Abstract
Aedes koreicus (Edwards) (Diptera: Culicidae) is an invasive mosquito species and potential vector of pathogens which has recently colonised a large part of northeastern Italy and other European countries. Several species of cyclopoid copepods are natural predators of mosquito larvae and can be useful biological control agents in artificial containers used as breeding sites by Aedes mosquitoes. However, to ensure behavioural efficiency of these agents, and to avoid the introduction of non-native species, predatory copepods should be selected from the local fauna. In this study, we evaluated the predation efficiency under laboratory conditions of two locally bred populations of cyclopoid copepod species, Macrocyclops albidus (Jurine, 1820) and Mesocyclops leuckarti (Claus, 1857) (Copepoda: Cyclopoida), which are two of the most common European species of cyclopoid copepods, against Ae. koreicus larvae. Predation experiments were also conducted with Aedes (Stegomyia) albopictus (Skuse) as a “reference”. In each predation test, one adult female copepod was placed with 50 first instar larvae of a single mosquito species in a Petri dish filled with 10 mL of water. After 24 hours, the mean number (± standard error) of first instar larvae killed by one M. albidus female was 18.6 ± 1.3 Ae. koreicus and 20.9 ± 1.3 Ae. albopictus, and the mean number killed by one M. leuckarti female was 25.8 ± 2.8 Ae. koreicus and 36.1 ± 4.2 Ae. albopictus. In addition, M. leuckarti was slighly less effective against Ae. koreicus than against Ae. albopictus after 48 hours, probably because first instar larvae of Ae. koreicus were larger than first instar larvae of Ae. albopictus. Our findings indicate for the first time that copepods are effective predators of first instar larvae of Ae. koreicus.
Introduction
In recent decades, non-native species of Aedes mosquitoes (Diptera: Culicidae) such as Aedes (Stegomyia) albopictus (Skuse) have colonised several areas of Europe. Among these, Aedes (Hulecoeteomyia) koreicus (Edwards) is a species native to Korea, Japan, northeastern China and eastern Russia (Tanaka et al. Citation1979). It was first detected in Belgium in 2008 and in Italy in 2011 (Capelli et al. Citation2011; Versteirt et al. Citation2012). Having colonised a large part of northeastern Italy, Ae. koreicus was also observed in Switzerland and western Russia in 2013, in southern Germany in 2015, and in Hungary in 2016 (Bezzhonova et al. Citation2014; Montarsi et al. Citation2015b; Suter et al. Citation2015; Werner et al. Citation2015; Kurucz et al. Citation2016). It has been suggested that Ae. koreicus transmits the Japanese encephalitis virus (Miles Citation1964) and Dirofilaria immitis, the agent of the dog heartworm (Montarsi et al. Citation2015a). However, despite its rapid expansion and potential role as vector of pathogens, information about the ecology and the control of this species is lacking. Aedes koreicus is a container-breeding mosquito, and larvae are mainly found in medium- to large-sized artificial containers permanently filled with organic-rich water, such as discarded tires, drums, concrete containers and construction equipment (Baldacchino et al. Citation2017). In addition, field observations show that this species is cold tolerant since it has been collected up to 1000 m above sea level (asl) (Baldacchino et al. Citation2017).
Permanent water containers used by Aedes mosquitoes can be treated by larvicidal products (e.g. Bacillus thuringiensis var. israelensis and insect growth regulators) as well as natural predators of mosquito larvae (Baldacchino et al. Citation2015). Cyclopoid copepods (Copepoda: Cyclopoida; hereafter referred to as copepods) are the most effective invertebrate mosquito predators (Veronesi et al. Citation2015). Because they survive for several months in artificial containers, they provide long-lasting control of mosquito populations (Marten & Reid Citation2007; Veronesi et al. Citation2015). Copepods prey mainly on first and second instar mosquito larvae, and the largest copepod species (> 1.4 mm body length) can kill up to 30–40 Aedes larvae per day (Marten & Reid Citation2007). Because copepods are usually numerically abundant in water bodies and mass production is simple and relatively inexpensive, they are considered cost-effective biological control agents (Lazaro et al. Citation2015). Even if several copepod species can survive droughts by special resting stages and are among the first organisms to recolonise temporary water after rainfalls (Bruno et al. Citation2001; Frisch & Green Citation2007; Kroeger et al. Citation2014), most of them are prone to desiccation and can only be used in permanent water containers.
To date, the predatory efficiency of about 50 copepod species belonging to 15 genera has been tested mostly in the laboratory, but also in the field, mainly against Aedes and Culex mosquitoes (Marten & Reid Citation2007; Veronesi et al. Citation2015). Two taxa of cyclopoids have proved to be particularly effective for controlling Aedes species such as Ae. albopictus: Macrocyclops albidus (Jurine, 1820) and several species of Mesocyclops (Marten & Reid Citation2007). While several laboratory and field studies have already been carried out on the use of M. albidus against Ae. albopictus in the United States, Brazil and, in one study, Italy (Marten Citation1990; Santos & Andrade Citation1997; Rey et al. Citation2004; Veronesi et al. Citation2015), no copepod species had been previously tested against Ae. koreicus larvae.
In order to avoid the introduction of non-native copepod species as control agents, two of the most common European copepod species, M. albidus and Mesocyclops leuckarti (Claus, 1857), were selected from the local fauna. Since different populations of the same copepod species can differ in predation behaviour (Marten & Reid Citation2007), these copepod species were mass-reared under laboratory conditions to assess their predation efficiency. Macrocyclops albidus and M. leuckarti live in temperate climatic regions: in Italy, M. leuckarti is most common below 500 m asl, whereas M. albidus has been found at altitudes of up to 1000 m asl (Stoch Citation2004). Both these copepods can survive in a wide range of water temperatures (0–40°C) and pH values (4.5–8), and in hypoxic conditions (Tinson & Laybourn-Parry Citation1985; Williams-Howze Citation1997; Nilssen & Waervagen Citation2000; Marten & Reid Citation2007). These ecological features make these two species suitable to use for biological control.
In the present study, we aimed to evaluate for the first time the predation efficiency of copepods against Ae. koreicus under laboratory conditions. Predation experiments were also conducted with Ae. albopictus larvae as a “reference” since copepods have already proven to be effective predators against this species. Thus, we assessed the predation efficiency of the two selected copepod populations of M. albidus and M. leuckarti, and compared their efficiency against Ae. koreicus and Ae. albopictus. We hypothesised that (i) both copepod species would be effective predators on the first instar larvae of Ae. koreicus, and (ii) their predation efficiency would be different according to the Aedes species larvae because of the size difference (i.e. Ae. koreicus larvae are larger than Ae. albopictus larvae).
Materials and methods
Copepod rearing
Macrocyclops albidus was collected from the littoral zone of Levico Lake in the province of Trento, Italy (46°00ʹ52.72″N, 11°16ʹ40.89″E) using a 100-μm-mesh plankton net (). In the laboratory, ovigerous females were isolated using a stereomicroscope and reared individually in 200-mL plastic cups filled with 50 mL well water, 50 mL protozoan culture (see below) and 1 g/L ground cat food (Friskies® adult, Nestlé Italiana S.p.A., Assago, Italy). The cups were kept in natural light conditions and placed in a thermostatic bath with a progressively increasing temperature (from 12 to 20°C) for 2 weeks to acclimatise copepods to room temperature. After the nauplius larvae hatched, the females were killed, dissected and mounted on permanent slides for identification to the species level following Dussart and Defaye (Citation2001). Nauplii from M. albidus were kept aside to start the cultures. Mesocyclops leuckarti from Crevalcore in the province of Bologna, Italy (44°43ʹ00″N, 11°09ʹ00″E) was commercially available and purchased online (http://www.eugea.it/). Subsequently, cultures of M. albidus and M. leuckarti were established to provide the large number of individuals needed for the experiments.
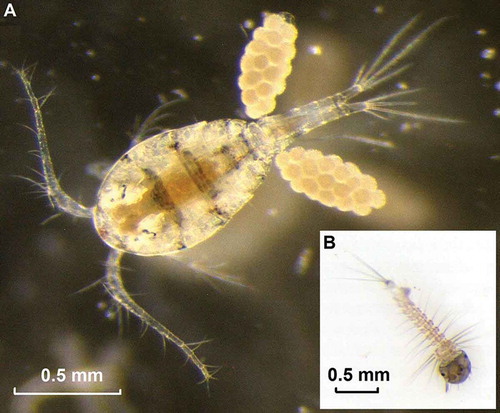
Figure 1. A, Ovigerous female of M. albidus, and B, first instar larva of Ae. koreicus (Photo: F. Baldacchino.)
The copepods were mass-reared under laboratory conditions according to the available literature (Suarez et al. Citation1992; Marten & Reid Citation2007). The cultures were maintained in 40-L tanks filled with 20 L of water pumped from a deep (40 m) well, and kept at room temperature and in natural light conditions. The copepods were fed weekly with pure protozoan cultures of commercially available Chilomonas paramecium and Paramecium caudatum (http://www.sciento.co.uk/). The former protozoan is the preferred food for naupliar and first copepod stages, whereas the latter protozoan is the preferred food for the last copepod stages and mature copepods. The protozoan cultures were maintained in semi-darkness at room temperature in 1-L glass jars and fed weekly with 1 g/L of ground cat food.
Mosquito rearing
Ae. albopictus and Ae. koreicus eggs were obtained from laboratory colonies established from mosquitoes collected in the field. Aedes albopictus and Ae. koreicus were originally collected in Trento, Italy (46°04′N, 11°07′E) and Villa-Agnedo, Italy (46°02′N, 11°32′E), respectively. The colonies were kept in a climatic chamber at 23 ± 1°C and 75 ± 5% relative humidity with a 16:8 (light:dark) photoperiod with crepuscular periods (1 h). Aedes albopictus adults were maintained in a 22 × 22 × 22 cm cage whereas Ae. koreicus adults were maintained in a 45 × 45 × 45 cm cage (Bugdorm, MegaView Science Co., Ltd., Taiwan). The larvae were reared in 500-mL plastic cups filled with dechlorinated water and were fed daily with ground cat food. Adults were supplied with cotton soaked in 10% sucrose solution, ad libitum. Adult females were fed twice a week with fresh whole cow blood using a Hemotek® blood-feeding system (Hemotek Ltd., Accrington, England) and were provided with filter paper in water-filled ovitraps for egg deposition. After 1–2 months, the eggs were immersed in plastic cups filled with 250 mL of dechlorinated water without adding food, and the resulting first instar larvae were used in the predation tests.
Predation tests
The comparative predation efficiency of M. albidus and M. leuckarti was assessed against Ae. koreicus and Ae. albopictus larvae under laboratory conditions as described by Marten (Citation1990). Copepod females and mosquito larvae came from laboratory colonies that were less than 6 months old. This predation experiment was conducted in the same climatic chamber used for breeding the mosquitoes. In each test, one female copepod, starved for 24–48 hours, was placed in a Petri dish (5 cm in diameter) filled with 10 mL of boiled well water. Subsequently, 50 first instar larvae (less than 24 hours old) of one of the two mosquito species were introduced to each Petri dish (). Petri dishes containing 50 first instar mosquito larvae but no copepod were used as a control. The number of living larvae was counted after 24 and 48 hours. At the end of each test, the copepod was dissected, mounted on a permanent slide and observed under a light microscope to confirm the species identification following Dussart and Defaye (Citation2001). Each test was replicated 15 times for each mosquito species and each copepod population, for a total of 60 tests.
Data analysis
Differences in the number of killed larvae were analysed at two different times, i.e. 24 hours and 48 hours, to compare: (i) the predation efficiency of the two copepod species on the same mosquito species, and (ii) the predation efficiency of the same copepod species on the two different mosquito species. As the data were not normally distributed, we used the non-parametric Mann–Whitney test to compare the median values of killed larvae. All statistical analyses were performed using PAST version 3.08 (Hammer et al. Citation2001). The mean number of larvae killed by a female copepod was also corrected using Abbott’s formula as follows: Corrected mean = 50 × (Meantest – Meancontrol)/(50 – Meancontrol). Abbott’s formula is a standard in bioassay evaluation to correct data for control response.
Results
In 24 hours under laboratory conditions, and regardless of mosquito species, M. albidus females killed and/or consumed almost half of the 50 first instar larvae (a mean of 17.8 and 20.1 Ae. koreicus and Ae. albopictus larvae, respectively), while M. leuckarti females killed more: well over half of the larvae (25.2 and 35.7 respectively; ). After 48 hours, the mean mortality rates increased in all the test combinations. Mortality rates in control tests were about 2.5% for both Aedes species after 24 hours, and 5.7% for Ae. albopictus and 20% for Ae. koreicus after 48 hours. Differences in mortality rates between treatments and controls were all significant after 24 and 48 hours. Mesocyclops leuckarti showed a significantly higher predation efficiency than M. albidus against Ae. albopictus (U = 55.5 and p = 0.02 after 24 h; U = 53.5 and p = 0.01 after 48 h), whereas the predation rates for the two copepod species against Ae. koreicus did not differ significantly (). In addition, the predation efficiency of M. albidus against both Aedes species did not differ significantly, whereas M. leuckarti was significantly more effective against Ae. albopictus than against Ae. koreicus after 48 hours (U = 65.5 and p = 0.05).
Table I. Number of Aedes koreicus or Ae. albopictus killed by one female of Macrocyclops albidus or Mesocyclops leuckarti in 24 and 48 hours.
Discussion
This study represents the first evidence of the predation efficiency of copepods against Ae. koreicus. Mesocyclops leuckarti and M. albidus killed 25.8 ± 2.8 and 18.6 ± 1.3 first instar Ae. koreicus larvae, respectively, in 24 hours in a standard test. These predation rates are lower than those considered “excellent” for mosquito control by Marten and Reid (Citation2007), which is defined as 40 out of 50 larvae killed by one copepod female in a 10-mL dish over 24 hours. Nonetheless, both species did kill more than 50% of the Ae. koreicus larvae in 48 hours. Suprisingly, we observed a relatively high mortality (20%) of first instar Ae. koreicus larvae in the Petri dishes without copepod females (i.e. control tests) after 48 hours. This might be explained by intra-specific competition between the larvae because of starvation and/or crowding (Armistead et al. Citation2008).
Mesocyclops leuckarti appears to be a particularly good candidate for controlling Ae. koreicus and Ae. albopictus in northern Italy, as it killed more than 50% of first instar larvae of both Aedes species in 24 hours. Macrocyclops albidus, on the other hand, seems less effective than M. leuckarti. This is quite surprising as M. albidus has proven to be a formidable predator of Ae. albopictus worldwide, with predation rates ranging from 28.4 to 45.0 larvae per copepod in 24 hours (Marten Citation1990; Rey et al. Citation2004; Veronesi et al. Citation2015). One explanation may be, as Marten and Reid (Citation2007) described, that different populations of the same copepod species may express differences in predation behaviour. Indeed, we did some preliminary tests on a population of M. leuckarti from Levico Lake, and unlike the population of M. leuckarti from Crevalcore, the population from Trentino did not prey effectively on the first instar larvae of Ae. albopictus (P. Visentin unpub. data). These results support the need for testing different local copepod populations in the laboratory to select the best species to use for mosquito control.
Our predation experiments in the laboratory using M. albidus and M. leuckarti against first instar larvae of Ae. koreicus and Ae. albopictus constitute a first step in assessing their eligibility as candidates for biological control agents in northern Italy. We found that both copepod species were effective predators although the predation efficiency of M. leuckarti was slightly lower against Ae. koreicus than against Ae. albopictus, possibly due to the larger size of Ae. koreicus larvae. Copepods might be promising biological control agents against Ae. koreicus since permanent water containers with organic-rich water are preferential larval sites for Ae. koreicus as well as suitable environments for copepods. However, laboratory tests represent the best-case scenario, and predation rates can be lower in natural conditions, where the systems can be larger or deeper, and where mosquito larvae can occur at much lower densities. Further investigations using large containers (e.g. buckets or plastic drums) in the field are warranted, as the use of copepods in natural conditions can be affected by many factors, such as the size of the container; the temperature; the availability of food sources other than mosquito larvae; and the presence of more than one copepod individual, leading to cannibalism (Veronesi et al. Citation2015), i.e. all factors which would probably reduce the predation efficiency (Marten & Reid Citation2007). Moreover, the growth and survival of copepod populations also need to be evaluated in the field. Previous studies have demonstrated that M. albidus develops well in tires or drums and is tolerant to a wide range of temperatures (Rey et al. Citation2004; Veronesi et al. Citation2015). More specifically, the predation efficiency of M. albidus and M. leuckarti against Ae. koreicus needs to be tested in cold temperate areas, as Ae. koreicus has been found at altitudes of up to 1000 m asl, where M. albidus is present, but which is higher than the known altitudinal limit for M. leuckarti; average temperatures at that altitude might therefore prevent the development of M. leuckarti.
Acknowledgements
We would like to thank Claudine Barategui and Francesca Bussola for their technical assistance in the laboratory.
Additional information
Funding
References
- Armistead JS, Arias JR, Nishimura N, Lounibos LP. 2008. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in Northern Virginia. Journal of Medical Entomology 45:629–637. DOI:10.1603/0022-2585(2008)45[629:ILCBAA]2.0.CO;2.
- Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, Rizzoli A. 2015. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Management Science 71:1471–1485. DOI:10.1002/ps.4044.
- Baldacchino F, Montarsi F, Arnoldi D, Barategui C, Ferro Milone N, Da Rold G, Capelli G, Rizzoli A. 2017. A 2-yr mosquito survey focusing on Aedes koreicus (Diptera: Culicidae) in Northern Italy and implications for adult trapping. Journal of Medical Entomology. In press. DOI:10.1093/jme/tjw216.
- Bezzhonova OV, Patraman IV, Ganushkina LA, Vyshemirskiĭ OI, Sergiev VP. 2014. The first finding of invasive species Aedes (Finlaya) koreicus (Edwards, 1917) in European Russia. Meditsinskaia parazitologiia i parazitarnye bolezni 1:16–19.
- Bruno MC, Loftus WF, Reid JW, Perry SA. 2001. Diapause in copepods (Crustacea) from ephemeral habitats with different hydroperiods in Everglades National Park (Florida, U. S. A.). Hydrobiologia 453/454:295–308. DOI:10.1023/A:1013161210836.
- Capelli G, Drago A, Martini S, Montarsi F, Soppelsa M, Delai N, Ravagnan S, Mazzon L, Schaffner F, Mathis A, Di Luca M, Romi R, Russo F. 2011. First report in italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasites & Vectors 4:188. DOI:10.1186/1756-3305-4-188.
- Dussart BH, Defaye D. 2001. Introduction to the Copepoda. In: Dumont HJF, coordinating editor. Guides to the identification of the Microinvertebrates of the continental waters of the world 16. Leiden: Backhuys Publisher.
- Frisch D, Green AJ. 2007. Copepods come in first: Rapid colonization of new temporary ponds. Fundamental and Applied Limnology / Archiv für Hydrobiologie 168:289–297. DOI:10.1127/1863-9135/2007/0168-0289.
- Hammer O, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9.
- Kroeger I, Liess M, Duquesne S. 2014. Temporal and spatial habitat preferences and biotic interactions between mosquito larvae and antagonistic crustaceans in the field. Journal of Vector Ecology 39:103–111. DOI:10.1111/jvec.2014.39.issue-1.
- Kurucz K, Kiss V, Zana B, Schmieder V, Kepner A, Jakab F, Kemenesi G. 2016. Emergence of Aedes koreicus (Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitology Research 115:4687–4689. DOI:10.1007/s00436-016-5229-5.
- Lazaro A, Han WW, Manrique-Saide P, George L, Velayudhan R, Toledo J, Runge Ranzinger S, Horstick O. 2015. Community effectiveness of copepods for dengue vector control: Systematic review. Tropical Medicine & International Health 20:685–706. DOI:10.1111/tmi.2015.20.issue-6.
- Marten GG. 1990. Evaluation of cyclopoid copepods for Aedes albopictus control in tires. Journal of American Mosquito Control Association 6:681–688.
- Marten GG, Reid JW. 2007. Cyclopoid copepods. Journal of the American Mosquito Control Association 23:65–92. DOI:10.2987/8756-971X(2007)23[65:CC]2.0.CO;2.
- Miles JA. 1964. Some ecological aspects of the problem of arthropod-borne animal viruses in the western pacific and South-East Asia regions. Bulletin of the World Health Organization 30:197–210.
- Montarsi F, Ciocchetta S, Devine G, Ravagnan S, Mutinelli F, di Regalbono AF, Otranto D, Capelli G. 2015a. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasites and Vectors 8. DOI:10.1186/s13071-015-0800-y.
- Montarsi F, Drago A, Martini S, Calzolari M, De Filippo F, Bianchi A, Mazzucato M, Ciocchetta S, Arnoldi D, Baldacchino F, Rizzoli A, Capelli G. 2015b. Current distribution of the invasive mosquito species, Aedes koreicus [Hulecoeteomyia koreica] in northern Italy. Parasites and Vectors 8:614. DOI:10.1186/s13071-015-1208-4.
- Nilssen JP, Waervagen SB. 2000. Superficial ecosystem similarities vs autecological stripping: The “twin species” Mesocyclops leuckarti (Claus) and Thermocyclops oithonoides (Sars) - Seasonal habitat utilisation and life history traits. Journal of Limnology 59:79–102. DOI:10.4081/jlimnol.2000.79.
- Rey JR, O’Connell S, Suarez S, Menendez Z, Lounibos LP, Byer G. 2004. Laboratory and field studies of Macrocyclops albidus (Crustacea: Copepoda) for biological control of mosquitoes in artificial containers in a subtropical environment. Journal of Vector Ecology 29:124–134.
- Santos LUD, Andrade CFSD. 1997. Survey of cyclopids (Crustacea, Copepoda) in Brazil and preliminary screening of their potential as dengue vector predators. Revista de Saude Publica 31:221–226. DOI:10.1590/S0034-89101997000300002.
- Stoch F. 2004. CKmap for Windows, Version 5.3. Ministry of Environment, Territory and Sea, Nature Protection Directorate. Available: http://ckmap.faunaitalia.it. Accessed Sep 2016 22.
- Suarez MF, Marten GG, Clark GG. 1992. A simple method for cultivating fresh-water copepods used in biological-control of Aedes aegypti. Journal of American Mosquito Control Association 8:409–412.
- Suter T, Flacio E, Fariña BF, Engeler L, Tonolla M, Müller P. 2015. First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasites and Vectors 8:402. DOI:10.1186/s13071-015-1010-3.
- Tanaka K, Mizusawa K, Saugstad ES. 1979. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contributions of the American Entomological Institute 16:1–987.
- Tinson S, Laybourn-Parry J. 1985. The behavioural responses and tolerance of freshwater benthic cyclopoid copepods to hypoxia and anoxia. Hydrobiologia 127:257–263. DOI:10.1007/BF00024230.
- Veronesi R, Carrieri M, MacCagnani B, Maini S, Bellini R. 2015. Macrocyclops albidus (Copepoda: Cyclopidae) for the biocontrol of Aedes albopictus and Culex pipiens in Italy. Journal of the American Mosquito Control Association 31:32–43. DOI:10.2987/13-6381.1.
- Versteirt V, De Clercq EM, Fonseca DM, Pecor J, Schaffner F, Coosemans M, Van Bortel W. 2012. Bionomics of the established exotic mosquito species Aedes koreicus in Belgium, Europe. Journal of Medical Entomology 49:1226–1232. DOI:10.1603/ME11170.
- Werner D, Zielke DE, Kampen H. 2015. First record of Aedes koreicus (Diptera: Culicidae) in Germany. Parasitology Research 115:1331–1334. DOI:10.1007/s00436-015-4848-6.
- Williams-Howze J. 1997. Dormancy in the free-living copepod orders Cyclopoida, Calanoida and Harpacticoida. Oceanography and Marine Biology: Annual Review 35:257–321.
