Abstract
Holoplanktonic polychaetes constitute an exception within this taxon, which as adults colonize all the whole variety of brackish water and marine habitats. Nevertheless, they form a distinctive group in marine zooplankton and play an important role in the pelagic trophic nets. Pelagic worms are little studied, especially in the Mediterranean Sea, while information regarding the distribution of the families Typhloscolecidae and Iospilidae along Italian coasts is a century out of date. This paper deals with the state of knowledge of these polychaetes; it summarizes the previous records, updating them with the new records here reported from Tyrrhenian Sea, in order to check whether they have a bearing on Italian fauna. The findings reported in this paper confirm the presence of the Typhloscolecidae, with the species Sagitella kowalewskyi and Typhloscolex muelleri, and of the Iospilidae, with the species Phalacrophorus pictus and Iospilus phalacroides, along the Italian coasts, validating, in each case, their current presence in the Italian fauna. The main morphological features of these species and a dichotomous key for their identification are also reported.
Introduction
Holoplanktonic polychaetes, which complete their entire life cycle in the water column, constitute an exception within this taxon, which typically includes a very high number of species, that as adults colonize all the variety of brackish water and marine bottoms. Nevertheless, they form a distinctive group in marine zooplankton and play the important role of predator in the pelagic trophic nets. Traditionally, pelagic polychaetes include seven families: Alciopidae, Lopadorhynchidae, Pontodoridae, Iospilidae, Tomopteridae, Poeobiidae and Typhloscolecidae (Fauchald Citation1977; Rouse & Pleijel Citation2001); the families Yndolacidae and Flotidae are not included here due to their uncertain phylogenetic relationship (Rouse & Pleijel Citation2001). Such exclusively pelagic worms are poorly studied, especially in the Mediterranean Sea, being seldom collected among plankton samples.
This paper deals with the state of knowledge of the Typhloscolecidae and the Iospilidae of the Italian fauna: it summarizes old records and updates them with the present records. This is in response to a recent editorial by Boero (Citation2011), which asked zoologists to address questions regarding “new species” as well as “old” ones and to check whether, in either case, they are still being found or have gone “missing”, all this to better assess regional biodiversity. The species from the Italian seas are here characterized with their main morphological features, and a dichotomous key for their identification is also provided.
The Typhloscolecidae consists of only three recognized genera: Sagitella Wagner, Typhloscolex Bush and Travisiopsis Levinsen, the first with only one species, the last two with six species, respectively (Fauchald Citation1977; Rouse & Pleijel Citation2001). They are holoplanktonic polychaetes characterized by a tapered, transparent body ranging from 2 to 40 mm in length with up to 50 segments, generally 20–25. Prostomium small, indistinct with a digitiform tip, typical nucal organs; segments with rudimentary lobes bearing a few simple chetae and pygidium with a pair of flattened anal cirri. Most studies relate to their systematics and distribution (Day Citation1967; Pleijel & Dales Citation1991; Fernández-Álamo Citation2004, Citation2006; Márquez-Rojas et al. Citation2013), while even basic knowledge about their biology and ecology remains lacking. Therefore, very little information is available on the distribution of the species of this family from the Italian seas: the only recorded data date back to the nineteenth century and to the 1920s (Bush Citation1851; Reibisch Citation1895; Lo Bianco Citation1904; Fauvel Citation1923).
The Iospilidae include pelagic polychaetes distinguished by slender and small size, prostomium without antennae, peristomium bearing a pair of ventral palps, all composite chaetae and an eversible pharynx, in some cases armed. Four genera are included in this family: Phalacrophorus Greeff, Iospilus Viguier, Pariospilus Viguier and Iospilopsis Augener, the first two genera with three and two species, respectively, and the latter two with only one species each, according to Fauchald (Citation1977) and Rouse and Pleijel (Citation2001). However, other authors (Day Citation1967; Dales & Peter Citation1972; Fernández-Álamo Citation2009a) recognized only the genera Phalacrophorus Greeff and Iospilus Viguier in the family, including Pariospilus Viguier in the genus Iospilus and Iospilopsis antillensis Augener as synonymous with Phalacrophorus uniformis Reibisch, Citation1895. Some papers deal with their geographical distribution and new records in Atlantic and Pacific oceans, but very few data concerning the Mediterranean Sea are available. In the case of the Italian seas, the records of Iospilidae also date back to the early 1900s (Lo Bianco Citation1904; Fauvel Citation1923).
Materials and methods
Plankton samples were collected by means of a net with a 40 cm mouth diameter and constructed throughout of nylon with a mesh aperture of 100 μm, with horizontal hauls in superficial waters in areas of 20–50 m depth along the northwestern coast of Isola d’Elba (Tuscany, Italy), from 2011 to 2015. The net was gently rinsed and samples were transferred to Petri plates containing sea water for sorting and observation in vivo. Identification of specimens was carried out under stereo and light microscopes. Sampling was carried out by Dr Marco Lucarelli (University of Rome “Tor Vergata”).
Results
Two species of Typholscolecidae for a total of 15 individuals were collected: five belonging to Sagitella kowalewskyi and 10 to Typhloscolex muelleri. They were recorded over 3 years between August and April. Two species of Iospilidae were also collected: Phalacrophorus pictus, with five individuals, and Iospilus phalachroides, with 18 individuals, respectively.
Class Polychaeta
Order Phyllodocida
Family Typhloscolecidae
Genus Sagitella Wagner, 1872
Type species: Sagitella kowalewskyi Wagner, 1872
Sagitella kowalewskyi Wagner, 1872
()
Main morphological features
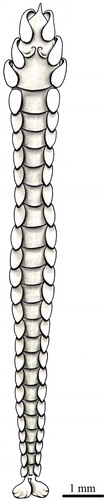
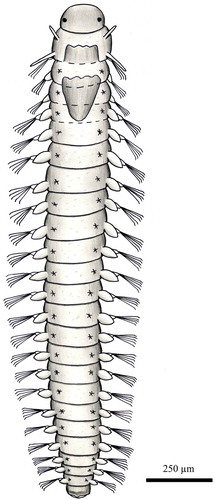
Body slender, cylindrical, tapered at both ends, transparent, with around 25–50 segments and 5–15 mm in length. Prostomium conical, anteriorly pointed, ending with a slender palpode. Eyes absent. Large ciliated flaps occurring to both sides of the prostomial end. A retort-shaped organ underneath the pharynx under a dorsal thickening. Behind this, two nuchal organs forming two semi-circular ridges curved in towards their posterior ends. The anteriormost two segments with a pair of foliaceous cirri; the following segments bearing rounded dorsal and ventral cirri. Parapodia slightly pronounced with rudimentary chaetigerous lobes with a single acicula and few simple chaetae, appearing at the third segment. Two large foliaceous anal cirri.
Type locality: Mediterranean (Gulf of Naples).
Geographic distribution
S. kowalewskyi is widely distributed in the Atlantic Ocean in northeastern, tropical and southern waters (Southern Citation1910; Fauvel Citation1916, Citation1923; Støp-Bowitz Citation1951; Day Citation1967; Orensanz & Ramirez Citation1973; Pleijel & Dales Citation1991; Fernández-Álamo Citation2006; Márquez-Rojas et al. Citation2013); in the Pacific Ocean in tropical and subtropical regions, along the north to southeastern coast and in northwestern waters (Chamberlin Citation1919; Okuda Citation1938; Dales Citation1957; Berkeley & Berkeley Citation1960; Tebble Citation1962; Ushakov Citation1974; Fernández-Álamo Citation2004; Fernández-Álamo & Sanvincente-Añorve Citation2005); and in the southwest Indian Ocean (Day Citation1967).
Mediterranean distribution
Tyrrhenian Sea, Gulf of Naples (Lo Bianco Citation1904), Corsica, Calvi (Fauvel Citation1916).
Genus Typhloscolex Bush, Citation1851
Type species: Typhloscolex muelleri Bush, Citation1851
Typhloscolex muelleri Bush, Citation1851
( and )
Main morphological features
Body anteriorly wide and posteriorly tapering, transparent, with around 15–25 segments and 2–5 mm long. Prostomium bearing a filiform palpode with a ventral cilindrical swelling. Broad semicircular ciliated lobes dorsally and ventrally on the prostomium, which looks like a plate perpendicular to the length axis of the body ending with the filiform palpode. Sides of head enfolded by a pair of flattened sinous cirri. A pair of similar flattened cirri on the two following segments; the others with foliaceous dorsal and ventral cirri. Small, not clearly differentiated parapodia. Two or three short acicular chaetae from the fifth segment, but usually present only in the posterior segments and rarely projecting beyond the parapodial lobe. Small and ovate anal cirri.
Type locality: Adriatic Sea, Trieste.
Geographic distribution
Common species in the Pacific Ocean, both in tropical sub-tropical regions and in Artic and Antartic waters (Chamberlin Citation1919; Fauvel Citation1936; Dales Citation1957; Berkeley & Berkeley Citation1960; Tebble Citation1962; Reish Citation1968; Ushakov Citation1974; Fernández-Álamo Citation2004; Fernández-Álamo & Sanvincente-Añorve Citation2005; Bilbao et al. Citation2008; Gagayev & Kosobokova Citation2012); Atlantic Ocean from northern to southern regions and Antartic waters (Treadwell Citation1943; Tebble Citation1960; Day Citation1967; Orensanz & Ramirez Citation1973; Ushakov Citation1974; Fernández-Álamo Citation2006, Citation2009b; Márquez-Rojas et al. Citation2013); and in the southwestern Indian Ocean (Day Citation1967, Citation1975).
Mediterranean distribution
North Adriatic Sea, Tyrrhenian Sea, Gulf of Naples (Lo Bianco Citation1904); Monaco, Gulf of Marseilles (Fauvel Citation1923; Peres Citation1954).
Family Iospilidae
Genus Phalacrophorus Greeff, 1879
Type species: Phalacrophorus pictus Greeff, 1879
Phalacrophorus pictus Greef, 1879
()
Main morphological features
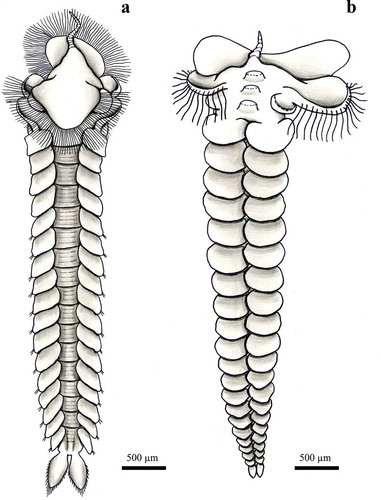
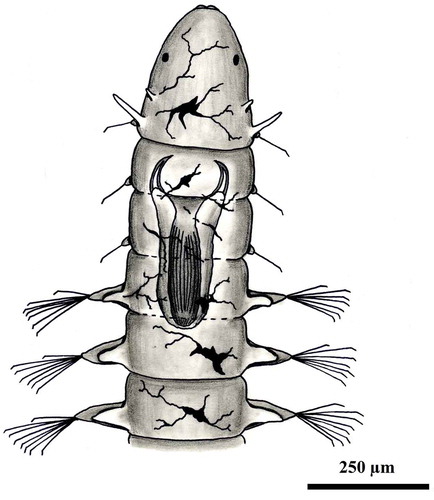
Body cylindrical, transparent, 3–6 mm long with 15–30 segments. Prostomium rounded without antennae and with one pair of brown eyes. First and second segments fused and bearing two pair of small tentacular cirri, the first pair much shorter than the second pair, the second segment only with setae. Two and three anterior segments with reduced parapodia with very few setae and without dorsal and ventral cirri. At fourth segment and posteriorly parapodia increasing in size. Dorsal and ventral cirri rounded, shorter than the podial lobes. Compound chaetae spinigerous with long pointed shaft-blades. Muscular pharynx armed with two curved chitinized jaws at the anterior end. Branched chromatophores ventral to the parapodia. Pygidium without appendages.
Type locality: Atlantic Ocean Canary Islands.
Geographic distribution
Atlantic Ocean from northern to southern regions and Arctic area (Reibisch Citation1895; Fauvel Citation1916, Citation1923; Friedrich Citation1950; Støp-Bowitz Citation1951; Orensanz & Ramirez Citation1973; Ushakov Citation1974; Druzhkov et al. Citation2000; Fernández-Álamo Citation2009a; Tovar-Faro et al. Citation2013); Pacific Ocean tropical, Arctic and Antarctic sectors (Ehlers Citation1913; Fauvel Citation1936; Treadwell Citation1943; Dales Citation1957; Tebble Citation1962; Ushakov Citation1974; Fernández-Álamo & Sanvincente-Añorve Citation2005; Fernández-Álamo Citation2006; Bilbao et al. Citation2008); Indian Ocean (Day Citation1975).
Mediterranean distribution
Bay of Alger (Viguier Citation1886), Tyrrhenian Sea, Gulf of Naples (Lo Bianco Citation1904), Gulf of Marseille (Peres Citation1954).
Genus Iospilus Viguier, Citation1886
Type species: Iospilus phalacroides Viguier, Citation1886
Iospilus phalacroides Viguier, Citation1886
()
Main morphological features
Small, transparent and slender body, 2–3 mm long with 15–20 segments. Prostomium rounded without antennae but with one pair of short palps. Two eyes. Four anterior segments with reduced parapodia. First and second segments fused and bearing two pairs of small tentacular cirri, the first much shorter than the second pair, and one pair of tufts of 2–3 chaetae. Parapodia of segments two and three with reduced chaetigerous lobes without dorsal and ventral cirri. Posterior segments with uniramous parapodia with short dorsal and ventral cirri and chaetigerous lobes with compound chaetae. All compound chaetae spinigerous with long shaft ending pointed. Eversible muscular pharynx unarmed. Starlike chromatophores. Pygidium large without appendages.
Type locality: Mediterranean Sea, Bay of Algiers.
Geographic distribution
Atlantic Ocean (Fauvel Citation1916; Jiménez-Cueto et al. Citation2006; Fernández-Álamo Citation2009a); Pacific Ocean (Treadwell Citation1943; Dales Citation1957; Fernández-Álamo & Sanvincente-Añorve Citation2005); Indian Ocean (Day Citation1967, Citation1975).
Mediterranean distribution
Bay of Algiers; Tyrrhenian Sea, Gulf of Naples (Lo Bianco Citation1904); southern Spanish coast (Fauvel Citation1916).
Discussion
Studies on biodiversity deal with different aspects of species – biogeographic, evolutive, genetic, ecological – but most of them quote descriptions of new species, suggesting an increase in biodiversity. Such additions extend the checklists of our fauna, but rarely do we pause to consider the “old” species, or the species listed as being present in a given geographical area in the past and now supposed to be still present (Boero Citation2011).
Typhloscolecidae and Iospilidae represent an interesting case study regarding this issue, being the records of the species belonging to these families from Italian seas published approximately a century ago. The same outdated records have been also reported in the checklist of Italian fauna of polychaetes (Castelli et al. Citation1995). Only a few recent data are available from the literature (Batistić et al. Citation2012), but extracted from the faunal list published for other purposes and missing any observations about the record of such polychaetes; these neglected species have hence passed unnoticed.
Holoplanktonic polychaetes prove to be widely distributed in all the oceans of the world; in particular, their distribution throughout the Atlantic and Pacific Oceans and in Arctic and Antarctic waters reflects the course of campaigns of oceanographic exploration. By contrast little is known on the distribution of pelagic polychaetes in the coastal and neritic environments. Subsequently, a lot of them are considered cosmopolitan (Tebble Citation1962; Ushakov Citation1974; Rouse & Pleijel Citation2001) and typical species of open sea waters (Støp-Bowitz Citation1948; Day Citation1967). Only recently have detailed studies focused on particular distributions of pelagic polychaetes in inshore localities of the Pacific and Atlantic Oceans. In fact, Fernández-Álamo (Citation1987, Citation2006) and Fernández-Álamo and Sanvincente-Añorve (Citation2005) explained the distribution pattern of the neritic assemblages of Typhloscolecidae and Iospilidae found along the Mexican Pacific coasts, suggesting that specific feeding habits and different tolerance to the variability in environmental conditions may influence these species along coastal waters.
In fact, notwithstanding their low abundance in the neritic waters, such pelagic polychaetes play an important role in the plankton food web. Some Typhloscolecidae are reported as predators on chaetognaths, while also being considered ectoparasites of such a zooplanktonic group (Øresland & Pleijel Citation1991; Øresland & Bray Citation2005). The same authors registered their maximum abundance in the areas with abundant neritic animals, such as preys or possible hosts; they also investigated the role of Typhloscolecidae as being possibly responsible for the headless chaetognaths frequently recorded in plankton samples from the Indian Ocean. Likewise, Márquez-Rojas et al. (Citation2013) recorded some Typhloscolecids and other pelagic polychaetes along the Atlantic continental shelf of the Venezuela coasts, where the mixing processes at the edge of the shelf cause nutrients to arise and promote a general increase in zooplankton abundance, this being a food supply for such carnivorous pelagic polychaetes. Regarding the phytophagous Iospilus phalacroides, which was reported to feed on diatoms (Orensanz & Ramirez Citation1973), Fernández-Álamo and Sanvincente-Añorve (Citation2005) explained its distribution in the neritic region according to the higher concentrations of phytoplankton along the Mexican Pacific coasts.
In recent years, studies of such holoplanktonic polychaetes have been undertaken in tropical Atlantic and Pacific areas (Fernández-Álamo & Sanvincente-Añorve Citation2005, Citation2006; Márquez-Rojas et al. Citation2013; Tovar-Faro et al. Citation2013), while no-up-to date information on their distribution in Mediterranean Sea is available for these families of polychates.
Our present data reported in this paper confirm the occurrence of the Typhloscolecidae, with the species Sagitella kowalewskyi and Typhloscolex muelleri, and of the Iospilidae, with the species Phalacrophorus pictus and Iospilus phalacroides, along the Italian coasts, proving the current presence of these species in the Italian fauna and validating the old records. Therefore, the findings of individuals of such pelagic polychaetes in the space of 4 years suggests that these species are a current and real component of the Italian fauna, and not just an occasional record.
The finding of such neglected species confirms that old records represent significant data in the monitoring on biodiversity. Investigation of the “old” species to check whether they are still being found remains a useful task for zoologists interested in examining the state of the fauna and changes in biodiversity (Boero Citation2011).
Pelagic polychaetes with the prostomium without appendages and eyes but ending with a median palpode; all chaetae simple Typhloscolecidae 2
-Pelagic polychaetes with the prostomium with two short palps and two eyes, without other appendages and palpode; all chaetae compound Iospilidae. 4
Prostomium with large transverse ciliated lobes dorsally and ventrally. Typholscolex muelleri
- Large transverse ciliated lobes absent 3
A pair of nuchal organs forming two semi-circular ridges posteriorly curved without projecting lobes; caruncle absent. Sagitella kowalewskii
- A pair of nuchal organs with free posteriorly projecting lobes; a caruncle between them usually present Travisiopsis*
Eversible pharynx unarmed . Iospilus phalacroides
- Eversible pharynx armed with a pair of curved jaws. 5
First 2–3 segments with reduced parapodia without dorsal and ventral cirri. Phalacrophorus pictus
- Up to 11 anterior segments with reduced parapodia without dorsal and ventral cirri. Phalacrophorus uniformis**
Key to families, genera and species
*Species of this genus are not included in Italian fauna.
**Species not included in Italian fauna.
References
- Batistić M, Jasprica N, Carić M, Čalić M, Kovačević V, Garić R, Njire J, Mikuš J, Bobanović-Ćolić S. 2012. Biological evidence of a winter convection event in the South Adriatic: A phytoplankton maximum in the aphotic zone. Continental Shelf Research 44:57–71. doi:10.1016/j.csr.2011.01.004.
- Berkeley E, Berkeley C. 1960. Some further records of pelagic Polychaeta from the Northeast Pacific North of latitude 40° N. and East of longitude 175° W., together with records of Siphonophora, Mollusca, and Tunicata from the same region. Canadian Journal of Zoology 38:787–799. doi:10.1139/z60-083.
- Bilbao M, Palma S, Rozbaczylo N. 2008. First records of pelagic polychaetes in southern Chile (Boca del Guafo - Elefantes channel). Latin American Journal of Aquatic Research 36:129–135. doi:10.3856/vol36.
- Boero F. 2011. New species are welcome, but… what about the old ones? Italian Journal of Zoology 78:1–2. doi:10.1080/11250003.2011.562708.
- Bush W. 1851. Beobachtungen ubre Anatomie und Entwickling einiger wirbellosen Seethiere. Berlin: Aug Hirschawald. 143 pp.
- Castelli A, Abbiati M, Badalamenti F, Bianchi CN, Cantone G, Gambi MC, Giangrande A, Gravina MF, Lanera P, Lardicci C, Somaschini A, Sordino P. 1995. Annelida Polychaeta, Pogonophora, Echiura, Sipuncula. In: Minelli A, Ruffo S, La Posta S, editors. Checklist delle specie della fauna italiana 19. Bologna: Calderini. pp. 1–45.
- Chamberlin RV. 1919. The Annelida Polychaeta. Memoirs Museum of Comparative Zoology of Harvard 48:1–514.
- Dales RP. 1957. Pelagic polychaetes of the Pacific Ocean. Bulletin Scripp Institute of Ocenography University of California 7:99–167.
- Dales RP, Peter G. 1972. A synopsis of the pelagic Polychaeta. Journal of Natural History 6:55–92. doi:10.1080/00222937200770071.
- Day JH. 1967. A monograph on the Polychaeta of Southern Africa. Part 1, Vol. 656. London: Trustees of the British Museum (Natural History). pp. 1–458.
- Day JH. 1975. Zooplancton de la region de Nosy-Bé. 10. The biology of planktonic Polychaeta near Nosy-Bé, Madagascar. Bulletin Scripp Institute of Ocenography University of California 2:99–168.
- Druzhkov NV, Marasaeva EF, Druzhkova EI, Båmstedt U. 2000. New records of the carnivorous pelagic polychaete, Phalacrophorus pictus borealis Reibisch, 1895 in the Arctic Ocean. Sarsia 85:467–469. doi:10.1080/00364827.2000.10414596.
- Ehlers E. 1913. Die Polychaetern-Sammlungen der Deutschen Sud-polar-Expedition 1901-1903. Deutschen-Sudpolar-Expedition 13:397–598.
- Fauchald K. 1977. The polychaete worms: Definition and keys to the orders, families and genera. Natural History Museum Los Angeles County Science Series 5:223–241.
- Fauvel P. 1916. Annélides Polychètes pélagiques provenant des campagnes des yachts Hirondelle et Princesse-Alice. Résultats des Campagnes Scientifiques. Imprimerie De Monaco Fascicule XLVIII:52–75.
- Fauvel P. 1923. Polychètes Errantes. Faune de France, Paris: Lechevalier P. pp. 488.
- Fauvel P. 1936. Polychètes. Resultats du voyage de la Belgica en 1897–1899, Zoologie. Expédition Antarctique Belgica, Anverns Buschmann: 1–46.
- Fernández-Álamo MA. 1987. Distribuciòn y abundancia de los poliquetos pelagicos (Annelida: Polychaeta) en el Golfo de Tehuantepec, México. In: Gómez Aguirre S, Arenas Fuentes V, editors. Contribution Hydrobiologie. México: UNAM. pp. 267–278.
- Fernández-Álamo MA. 2004. Distribution of holoplanktonic typhloscolecids (Annelida-Polychaeta) in the eastern tropical Pacific Ocean. Journal of Plankton Research 26:647–657. doi:10.1093/plankt/fbh063.
- Fernández-Álamo MA. 2006. Composition, abundance and distribution of holoplanktonic polychaetes from the expedition “El Golfo 6311-12” of Scripp Institution of Oceanography. Scientia Marina 70S3:209–215.
- Fernández-Álamo MA. 2009a. Iospilidae Bergström, 1914. In: de León-Gonzáles JA, Bastida-Zavala JR, Carrera-Parra LF, García-Garza ME, Peña-Rivera A, Salazar-Vallejo SI, Solís-Weiss V, editors. Poliquetos de México y Americal Tropical. México: Universitad Autónoma de Nuevo León, Monterrey. pp. 245–249.
- Fernández-Álamo MA. 2009b. Typhloscolecidae, Uljanin, 1878. In: de León-Gonzáles JA, Bastida-Zavala JR, Carrera-Parra LF, García-Garza ME, Peña-Rivera A, Salazar-Vallejo SI, Solís-Weiss V, editors. Poliquetos de México y Americal tropical. México: Universitad Autónoma de Nuevo León, Monterrey. pp. 671–675.
- Fernández-Álamo MA, Sanvincente-Añorve L. 2005. Holoplanktonic polychaetes from the Gulf of Tehuantepec, Mexico. Cahiers Biologie Marine 46:227–239.
- Friedrich H. 1950. Vorkommen und Verbreitung der pelagischen Polychaeten im Atlantischen Ozean. Auf Grund der Fäuge der Meteor Expedition. Kiel Meeresforschunginstitut 7:5–23.
- Gagayev SY, Kosobokova KN. 2012. Species composition and distribution of pelagic polychetes in the Arctic Basin. Zoological Journal 91:1457–1464.
- Jiménez-Cueto S, Suárez-Morales E, Salazar-Vallejo S. 2006. Iospilids (Polychaeta: Iospilidae) from the northwest Caribbean Sea, with observations on reproductive structures. Zootaxa 1211:53–68.
- Lo Bianco S. 1904. Pelagische Tiefseefischerei der “Maja” in der Umgebung von Capri. Beiträge Zoologische des Meeres und seiner Bewohner I. Jena: Gustav Fischer. pp. 91.
- Márquez-Rojas B, Díaz-Díaz O, Balza MA. 2013. Holoplanctonic polychaetes (Annelida: Polychaeta) from Venezuela. Pan-American Journal of Aquatic Science 8:160–165.
- Okuda S. 1938. Polychaeteus annelids from the vicinity of the Mitsui Institute of Marine Biology. Japanese Journal of Zoology 8:75–105.
- Orensanz JM, Ramirez FC. 1973. Taxonomía y distribución de los poliquetos pelágicos del Atlántico Sudoccidental. Boletin de Instituto de Biologia Marina de Universidades Nacionales de Buenos Aires 21:30–57.
- Øresland V, Bray RA. 2005. Parasites and headless chaetognaths in the Indian Ocean. Marine Biology 147:725–734. doi:10.1007/s00227-005-1618-5.
- Øresland V, Pleijel F. 1991. An ectoparasitic typhloscolecid polychaeteon the chaetognath Eukronhia hamaca from the Antartic Peninsula. Marine Biology 108:429–432. doi:10.1007/BF01313652.
- Peres JM. 1954. Contribution a l’etude des Annélides Polychètes de la Mer Méditerranée occidentale. Recueil Des Travaux De La Station Marine D’endoume 13:83–154.
- Pleijel F, Dales RP. 1991. Polychaetes: British Phyllodocoideans, Typhloscolecoideans and Tomopteroideans. Synopses of the British Fauna 45:1–202.
- Reibisch JG. 1895. Die pelagischen Phyllodociden und Typhloscolediden der Plankton-Expedition. Ergebnisse der Deutschen Plankton-Expedition 2:1–63.
- Reish DJ. 1968. A biological survey of Bahia de Los Angeles, Gulf of California, México. II. Benthic polychaetous annelids. Transactions of San Diego Society of Natural History 15:67–106. doi:10.5962/bhl.part.12054.
- Rouse GW, Pleijel F. 2001. Polychaetes. Oxford: Oxford University Press. 353 pp.
- Southern R. 1910. A preliminary note on the Alciopinae, Tomopteridae and Typhloscolecidae from the Atlantic adyacent to Ireland. Annales of Magazine of Natural History of London 8:428–429.
- Støp-Bowitz C. 1948. Polychaeta from the Michael Sars North Atlantic Deep-sea Expedition, 1910. Report Sars National Deep-sea Expedition 8:54–85.
- Støp-Bowitz C. 1951. Polychètes pèlagiques de l’expédition suédoise Antartique 1901-1903. Results Sweden Antartique Expedition 4:1–14.
- Tebble N. 1960. Distribution of pelagic polychaetes in the South Atlantic Ocean. Discovery Report 30:161–300.
- Tebble N. 1962. The distribution of pelagic polychaetes across the North Pacific Ocean. Bulletin of British Museum (Natural History) Zoology 7:371–492.
- Tovar-Faro B, Leocádio M, De Paiva PC. 2013. Distribution of Iospilidae (Annelida) along the eastern Brazilian coast (from Bahia to Rio de Janiero. Latin American Journal Aquatic Research 41:323–334.
- Treadwell AL. 1943. Scientific results of cruise VII of the “Carnegie” during 1928-1929 under command of captain J.P. Ault. Biology 4. Biological results of the last cruise of Carnegie: Polychaetous annelids. Carnegie Institution, Washington 555:29–59.
- Ushakov PV. 1974. Polychaetes of the suborder Phyllodociformia of the Polar Basin and the Northwestern part of the Pacific. Akad Nauk SSR Zoologicheskii Institut Fauna USSR 102:183–250. (translated from the Russian by the Israel Program for Scientific Translation Jerusalem).
- Viguier C. 1886. Études sue les animaux inférieurs de la baie d’Alger. Archives Zoologique Expédition Général Paris 4:347–442.