Abstract
The paper focuses on the long-term taxonomic composition and distribution of the shallow-water sponge fauna from the meromictic–anchialine coastal basin Faro Lake (Southern Italy), comparing recent qualitative field data with literature data over a 50-year period. The Faro Lake shallow water currently hosts 24 conspicuous species of Porifera belonging to 21 genera, 18 families, eight orders, three subclasses and two classes, i.e. Demospongiae (23) and Calcarea (one). The comparison between the present and past status of the sponge fauna showed a high turnover, with 15 new colonizers and only nine persistent species. Thirteen species reported in the literature are missing, suggesting the occurrence of remarkable changes in the faunal composition during the last 50 years. The analysis of the geographic distribution of each species allowed us to outline the prevalent North Atlantic affinity of the sponge community. Worthy of note is the new record of the alien calcareous sponge Paraleucilla magna of cryptogenic origin.
Introduction
Long-term variations of biodiversity values could be indicative of environmental and/or climatic changes. The availability of data focusing on the Mediterranean Sea from the past is basic to perform comparisons for determining possible variations in faunal composition and species richness that may have caused loss or turnover of biodiversity values (Gaino & Pronzato Citation1989; Gaino et al. Citation1992; Pronzato Citation1999; Cerrano et al. Citation2000; Occhipinti-Ambrogi & Savini Citation2003; Bianchi & Morri Citation2004; Garrabou et al. Citation2009; Por Citation2009; Bertolino et al. Citation2012, Citation2014a, Citation2016; Pronzato et al. Citation2012; Duarte et al. Citation2013; Sukhotin & Berger Citation2013; Bianchi et al. Citation2014a,b).
Enclosed salty coastal basins represent excellent models of aquatic systems to test hypotheses on possible temporal variations of sponge fauna in the Mediterranean Sea.
We have chosen the Faro Lake meromictic–anchialine basin to evaluate a qualitative long-term change in the benthic community by comparing the present sponge fauna with that described by Labate and Arena (Citation1964) more than 50 years ago. To achieve this aim we have repeated, as closely as possible, the sampling plan performed in the first investigation.
Study area
Faro Lake (38°16ʹ07ʹʹN, 15°38ʹ13ʹʹE) is a small (0.263 km2) temperate coastal basin within the Natural Reserve of Cape Peloro (NE Sicily; ). This basin, despite its limited size, is the deepest coastal lake in Italy, reaching a maximum depth of 29 m.
Figure 1. A–C, map of Faro Lake in North–East Sicily at different magnifications (arrows); D, sampling sites in 2013 as tentative replicas of the 1957–63 sampling plan (black dots). Sampling sites: 3 and 7 along the rocky coast near the entrance of the Margi Canal joining the Lake to the Ganzirri Lake; 8 at the entrance of the Eastern canal joining the Lake to the Messina Strait‐Ionian Sea; 9 at the entrance of the Northern canal joining the Lake to the Tyrrhenian Sea. Additional stations scattered in shallow water (0.1–3 m) of the Lake are indicated by 1, 2, 4, 5, 6, and 10. Isobathic spacing = 3 m.
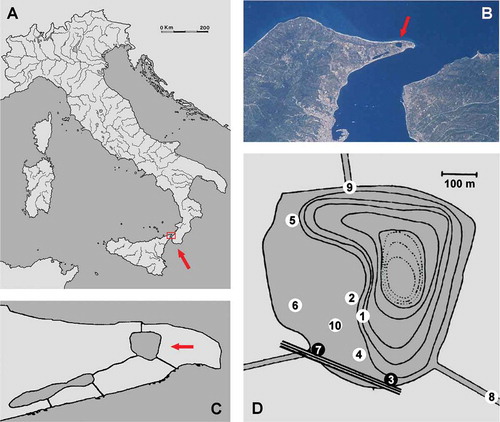
The basin is connected to the sea by two small, narrow, shallow canals (less than 1 m deep), namely a northern canal opened in 1960 on the Tyrrhenian side, notably silty and lentic for most of the year, and a north-eastern lotic canal with permanent water connected to the Messina Strait. A third north-western canal (Margi Canal) is connected to the neighbouring Ganzirri Lake ().
Faro Lake is a rare example of a meromictic basin characterized by a persistent physico-chemical stratification of the deeper water column, matching in part the definition of anchialine system. The water body is almost permanently stratified, the first 10–15 m being well mixed and the deeper layer having much lower water movement. Salinity ranges from 26 to 36 PSU, and temperature from 10 to 30°C in the upper layer (10–15 m). Below 15 m depth, both these parameters become stable with a salinity ca. 38 PSU and temperature ca. 15°C. Similarly, pH is 7.9–8.6 in the upper layer, and 7.0–7.4 below 20 m. The surface water redox potential varies from +120 to +190 mV and from −300 to −360 mV in the deeper water (Abruzzese & Genovese Citation1952; Saccà et al. Citation2008). Both water column and sediments are classified as mesotrophic and support significant microbial productivity (Maugeri et al. Citation2001; Leonardi et al. Citation2009).
The hydrodynamic regime of this coastal basin with quite constant water level is conditioned by the tidal regime of the Messina Strait (). Water mass exchanges are driven by the tide of the strait through the canals. Environmental conditions of the Messina Strait are peculiar (Bôhm et al. Citation1987; Cescon et al. Citation1997). Between Taormina Cape and Messina the surface layers of the strait are significantly colder than those observed in its boundaries, as a consequence of upwelling phenomena bringing deep water to the surface. In the strait, the water surface temperatures in summer are, on average, 4–10°C lower than in the surrounding water; the intense and alternating current, the low temperature and the abundance of nitrogen and phosphorus salts transported to the surface from deep water determine the availability of a large amount of organic matter used both by pelagic and benthic coastal organisms. All this determines an ecological rearrangement in the shallow-water coastal communities of the strait, tending to simulate an “Atlantic” type situation (see Drew Citation1974; Bianchi Citation2004).
Materials and methods
Conspicuous photophilic sponges were collected by snorkelling (between February and August 2013) from hard substrata in shallow water (0–5 m of depth) of Faro Lake (FL) mainly focusing as well as possible on replicas of previous collections by Labate and Arena (Citation1964) ( and ).
Replicas of sampling sites ( and ) are as follows: (a) FL3, along the coast facing south (arches of the viaduct, 0.1–0.3 m of depth); (b) FL7, near the entrance of the Margi Canal, north-western canal to the Ganzirri Lake (0.1–0.3 m of depth).
An additional eight shallow-water stations (0.1–5 m of depth) were sampled in the lake (FL1, FL2, FL4, FL5, FL6 and FL10), and in the two canals joining the lake with the Messina Strait (FL8) and the Tyrrhenian Sea (FL9), respectively (). More precise samplings performed by Labate and Arena (Citation1964) were replicated in stations FL3 and FL7 (Figures 1 and 2). Other previously sampled sites were not replicated because the latter authors did not provide their precise position in the lake. In any case, the present survey does not claim quantitative sampling, but simply aims to verify a qualitative sponge fauna long-term change.
The dry preserved sponges were observed to characterize macrotraits such as growth form, consistency, colour, surface traits, distribution and shape of inhalant and exhalant apertures. They were studied by light microscopy (LM) and/or scanning electron microscopy (SEMVega3 TESCAN, LMU type) by standard methods. Dissected sponges were examined by LM to analyse the skeletal arrangement in the ectosome and choanosome and the spicule morphotraits. The skeleton was processed by dissolution of the organic matter in boiling 65% nitric acid, suspended in alcohol and dropped onto slides for LM following standard methods to characterize microtraits of megascleres and microscleres. Taxonomic decisions, while keeping in mind the Porifera checklist of the Italian seas (Pansini & Longo Citation2008) and the Fauna d’Italia (Pansini et al. Citation2011), refer to the World Porifera Database (Van Soest et al. Citation2016) just updated after Morrow and Cárdenas (Citation2015).
For all the species recorded in both surveys the geographic range was considered, in order to highlight possible variation of geographic affinity (e.g. Lusitanic vs. Senegalese) of past vs. present fauna ( and II). Data on geographic range are from Van Soest et al. (Citation2016) and Pulitzer–Finali’s unpublished archive.
Table I. List of Porifera species (in alphabetical order) recorded in the Faro Lake over 50 years. Alien species and Mediterranean endemics are indicated by A and by asterisks (*), respectively.
Results
Faro Lake presently harbours 24 species in 10 sites (), 23 of which are Demospongiae, and one Calcarea. Among Demospongiae the three currently accepted subclasses sensu Morrow and Cárdenas (Citation2015) – Heteroscleromorpha, Keratosa and Verongimorpha – are represented by eight orders, 18 families and 21 genera. The order Poecilosclerida is the most represented with six species (six genera, six families). The orders Haplosclerida (three genera, two families) and Suberitida (three genera, two families) are represented by four species each. The orders Axinellida (two genera, two families), Bubarida (two genera, one family), and Dictyoceratida (two genera, two families) are represented by two species each, while the orders Chondrosida, Tethyida and Tetractinellida are each represented by one species only. The class Calcarea is represented only by Paraleucilla magna Klautau, Monteiro & Borojevic, 2004, an alien species recently introduced in the Mediterranean Sea (Longo et al. Citation2007; Longo & Pronzato Citation2011; Bertolino et al. Citation2014b).
Some species, i.e. Haliclona (Reniera) cf. cinerea, Haliclona sp., Halichondria (Halichondria) bowerbanki, Halichondria (Halichondria) panicea, Hymeniacidon perlevis and Suberites massa, were observed to form flourishing populations (data on their abundance are out of the aim of this paper).
The sponge fauna reported by Labate and Arena (Citation1964) refers to samples collected in not-well-defined shallow-water sites in two surveys (1957, 1963) on large rocky bottom areas of the lake (). The number of species recorded in that period was 22 (), 10 of which were considered “more abundant”, as reported in with no further indications. These species belong to 13 orders, 14 families, and 19 genera ().
Figure 2. Faro Lake. Sampling sites in the original illustration of 1957–63 (modified from Labate and Arena Citation1964). The 10 species evidenced by symbols are here interpreted as the most abundant/conspicuous in comparison with the lists reported in –. The indication of 19 spp. is not reported or better explained in the original paper. We report these historical data as indications without any concrete abundance validity. Isobathic spacing = 3 m.
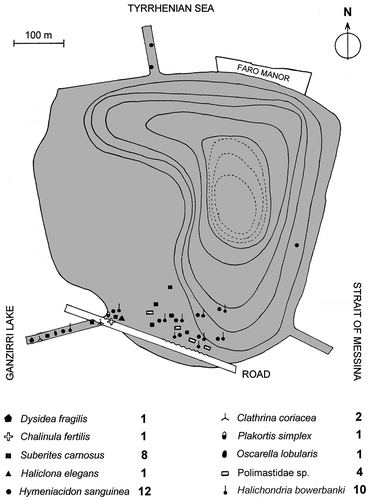
The number of species shared by both surveys (persistent) is nine (1957–1963 vs. 2013; ). There were 13 species no longer recorded (missing), while there were 15 new records (new colonizers) with a turnover rate of abundantly over 50% of the species ().
As to the biogeographic affinity of the three groups of species – i.e. missing, persistent and new colonizers – the majority of them show an Atlantic–Mediterranean biogeographic pattern. The 13 missing taxa are all recorded in the Mediterranean Sea and the North Atlantic. Eight of them are reported also from Senegal and the Gulf of Guinea (); one species is reported also from the Red Sea, one from the Arctic and one from the Pacific.
Table II. Geographic range of the species of Porifera recorded in the Faro Lake during the last 50 years.
Among the nine persistent taxa, all previously recorded in the Mediterranean Sea and the North Atlantic, five are reported also from West Africa, Senegal and the Gulf of Guinea (). One species (Chalinula renieroides) is reported also from the Caribbean, and one (Dysidea fragilis) also from the Arctic. Two species, i.e. Haliclona (Reniera) cf. cinerea and Halichondria (Halichondria) panicea, are known also from the Arctic and the Pacific, and one (Hymeniacidon perlevis) also from the Arctic, the Pacific and the Caribbean ().
Among the recent colonizers, one species (Paraleucilla magna), recently recorded in the Mediterranean Sea, is a possible tropical alien of cryptogenic origin. All the other species belong to the Mediterranean and North Atlantic fauna and only three are Mediterranean endemics, i.e. Dictyonella marsilii, Sarcotragus fasciculatus and Hymedesmia (Hymedesmia) castanea. Six of them are reported also from Senegal and the Guinea Gulf ().
Summarizing, most of the persistent species (living in Faro Lake for more than 50 years) have a geographic range that exceeds the Atlantic–Mediterranean area (). The missing species are prevalently North Atlantic–Mediterranean, with more than the 60% of them also recorded in Senegalese water (). The geographic range of colonizer species is prevalently North Atlantic–Mediterranean with a lower percentage (less than 50%) also recorded in Senegalese water ().
Discussion
The Faro Lake shallow water currently hosts 24 species of Porifera belonging to two classes, three subclasses, eight orders, 18 families and 21 genera. The disappearance of 13 species and the presence of 15 new colonizers testify to a notable change in the lake sponge fauna in a period of ca. 50 years. Only nine species are persistent ( and II). Among the new colonizers, one is an invasive alien species of cryptogenic origin.
All of the species present in the lake belong to the Mediterranean and North-East Atlantic sponge fauna. The prevalently North Atlantic biogeographic affinity of the sponge species living in the Faro Lake can be related to an “Atlantic-type condition”, due to the presence of relatively colder water (4–10°C colder than the neighbouring water not affected by upwelling phenomena) influencing the shallow-water coastal communities of the Messina Strait (between Taormina Cape and Messina). Indeed, many typically Atlantic species, e.g. kelp, although occasionally present in other areas of the Mediterranean Sea, are able to form well-structured underwater forests only in the Messina Strait (Drew Citation1974). Moreover, our data match the biogeographic analysis of the Italian seas by Bianchi (Citation2004) that referred to the Messina Strait as “microsector rich in Atlantic Pliocene relics”.
As for the geographical distribution, we take into account the 31 taxa identified at the species level in both surveys (1957–1963 and 2013), subdivided into three groups as reported in . We can observe the South Atlantic extension of the geographic range in 8/9 of the missing species, 5/9 of the persistent and 7/13 of the colonizers (including the cryptogenic one). The percentage of species with South Atlantic–Senegalese affinity seems to have decreased over time: 88.8% for the missing species, 55.5% for the persistent species and 53.8% for the new colonizers ().
Only the group of new colonizers counts three species endemic to the Mediterranean Sea.
Among the nine species whose distribution exceeds the Mediterranean and North-East Atlantic area, the majority (seven) are reported also from Arctic coastal water [Haliclona (Reniera) cf. cinerea, Myxilla (Myxilla) rosacea, Halichondria (Halichondria) panicea, Hymeniacidon perlevis, Suberites carnosus, Tethya aurantium and Dysidea fragilis], five also from the Indo-Pacific [Haliclona (Reniera) cf. cinerea, Ciocalypta penicillus, Halichondria (Halichondria) panicea, Hymeniacidon perlevis and Tedania (Tedania) anhelans], two species also from the Caribbean (Chalinula renieroides and Hymeniacidon perlevis) and one species also from the Red Sea (Spirastrella cunctatrix). Once again these data confirm the strengthening of the North-East Atlantic affinity of the Faro Lake sponge fauna.
The highest number (5/9 = 55.5%) of extra-Atlantic–Mediterranean species is found among the persistent group, while there are 3/9 (33.3%) among the missing group and 3/13 (20.1%) among the colonizers. Also, in this case, the data are in agreement with the high climatic tolerance of widespread sponge species ().
Taking into account the data concerning the missing species (13) and the new colonizers (15), values of biodiversity loss and renewal are high and similar in species number.
The presence of one alien species of cryptogenic origin confirms the Faro Lake as a hotspot for the entry and spread of alien marine species in the Central Mediterranean (see Cosentino et al. Citation2009). By now a total of 11 non-indigenous benthic invertebrates are considered to be established in this coastal basin (Zenetos et al. Citation2010; Giangrande et al. Citation2012; present paper).
There are not many data on the sponge faunal assemblage in the Faro Lake boundaries. Similar data are reported by Mastrototaro et al. (Citation2010) but unfortunately they are not comparable with ours, being focused on very deep, open-sea sponge fauna. In any case, this sponge assemblage also shows a North Atlantic affinity.
Conclusive remarks
Faro Lake is a stressed, confined, highly variable environment. Its abiotic parameters fluctuate greatly depending on the season, and can be very different from year to year.
This ecological unpredictability determines the presence of a highly tolerant sponge fauna subject to a high turnover. From a biogeographic point of view, no particular faunistic changes were evidenced; both missing and colonizer species show a prevalent North Atlantic–Mediterranean biogeographic pattern, with an evident southern extension to the warmer area of Eastern Tropical Atlantic (West African coast, Senegal and Guinea Gulf). This is true more for the past than for the present species. The persistent species are those with lower or no biogeographic significance, being the more tolerant and widespread.
The presence of a first-reported alien sponge species of cryptogenic origin (Paraleucilla magna) weakly corroborates the hypothesis of a possible tendency to tropicalization. A relationship between the mussel culture in Faro Lake and the introduction of P. magna, with the species being invasive in the mussel farms of Taranto (Longo et al. Citation2007) and in other areas of the Mediterranean Sea (Bertolino et al. Citation2014b), is highly probable. In any case, a possible explanation of its appearance almost simultaneously in Brazilian waters and in various Mediterranean locations (Bertolino et al. Citation2014b) is problematic.
As to a possible influence of global climatic change, no significant evidence was detected. Comparing the present situation with that of 50 years ago, only a weakening of the Southern affinity for the current sponge assemblage is noticeable. Moreover, the persistent species group seems to reflect a higher tolerance for warm water.
Considering the process of faunal renewal in this relatively isolated habitat, it is still useful to evaluate the replacement of species (extinction vs. colonization) in Faro Lake based on the MacArthur and Wilson theory (Citation1967). In our case the inland basin is small, close to the sea, and not completely isolated because of the presence of narrow, temporary canals acting as corridors to the open sea and potential suppliers of new colonizers from the adjacent seas. Moreover, the environmental conditions in this confined water body are largely variable and different from the surrounding open seawater.
Following the “equilibrium theory”, the sponge fauna of Faro Lake, despite the high percentage of species extinction (59.1%) and colonization (62.5%), seems numerically quite stationary. The changes in the number of taxa are very low, almost at the species level in the present survey, i.e. two more species (24 vs. 22 = 8.3%); three more genera (22 vs. 19 = 13.6%); four more families (14 vs. 18 = 22.2%); three fewer orders (13 vs. 10 = 23.1%). A slight increasing trend of taxon richness seems to be evident. These data, although focusing on an aquatic biotope, agree with MacArthur and Wilson (Citation1967). The biotic potential of Faro Lake, in terms of sponges, does not seem to overcome the maximum of 25 conspicuous species.
The persistence of this species richness in Faro Lake, as regards the sponge fauna, despite the small size of this vulnerable site and the notably increased anthropic impact along its coast (), underlines a long-term faunal equilibrium. After over 50 years, a shifting from Senegalese to North Atlantic biogeographic affinity seems to be evidenced.
Figure 3. Faro Lake landscape through time. A, view from West to East, with the Calabria coast in the background (ca. 1900); B, ground view from the Southern road, with the Western coast in the background (ca. 1850); C, the same view of A after 50 years (ca. 1950); D, aerial view (northwards) of the Faro Lake (present day). The long term shellfish farming is evident in all images. Human settlement was not negligible over 150 years. See also Figure 2 for orientation.

Acknowledgements
Maria Vittoria Marra is currently a PhD student at the National University of Ireland Galway and is funded through a Tony Ryan fellowship (NUI Galway). The authors want to thank the “Provincia Regionale di Messina for permissions, the “Farau” Mussel farm for facilities and Mr Sergio De Matteo (Università degli Studi di Messina) for logistical support during samplings.
Additional information
Funding
References
- Abruzzese D, Genovese S. 1952. Osservazioni geomorfologiche e fisico-chimiche sui laghi di Ganzirri e Faro. Bollettino di Pesca, Piscicoltura e Idrobiologia 28:75–92.
- Bertolino M, Betti F, Bo M, Cattaneo-Vietti R, Pansini M, Romero J, Bavestrello G. 2016. Changes and stability of a Mediterranean hard bottom benthic community over 25 years. Journal of the Marine Biological Association of the United Kingdom 96:341–350. DOI:10.1017/S0025315415001186.
- Bertolino M, Calcinai B, Capellacci S, Cerrano C, Lafratta A, Pansini M, Penna A, Bavestrello G. 2012. Posidonia oceanica meadows as sponge spicule traps. Italian Journal of Zoology 79:231–238. DOI:10.1080/11250003.2011.614641.
- Bertolino M, Calcinai B, Cattaneo-Vietti R, Cerrano C, Lafratta A, Pansini M, Pica D, Bavestrello G. 2014a. Stability of the sponge assemblage of Mediterranean coralligenous concretions along a millennial time span. Marine Ecology 35:149–158. DOI:10.1111/maec.2014.35.issue-2.
- Bertolino M, Longo C, Marra MV, Corriero G, Pansini M. 2014b. Paraleucilla magna Klautau et al., 2004 (Porifera, Calcarea), an alien species extending its range in the Mediterranean Sea. Biologia Marina Mediterranea 21:109–110.
- Bianchi CN. 2004. Proposta di suddivisione dei mari italiani in settori biogeografici. Notiziario della Società Italiana di Biologia Marina 46:57–59.
- Bianchi CN, Corsini-Foka M, Morri C, Zenetos A. 2014a. Thirty years after – dramatic change in the coastal marine habitats of Kos Island (Greece), 1981–2013. Mediterranean Marine Science 15:482–497. DOI:10.12681/mms.678.
- Bianchi CN, Morri C. 2004. Climate change and biological response in Mediterranean Sea ecosystems: A need for broad-scale and long-term research. Ocean Challenge 13:32–36.
- Bianchi CN, Morri C, Pronzato R. 2014b. The other side of rarity: Recent habitat expansion and increased abundance of the horny sponge Ircinia retidermata (Demospongiae: Dictyoceratida) in the southeast Aegean. Italian Journal of Zoology 81:564–570. DOI:10.1080/11250003.2014.920929.
- Bôhm E, Magazzù G, Wald L, Zoccolotti ML. 1987. Coastal currents on the Sicilian shelf south of Messina. Oceanologica Acta 10:137–142.
- Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F. 2000. A catastrophic mass–mortality episode of gorgonians and other organisms in the Ligurian Sea (North–western Mediterranean), summer 1999. Ecology Letters 3:284–293. DOI:10.1046/j.1461-0248.2000.00152.x.
- Cescon B, Azzaro F, Creazzo S, Decembrini F, Magazzù G. 1997. Processes affecting upwelling and primary productivity of the Messina Straits. Bollettino di Geofisica Teorica e Applicata 38:137–148.
- Cosentino A, Giacobbe S, Potoschi A. 2009. The CSI of the Faro coastal lake (Messina): A natural observatory for the incoming of marine alien species. Biologia Marina Mediterranea 16:132–133.
- Drew EA. 1974. An ecological study of Laminaria ochroleuca Pyl. growing below 50 metres in the straits of Messina. Journal of Experimental Marine Biology and Ecology 15:11–24. DOI:10.1016/0022-0981(74)90059-8.
- Duarte L, Viejo RM, Martínez B, deCastro M, Gómez-Gesteira M, Gallardo T. 2013. Recent and historical range shifts of two canopy-forming seaweeds in North Spain and the link with trends in sea surface temperature. Acta Oecologica 51:1–10. DOI:10.1016/j.actao.2013.05.002.
- Gaino E, Pronzato R. 1989. Ultrastructural evidence of bacterial damage to Spongia officinalis fibres (Porifera, Demospongiae). Diseases of Aquatic Organisms 6:67–74. DOI:10.3354/dao006067.
- Gaino E, Pronzato R, Corriero G, Buffa P. 1992. Mortality of commercial sponges: Incidence in two Mediterranean areas. Bolletino di Zoologia 59:79–85. DOI:10.1080/11250009209386652.
- Garrabou J, Coma R, Bensoussan N, Bally M, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido N, Torrents O, Zabala M, Zuberer F, Cerrano C. 2009. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Global Change Biology 15:1090–1103. DOI:10.1111/gcb.2009.15.issue-5.
- Giangrande A, Cosentino A, Presti CL, Licciano M. 2012. Sabellidae (Annelida) from the Faro coastal lake (Messina, Ionian Sea), with the first record of the invasive species Branchiomma bairdi along the Italian coast. Mediterranean Marine Science 13:283–293. DOI:10.12681/mms.310.
- Labate M, Arena P. 1964. La fauna dei Poriferi nei laghi di Ganzirri e Faro (Messina). Archivio Zoologico Italiano 49:249–280.
- Leonardi M, Azzaro F, Azzaro M, Caruso G, Mancuso M, Monticelli LS, Maimone G, La Ferla R, Raffa F, Zaccone R. 2009. A multidisciplinary study of the Cape Peloro brackish area (Messina, Italy): Characterisation of trophic conditions, microbial abundances and activities. Marine Ecology 30:33–42. DOI:10.1111/mae.2009.30.issue-s1.
- Longo C, Mastrototaro F, Corriero G. 2007. Occurrence of Paraleucilla magna (Porifera: Calcarea) in the Mediterranean Sea. Journal of the Marine Biological Association of the UK 87:1749–1755. DOI:10.1017/S0025315407057748.
- Longo C, Pronzato R. 2011. Class Calcarea. In: Pansini M, Manconi R, Pronzato R, editors. Porifera I. Calcarea, Demospongiae, Hexactinellida, Homoscleromorpha. Fauna d’Italia. Vol. 46. Bologna: Calderini–Il Sole 24 Ore. pp. 117–244.
- MacArthur RH, Wilson EO. 1967. The theory of the Island biogeography. Princeton, NJ: Princeton University Press.
- Mastrototaro F, D’Onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A. 2010. Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Research Part II: Topical Studies in Oceanography 57:412–430. DOI:10.1016/j.dsr2.2009.08.021.
- Maugeri TL, Gugliandolo C, Caccamo D, La Rosa T. 2001. Biomass and productivity of microbial communities in the brackish environments of Ganzirri and Faro (Messina). Biologia Marina Mediterranea 8:316–321.
- Morrow C, Cárdenas P. 2015. Proposal for a revised classification of the Demospongiae (Porifera). Frontiers in Zoology 12:1–27. DOI:10.1186/s12983-015-0099-8.
- Occhipinti-Ambrogi A, Savini D. 2003. Biological invasions as a component of global change in stressed marine ecosystems. Marine Pollution Bulletin 46:542–551. DOI:10.1016/S0025-326X(02)00363-6.
- Pansini M, Longo C. 2008. Porifera. Biologia Marina Mediterranea 15(suppl.):42–66.
- Pansini M, Manconi R, Pronzato R, editors. 2011. Porifera I. Calcarea, Demospongiae, Hexactinellida, Homoscleromorpha. Fauna d’Italia. Vol. 46. Bologna: Calderini–Il Sole 24 Ore.
- Por FD. 2009. Tethys returns to the Mediterranean: Success and limits of tropical re-colonization. BioRisk 3:5–19. DOI:10.3897/biorisk.3.30.
- Pronzato R. 1999. Sponge fishing, disease and farming in the Mediterranean Sea. Aquatic Conservation: Marine and Freshwater Ecosystems 9:485–493. DOI:10.1002/(ISSN)1099-0755.
- Pronzato R, Ledda FD, Manconi R. 2012. Mediterranean horny sponges: How to drive a never-ending story of exploitation toward a suitable management and conservation? In: Lucas-Borja ME, editor. Endangered species: Habitat, protection and ecological significance. Hauppauge, NY: Nova Science Publishers. pp. 77–108.
- Saccà A, Guglielmo L, Bruni V. 2008. Vertical and temporal microbial community patterns in a meromictic coastal lake influenced by the straits of Messina upwelling system. Hydrobiologia 600:89–104. DOI:10.1007/s10750-007-9179-x.
- Sukhotin A, Berger V. 2013. Long-term monitoring studies as a powerful tool in marine ecosystem research. Hydrobiologia 706:1–9. DOI:10.1007/s10750-013-1456-2.
- Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, De Voogd NJ, Alvarez De Glasby B, Hajdu E, Pisera AB, Manconi R, Schönberg C, Janussen D, Tabachnick KR, Klautau M, Picton B, Kelly M, Vacelet J, Dohrmann M, Díaz MC, Cárdenas P 2016. World Porifera database. Available: http://www.marinespecies.org/porifera. Accessed Jun 2016 21.
- Zenetos A, Gofas S, Verlaque M, Çinar ME, García Raso JE, Bianchi CN, Morri C, Azzurro E, Bilecenoglu M, Froglia C, Siokou I, Violanti D, Sfriso A, San Martín Peral G, Giangrande A, Katagan T, Ballesteros E, Ramos-Esplá AA, Mastrototaro F, Ocaña O, Zingone A, Gambi MC, Streftaris N. 2010. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterranean Marine Science 11:381–493. DOI:10.12681/mms.87.
