir Bay, Turkey (3170 ind. m–2; Çinar et al. Citation2005) and the Sea of Marmara (3390 ind. m–2; Karhan et al. Citation2008), while in the north-western part of the Black Sea densities reached 150,000 ind. m–2 (Surugiu Citation2005). The results of this study highlight that P. cornuta represents on average about 1.2% of macrofauna abundance in sampled stations, and, even when reaching higher densities, its relative abundance rarely exceeded 8% of total macrofauna. On the contrary, studies on benthic communities from the eastern Mediterranean and the Sea of Marmara revealed high levels of P. cornuta, which has been ascribed to changes in the species composition of benthic assemblages (Çinar et al. Citation2005; Karhan et al. Citation2008; Dagli et al. Citation2011).
The detection of specimens of P. cornuta in 2009 and 2014 in shallow marine waters seems to indicate that this species is able to spread out along the coast, and suggests that its distribution in the North Adriatic basin may be much broader than that described in this paper. The complex system of lagoons and brackish water environments, interconnected along the coast by a net of channels and river mouths, makes the whole region highly suitable for the ecological characteristics of this species.
In North Adriatic lagoons, P. cornuta colonises sandy mud and silty mud soft bottoms with communities dominated by the deposit-feeder polychaete Streblospio shrubsolii, with Oligochaeta Tubificidae and Chironomus larval stages. Higher abundance is common in areas characterised by the presence of the polychaetes Hediste diversicolor (O.F. Müller, 1776), Heteromastus filiformis (Claparède, 1864) and Capitella spp. Blainville, 1828. The same species association was found in disturbed soft-bottom communities of the Sea of Marmara (Karhan et al. Citation2008). This confirms the opportunistic traits of P. cornuta, an early recoloniser after disturbance events (Tagliapietra et al. Citation1998).
The results of the present study indicate that the population of P. cornuta may increase quickly owing to high larval output, and reproducing even in winter. Other studies in controlled conditions showed that development time in the egg capsules, from deposition of eggs to release of three-chaetiger larvae, is around 4 to 5 days at 20°C (Rice et al. Citation2008). Anger et al. (Citation1986) found that at 12°C the development time from hatching to metamorphosis was 1.4–3.1 times slower than at 18°C. Counts of capsules per sample from Marinetta lagoon in March 2015 and the average number of eggs per capsule can be used to estimate the potential larval production of P. cornuta. Considering an average temperature of 11°C, and a development time from egg deposition to hatching larvae 3 times greater than the one found at 20°C by Rice et al. (Citation2008), it is possible to expect a production of 452,400 (± 34,200 SE) larvae m–2 in 2 weeks. This production level is likely to be an underestimate because of the net mesh used to filter the samples, which may have failed to retain some egg capsules. Indeed, this species is capable of higher production rates, up to 884,000 larvae m–2 in 2 weeks (Zajac Citation1991b). In this study, females hatching egg capsules often had additional eggs in the coelomic cavity, confirming that P. cornuta may produce more broods in a single season. The high reproductive potential is often balanced by high mortality rates during larval settlement and development (Zajac Citation1991b), but such a high reproductive output allows P. cornuta to maintain established populations and occasionally reach high densities.
In conclusion, the presence of established populations in three distinct North Adriatic lagoons, the identification of specimens collected along the coast in 2009 and the previous records in the literature support the hypothesis that P. cornuta first began colonising the Gulf of Venice area before the 1990s. A revision of historical and reference collections might shed some light on the timeline and the introduction pathways of this species. Additional records and molecular studies across Mediterranean populations could help to define the spatio-temporal dynamics of the possible multiple introductions of this species.
Acknowledgements
I am very grateful to Dr. Vasily I. Radashevsky for confirming the identification of Polydora cornuta, and for providing relevant literature and comments. My thanks also go to Emiliano Verza, Danilo Trombin and Riccardo Leonardi for their valuable help in management and logistic support for field activities. I am very grateful to Dr. Agavni Petrosyan for correcting the English manuscript. My acknowledgment goes to four anonymous referees who greatly improved the manuscript with invaluable suggestions and comments. I am much indebted to Andrea Bosi for his precious advices on microscopy techniques: this paper is dedicated to him.
References
- Anger K, Anger V, Hagmeier E. 1986. Laboratory studies on larval growth of Polydora ligni, Polydora ciliata, and Pygospio elegans (Polychaeta, Spionidae). Helgoländer Meeresuntersuchungen 40:377–395. doi:10.1007/BF01983819.
- Basset A, Galuppo N, Sabetta L. 2007. Environmental heterogeneity and benthic macroinvertebrate guilds in italian lagoons. Transitional Waters Bulletin 1:48–63. doi:10.1285/i1825226Xv1n1p48.
- Blake JA. 1969. Reproduction and larval development of Polydora from northern New England (Polychaeta: Spionidae). Ophelia 7:1–63. doi:10.1080/00785326.1969.10419288.
- Blake JA. 1971. Revision of the genus Polydora from the East coast of North America (Polychaeta: Spionidae). Smithsonian Contributions to Zoology 75:1–32. doi:10.5479/si.00810282.75.
- Blake JA, Kudenov JD. 1978. The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera. Memoirs of the National Museum of Victoria 39:171–280.
- Blake JA, Maciolek NJ. 1987. Redescription of Polydora cornuta Bosc (Polychaeta: Spionidae) and designation of a neotype. Proceedings of the Biological Society of Washington 7:11–15.
- Bosc LAC. 1802. Histoire naturelle des vers, contenant leur description et leur moeurs avec figures dessinées d’après nature. 3 vols. Paris: Deterville Libraire.
- Casellato S, Ragazzo S. 1997. Distribuzione dei policheti nella laguna di Venezia: Correlazioni con la qualità dei sedimenti. In: Anelli A, Ferrari I, Rossetti G, Vezzosi M, editors. S.It.E. Atti XVIII. Atti Ottavo Congresso Nazionale della Società Italiana di Ecologia (S.It.E.). Parma; 10-12 settembre 1997. Parma: Edizioni Zara. pp. 233–234.
- Castelli A, Bianchi CN, Cantone G, Çinar ME, Gambi MC, Giangrande A et al. 2008. Annelida Polychaeta. In: Relini G, editor. Checklist della flora e della fauna dei mari italiani. S.I.B.M. Biologia Marina Mediterranea 15(Supplement 1):323–373.
- Çinar ME, Ergen Z, Dagli E, Petersen ME. 2005. Alien species of spionid polychaetes (Streblospio gynobranchiata and Polydora cornuta) in Izmir Bay, eastern Mediterranean. Journal of the Marine Biological Association of the United Kingdom 85:821–827. doi:10.1017/S0025315405011768.
- Dagli E, Çinar ME, Ergen Z. 2011. Spionidae (Annelida : Polychaeta) from the Aegean Sea (eastern Mediterranean). Italian Journal of Zoology 78:49–64. doi:10.1080/11250003.2011.567828.
- Dauer DM, Maybury CA, Ewing RM. 1981. Feeding behavior and general ecology of several spionid polychaetes from the Chesapeake Bay. Journal of Experimental Marine Biology and Ecology 54:21–38. doi:10.1016/0022-0981(81)90100-3.
- Dean WE. 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. Journal of Sedimentary Research 44:242–248. doi:10.1306/74D729D2-2B21-11D7-8648000102C1865D.
- Eurostat. 2015. Transport database. Maritime transport - Vessel traffic - Quarterly data - Main ports - Number and gross tonnage of vessels - by type and size of vessels - Direction: inwards only - year 2006 onwards. Available: http://ec.europa.eu/eurostat/web/transport/data/database. Accessed 29 Sept 2015.
- Grassle JF, Grassle JP. 1974. Opportunistic life histories and genetic systems in marine benthic polychaetes. Journal of Marine Research 32:253–284.
- Karhan SÜ, Kalkan E, Simboura N, Mutlu E, Bekbölet M. 2008. On the occurrence and established populations of the alien polychaete Polydora cornuta Bosc, 1802 (Polychaeta: Spionidae) in the Sea of Marmara and the Bosphorus Strait (Turkey). Mediterranean Marine Science 9:5–19. doi:10.12681/mms.140.
- Light WJ. 1978. Spionidae, Annelida, Polychaeta (Invertebrates of the San Francisco Bay estuary system). California: California Academy of Sciences, Pacific Grove. 211 pp.
- Loosanoff VL, Engle JB. 1941. Polydora in oysters suspended in the water. Biological Bulletin 85:69–78. doi:10.2307/1538270.
- Mikac B. 2015. A sea of worms: polychaete checklist of the Adriatic Sea. Zootaxa 3943:1–172. doi:10.11646/zootaxa.3943.1.1.
- Mistri M, Munari C. 2008. BITS: a SMART indicator for soft-bottom, non-tidal lagoons. Marine Pollution Bulletin 56:587–599. doi:10.1016/j.marpolbul.2007.12.002.
- Mustaquim J. 1986. Morphological variation in Polydora ciliata complex (Polychaeta: Annelida). Zoological Journal of the Linnean Society 86:75–88. doi:10.1111/j.1096-3642.1986.tb01808.x.
- Occhipinti-Ambrogi A, Marchini A, Cantone G, Castelli A, Chimenz C, Cormaci M et al. 2011. Alien species along the Italian coasts: an overview. Biological Invasions 13:215–237. doi:10.1007/s10530-010-9803-y.
- Petersen ME. 1998. Pholoe (Polychaeta: Pholoidae) from northern Europe: a key and notes on the nearshore species. Journal of the Marine Biological Association of the United Kingdom 78:1373–1376. doi:10.1017/S002531540004457X.
- Pranovi F, Da Ponte F, Torricelli P. 2008. Historical changes in the structure and functioning of the benthic community in the lagoon of Venice. Estuarine, Coastal and Shelf Science 76:753–764. doi:10.1016/j.ecss.2007.08.006.
- Radashevsky VI. 2005. On adult and larval morphology of Polydora cornuta Bosc, 1802 (Annelida: Spionidae). Zootaxa 24:1–24.
- Radashevsky VI, Hsieh H. 2000. Polydora (Polychaeta: Spionidae) species from Taiwan. Zoological Studies 39:203–217.
- Radashevsky VI, Selifonova ZP. 2013. Records of Polydora cornuta and Streblospio gynobranchiata (Annelida, Spionidae) from the Black Sea. Mediterranean Marine Science 14:261–269. doi:10.12681/mms.415.
- Rasmussen E. 1973. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia 11:1–507. doi:10.1080/00785326.1973.10430115.
- Rice SA. 1980. Ultrastructure of the male nephridium and its role in spermatophore formation in spionid polychaetes (Annelida). Zoomorphologie 95:181–194. doi:10.1007/BF00998121.
- Rice SA. 1991. Reproductive isolation in the Polydora ligni complex and the Streblospio benedicti complex (Polychaeta: Spionidae). Bulletin of Marine Science 48:432–447.
- Rice SA, Karl S, Rice KA. 2008. The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct. Invertebrate Biology 127:45–64. doi:10.1111/j.1744-7410.2007.00104.x.
- Rice SA, Simon JL. 1980. Intraspecific variation in the pollution indicator polychaete Polydora ligni (Spionidae). Ophelia 19:79–115. doi:10.1080/00785326.1980.10425509.
- Simboura N, Sigala K, Voutsinas E, Kalkan E. 2008. First occurrence of the invasive alien species Polydora cornuta Bosc, 1802 (Polychaeta: Spionidae) on the coast of Greece (Elefsis Bay; Aegean Sea). Mediterranean Marine Science 9:119–124. doi:10.12681/mms.138.
- Solidoro C, Pastres R, Canu DM, Pellizzato M, Rossi R. 2000. Modelling the growth of Tapes philippinarum in Northern Adriatic lagoons. Marine Ecology Progress Series 199:137–148. doi:10.3354/meps199137.
- Surugiu V. 2005. Inventory of inshore polychaetes from the Romanian coast (Black Sea). Mediterranean Marine Science 6:51–73. doi:10.12681/mms.193.
- Surugiu V. 2012. Systematics and ecology of species of the Polydora-complex (Polychaeta: Spionidae) of the Black Sea. Zootaxa 3518:45–65.
- Tagliapietra D, Minelli A. 2009. Acquatic invertebrates. In: Ruffo S, Stoch F, Garside A, Walton G, editors. Lagoons, estuaries and deltas. Udine: Museo Friulano di Storia Naturale. pp. 39–63.
- Tagliapietra D, Pavan M, Wagner C. 1998. Macrobenthic community changes related to eutrophication in Palude della Rosa (Venetian Lagoon, Italy). Estuarine, Coastal and Shelf Science 47:217–226. doi:10.1006/ecss.1998.0340.
- Tena J, Capaccioni-Azzati R, Porras R, Torres-Gavilá FJ. 1991. Cuatro especies de poliquetos nuevas para las costas mediterráneas españolas en los sedimentos del antepuerto de Valencia. Miscellanea Zoologica 15:29–41.
- Zajac RN. 1991a. Population ecology of Polydora ligni (Polychaeta: Spionidae). II. Seasonal demographic variation and its potential impact on life history evolution. Marine Ecology Progress Series 77:207–220. doi:10.3354/meps077207.
- Zajac RN. 1991b. Population ecology of Polydora ligni (Polychaeta: Spionidae). I. Seasonal variation in population characteristics and reproductive activity. Marine Ecology Progress Series 77:197–206. doi:10.3354/meps077197.
- Zenetos A, Çinar ME, Pancucci-Papadopoulou MA, Harmelin JG, Furnari G, Andaloro F et al. 2005. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterranean Marine Science 6:63–118. doi:10.12681/mms.186.
- Zenetos A, Gofas S, Morri C, Rosso A, Violanti D, García Raso JE et al. 2012. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterranean Marine Science 13:328–352. doi:10.12681/mms.327.