?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chironomidae larvae may represent more than 70% of total Arthropoda numbers in hypersaline waters. Crimea, the largest peninsula of the Black Sea, has more than 50 hypersaline water bodies of marine and continental origin. Chironomidae larvae are common components of their ecosystems, but they still are poorly understood. This paper summarizes the results of a long-term study (2007–2016) of chironomids in Crimean hypersaline waters. More than 400 samples from 38 water bodies were used for analysis. The maximum salinity of water bodies containing Сhironomidae larvae was between 320 and 340 g/L. At first it was shown that Baeotendipes noctivagus (Kieffer, 1911) is the most halotolerant chironomid species in the world. Frequency of larvae occurrence varied and was negatively dependent on salinity. Four chironomid species were found: B. noctivagus, Cricotopus gr. cylindraceus (Kieffer, 1908), Tanytarsus gr. mendax Kieffer, 1925 and Paratanytarsus sp. Ceratopogonidae larvae were also found twice, at salinities of 150 and 270 g/L. B. noctivagus was the most common species, which occurred in 81% of samples with chironomids. Abundance of larvae fluctuated widely and reached high numbers: in plankton – to 8 thousand/m3, in floating green algae mats – up to 3 thousand/m2, and in benthos – up to 9 thousand/m2. Nonlinear dependence of chironomid abundance from salinity was observed; maximum abundance was at salinity levels of between 150 and 170 g/L. The average weight of larvae of 0.05–1.50 mm in length varied little in the samples; however, larvae of greater length had a significantly different average weight. Larvae of 8 mm in the samples had the average actual weight, which ranged from 0.750 to 2.203 mg.
Introduction
Hypersaline water bodies are among the most extreme habitats on the planet, and therefore biodiversity in them is not very high (Vareschi Citation1987; Williams Citation1998; Wharton Citation2002; Belmonte et al. Citation2012). The majority of animal species successfully living in such harsh conditions are Arthropoda, which are represented by crustaceans and insects (Bayly Citation1972; Brock & Shiel Citation1983; Belmonte et al. Citation2012). The greatest variety and number of insects are represented by Diptera (Tipulidae, Culicidae, Simuliidae, Stratiomyidae, Ceratopogonidae, Ephydridae, Dolichopodidae, Chironomidae), among which in hypersaline lakes Ephydridae and/or Chironomidae usually dominate (Beadle Citation1969; Bayly Citation1972; Brock & Shiel Citation1983; Przhiboro & Shadrin Citation2012; Przhiboro Citation2014). Chironomidae larvae can represent more than 70% of the total number of Arthropoda in hypersaline waters (Kokkinn & Williams Citation1988; El-Shabrawy & El Sayed Citation2005).
As a component of diverse water ecosystems, the larvae of Chironomidae play an important functional role in the various inland waters and in the diet of water birds (Balushkina Citation1987; Armitage et al. Citation1995), can contribute to the spread of pathogenic organisms (Broza et al. Citation2005), and can cause allergic reactions in humans (Armitage et al. Citation1995). These factors indicate the importance of their comprehensive study. To date there are more than 15,000 described species of Chironomidae. Their larvae occupy a variety of aquatic habitats, including some very extreme, at latitudes ranging from 81°49ʹN to 68°00ʹS (Armitage et al. Citation1995). The maximum altitude at which there are representatives of chironomids is 5600 m above sea level (Himalayas), where Diamesa spp. live at subzero temperatures in water drainage channels in glaciers (Kohshima Citation1984; Sæther & Willassen Citation1987). There are typical marine species, such as Clunio marinus Haliday, 1855 (Palmégn & Lindeberg Citation1959), or the genus of exclusively marine flightless midges Pontomyia Edwards, 1926 (Huang & Cheng Citation2011). Chironomidae larvae also inhabit hypersaline waters of different regions; it has been shown that larvae of chironomids can reach high abundance and dominate (in density and biomass) in water bodies at salinities of up to 70–120 g/L (Szadziewski & Hirvenoja Citation1981; Drake & Arias Citation1995; Balushkina et al. Citation2009; Zerguine Citation2014). Tanytarsus barbitarsis Freeman, 1961 dwells in Australian waters at a salinity of up to 177 g/L and can reach high abundance – up to 200,000 ind./m2 (Kokkinn Citation1986).
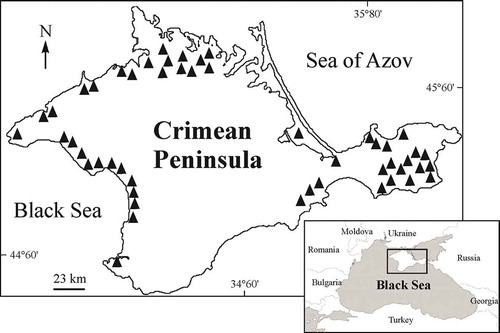
Crimea, which is the largest peninsula of the Black Sea (27,000 km2), has more than 50 hypersaline water bodies of marine and continental origin (Kurnakov et al. Citation1936; Shadrin Citation2009) (). Studies of biota, including Chironomidae, in hypersaline Crimean lakes, were previously carried out on eight lakes (Balushkina & Petrova Citation1989; Ivanova et al. Citation1994; Balushkina et al. Citation2009; Shadrin et al. Citation2010; Litvinenko & Shlyakhov Citation2011; Belmonte et al. Citation2012). It was shown that chironomid larvae are common components of the ecosystems of seven of them. The larvae were not found in Lake Koyashskoye, which has low productivity and where salinity did not fall below 160 g/L during the study period (Balushkina et al. Citation2009; Belmonte et al. Citation2012). The total number of chironomid larvae in seven lakes was very high in some cases, reaching 15,250 ind./m2 (Balushkina et al. Citation2009) and even 69,000 ind./m2 (Litvinenko & Shlyakhov Citation2011). Species identification of chironomid larvae was made in only two studies, and only Baeotendipes tauricus Tshernovskij – junior synonym of Baeotendipes noctivagus (Kieffer, 1911) – was found (Balushkina & Petrova Citation1989; Balushkina et al. Citation2009). There is commercial harvesting of chironomid larvae in some Crimean hypersaline water bodies to use them as food for ornamental fish. Despite the important environmental and economic role of Chironomidae they are still poorly understood in the hypersaline waters of the Crimea, and have been studied in only a few water bodies.
The authors conducted chironomid larva research in the hypersaline waters of Crimea between 2007 and 2015. The goal of this paper is to describe, analyze and discuss the results of this long-term research, comparing them with published data from other regions of the planet.
Materials and methods
Study area
Fifty relatively large and many smaller hypersaline water bodies are located in Crimea; they include Bay Sivash (the Sea of Azov) which is the largest lagoon in Europe (around 2560 km2). By origin and ionic composition, the Crimean natural water bodies are divided into marine (talassohaline) and continental (atalassohaline-sulfate). In addition to natural water bodies, on the Kerch peninsula there are artificial water bodies that are increasing in salinity and becoming hypersaline due to increasing climatic aridity (; Kurnakov et al. Citation1936; Shadrin Citation2009; Anufriieva et al. Citation2014). All Crimean lakes are shallow and polymixic, and vary in size, range of abiotic factors (salinity, temperature, pH, etc.) and biotic composition. In 2000–2015, biota and ecology of saline water bodies in Crimea were studied and results of this were partially published (Zagorodnyaya et al. Citation2008; Balushkina et al. Citation2009; Belmonte et al. Citation2012; Shadrin & Anufriieva Citation2013a; Anufriieva et al. Citation2014; etc.).
Sampling and processing
A total of 416 samples were collected from 38 water bodies in 2007–2015; 389 samples of zooplankton, 12 benthic samples and 15 samples of floating filamentous algae mats. Of these samples, 324 were collected from hypersaline water bodies and 92 from brackish waters. It has been shown in previous studies that in hypersaline conditions (Zagorodnyaya et al. Citation2008; Belmonte et al. Citation2012), most of the benthic animals, including chironomid larvae, are present in the water column rather than in the benthos; therefore, larvae were taken primarily from plankton samples. There are two reasons why benthic animals occupy the water column: (1) increased water density under high salinity; (2) the common presence of anoxic conditions near the bottom in hypersaline waters. Quantitative samples of zooplankton were collected by filtration of at least 50–100 L of water through a plankton net with a mesh size of 110 microns. Simultaneously with biota sampling salinity, temperature and pH were measured using a refractometer (Kelilong WZ212) and a pH meter (PHH-830). Samples were fixed with 4% formalin. A smaller part of the samples was qualitative. Benthic samples were collected in areas with a depth of 0.2–0.6 m by means of a benthic tube (area 0.018 m2). Samples of floating filamentous algae mats were collected from an area of 0.25 m2. Samples were dried to air-dry mass, which was converted into absolute dry weight (adm) using a coefficient of 0.93 (Korelyakova Citation1977). The mass of sampled algae mat was determined on an electronic balance. The relative number of animals in the mats was determined by dividing the number of counted individuals by the weight of the mat piece. The samples were processed using an Olympus SZ-ST and LOMO MBS-9 stereo microscope. In 27 samples from hypersaline water bodies (), larva species were identified using articles and keys for identification (Pankratova Citation1970, Citation1983; Hirvenoja Citation1973; Wiederholm Citation1983; Makarchenko & Makarchenko Citation1999). Developmental stage was evaluated and size was measured. The length of the chironomid larvae was measured under a STEMI DV4 (Zeiss) stereo microscope with an ocular micrometer. The larval mass was determined by the weighing of larvae (pre-dried on the filter paper) on a torsion balance WT-250. Large individuals were weighed individually; small individuals of similar size were weighed together and average mass was calculated.
Table I. Characteristics of Crimean hypersaline water bodies, where Chironomidae larvae were identified.
Data processing
To assess the frequency of occurrence of chironomid larvae in different ranges of salinity, all 416 samples from brackish and hypersaline water bodies were used:
where Kc is the number of samples in a certain range of salinity, which contained chironomids; and K is the total number of samples in the interval.
To analyze the relationship between chironomid abundance and environmental factors, only quantitative samples () were used. Taking into account the temperature correction coefficient Q10 = 2.25, production was calculated using the formula for growth of chironomid larvae (Balushkina Citation1987):
where P – production, J/m2·day or J/m3·day, N – abundance, ind./m2 or ind./m3, W – individual mass, mg.
Data were subjected to standard statistical processing. The regression equations between chironomid abundance and environmental factors were calculated by the least squares method in the standard program MS Excel 2007. The significance of differences in mean values was evaluated by Student’s t-test, and the confidence level of the correlation coefficients was determined (Müller et al. Citation1979).
Results
Chironomids are common inhabitants of the Crimean hypersaline lakes, among which there are water bodies both maritime and continental in origin. Chironomids are also abundant in Bay Sivash and in saline/hypersaline artificial ponds. The maximum salinity at which Chironomidae larvae were found was between 320 and 340 g/L. Frequency of larva occurrence at different salinities varied and was negatively dependent on salinity if higher than 30–50 g/L (). Dependency may be reliably approximated by a linear equation (R = 0.935; p = 0.0005):
Figure 2. Dependence of frequency of Chironomidae larvae occurrence on salinity in Crimean hypersaline waters.
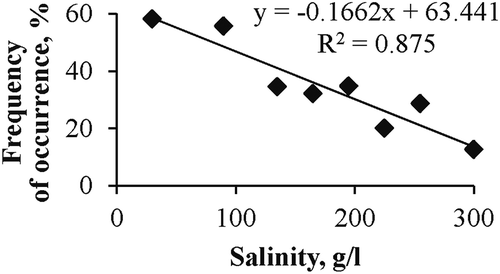
where Y is the frequency of occurrence (%); and S is the salinity (g/L; average of interval).
Thus, 87% of probability to find chironomid larvae in plankton at a salinity of above 50 g/L was determined by salinity. It should be noted that if salinity was below 50 g/L, Chironomidae larvae were usually 5–20 times less abundant in plankton samples than in the benthos or floating mats. There were also benthic or mat samples without chironomid larvae, despite their presence in the plankton. With a rise of salinity above 100–110 g/L, the portion of cases in which the most part of chironomid larvae lived in the plankton increased. The largest number of samples (127) was collected in Lake Chersonessus (near Sevastopol) from 2007 to 2015. The seasonal occurrence of larvae in plankton was studied; chironomid larvae were absent in samples collected from November to March. In April they were present, but not every year. Chironomid larvae were not observed during these months in other lakes.
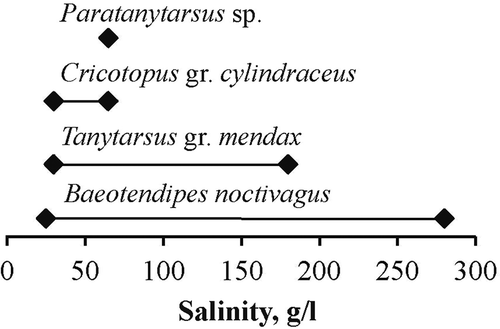
In total, four chironomid species were found in 27 samples from hypersaline waters: B. noctivagus, Cricotopus gr. cylindraceus (Kieffer, 1908), Tanytarsus gr. mendax Kieffer, 1925 and Paratanytarsus sp. Ceratopogonidae larvae were found twice, at salinities of 150 and 270 g/L, but were not identified to the species group level. B. noctivagus was the most common chironomid species, which was represented in 81% of chironomid samples at salinities between 25 and 280 g/L. Cricotopus was represented in 22% of samples at salinities between 30 and 65 g/L. Tanytarsus was in 8% of chironomid samples at salinities between 30 and 180 g/L, and Paratanytarsus occurred once at a salinity of 58 g/L (). As a rule, only one chironomid species was represented in a sample, and only in two samples was B. noctivagus found together with other species: once with Cricotopus at a salinity of 65 g/L, when Cricotopus contributed 66% of the total number of chironomids; the other with Tanytarsus at a salinity of 180 g/L, when Tanytarsus represented about 1% of the total abundance. Cricotopus and Tanytarsus were found together in one sample at a salinity of 30 g/L.
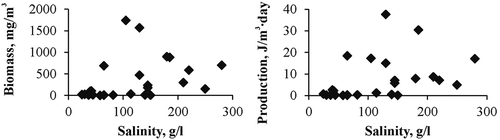
Abundance of Chironomidae larvae fluctuated widely (, ; ) and reached high values: in plankton, up to 8 thousand/m3 or 3 thousand/m2 (calculated taking into account depth), in floating mats, up to 3 thousand/m2, and on the benthos, up to 9 thousand/m2. As an example, in August 2015 in Bay Sivash at salinity between 61 and 65 g/L and temperature of 30°C, the abundance of Chironomidae larvae in plankton was 14 ind./m3 (B. noctivagus), in floating mats it was 2820 ind./m2 (Cricotopus) and in the benthos it was 1667 ind./m2 (B. noctivagus). In this case, low abundance of Chironomidae larvae in plankton was probably due to a high concentration of juvenile fish – Knipowitschia caucasica (Berg, 1916) and Atherina boyeri Risso, 1810 (Shadrin et al. Citation2016). It should be noted that the average weight of animals in biotopes at this time also varied: 0.40 mg in plankton, 0.25 mg in mats and 0.75 mg on the bottom. Typically, the abundance of chironomid larvae was higher in the floating mats and in the benthos than in the plankton, though often there were some cases when chironomids massively presented in the plankton but had very low numbers in the mats or on the bottom.
Table II. Abundance, biomass and production of chironomid larvae in Crimean hypersaline waters.
Figure 4. Dependence of log concentration of Chironomidae larvae on (a) salinity and (b) temperature in plankton of Crimean hypersaline waters.
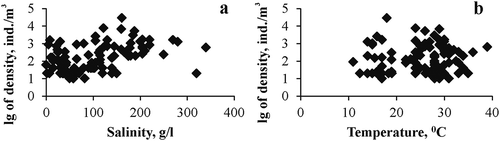
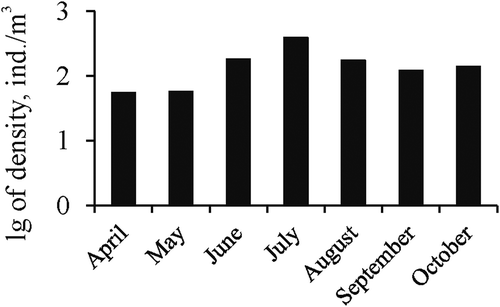
Due to the small number of floating mat and sediment samples, subsequent analysis of abundance dependence on salinity was made only for plankton samples (). In general, for all lakes nonlinear dependence of chironomid larvae abundance from salinity was observed; maximum abundance was in the salinity range of 150–170 g/L. In the samples taken only from Lake Chersonessus, a significant trend of an increase of larva abundance in plankton up to 120 g/L was noted. Dependence is close to linear (R = 0.689; p = 0.005), and up to 48% of the total variability in the chironomid larva numbers in the lake plankton can be explained by the variability of salinity. The dependence of the population density on temperature was also nonlinear (), and the largest number of individuals was observed in the range of 16–29°C. Abundance did not correlate with pH. In the period when the larvae were represented in plankton there was a general trend of increase in the average abundance for all lakes and every year from April to July, with a subsequent decrease in October (). In Lake Chersonessus the peak of the chironomid larva abundance occurred also in July.
Figure 5. The average monthly abundance of Chironomidae larvae in plankton of Crimean hypersaline waters.
B. noctivagus larva length varied among the samples from 1.5 to 12.0 mm, and the body weight from 0.036 to 4.880 mg (). Average weight of larvae of 0.05 to 1.50 mm varied little among the samples; larvae of greater length had significantly (p = 0.001) different average weight with pairwise comparison of samples. The analysis of the entire set of samples showed that there is a non-significant trend of a weight decrease of same-length larvae with a salinity increase. As an example, in Lake Kiyatskoye in August 2014 and 2015, salinity was similar at 180 g/L and 185 g/L, respectively. However, individual average mass of same-length larvae was significantly different (p = 0.001) in 2014 and 2015. In 2014, larvae of 4 mm had a mass of 0.445 mg, 6 mm – 1.044 mg and 8 mm – 2.333 mg; and in 2015, length of 4 mm – 0.244 mg, 6 mm – 0.567 mm and 8 mg – 0.750 mg. Dependence of weight on length is described by equations such as:
Table III. Coefficients of power dependence (Equation 3) “mass (mg) – length (mm)” of Baeotendipes noctivagus larvae in different Crimean hypersaline habitats.
where W – body weight (mg); L – length (mm); and a and b are coefficients.
Paired comparison showed that equation coefficients “b” in most cases were significantly (p = 0.001–0.0001) different and the coefficients “a” were not significantly different; the average value of the coefficient “a” was calculated. The “b” coefficients were recalculated for all of the equations using this average value of “a” (). The biomass and production of chironomid larvae was calculated taking into account all data (). The maximum biomass of larvae in the plankton reached 2560 mg/m3, and dependence on salinity had a dome-shaped form (). Maximum production reached 37.5 J/m3 day, and also had a dome-shaped form of dependence on salinity ().
Discussion
As seen from new and previously published (Ivanova et al. Citation1994; Zagorodnyaya et al. Citation2008; Balushkina et al. Citation2009; Belmonte et al. Citation2012) data, Chironomidae larvae are a common component of ecosystems in Crimean hypersaline waters and play an important role in production processes. Larvae with a wide range of sizes from 2.5 to 9.5 mm were abundantly represented in water bodies of salinity up to 280 g/L, indicating the active state of larval chironomid populations. Even though larvae have been found at salinities between 320 and 340 g/L, this does not mean that they can function properly in such salinity. It has been shown previously that larvae of some Chironomidae species may spend a period of time in a dormant state, anhydrobiosis (Suemoto et al. Citation2004; Cornette & Kikawada Citation2011). It can be assumed that some B. noctivagus stages can move into anhydrobiosis due to drying or very high salinity.
The massive presence of Chironomidae larvae in the hypersaline waters of Crimea is not an exception; this phenomenon has been observed in different regions (see , where a list is given of Chironomidae species the larvae of which are able to live in hypersaline waters). Approximately 38 species belonging to different subfamilies may exist at a salinity of more than 35 g/L, and 16 species among them occur at a salinity of more than 100 g/L. Only three species were found at a salinity of 150 g/L and higher. E. K. Suworow (Citation1908) found active stages of chironomid larvae (Chironomus sp.?) in Lake Bulak (near Caspian Sea) at a salinity of 285 g/L. Now B. noctivagus can be considered the most halotolerant Chironomidae species in the world, inhabiting in an active state hypersaline waters with a salinity of up to 280 g/L, and possibly up to 340 g/L. It is likely that Suworow found B. noctivagus larvae in Lake Bulak, which is within the area of distribution of this species.
Table IV. Chironomid species in hypersaline waters worldwide.
A question arises: what adaptations allow some chironomid species to exist at high salinity? It is known that arthropods can use two strategies of osmo-adaptation to exist in hypersaline habitats (Khlebovich & Aladin Citation2010). Osmo-regulating animals use mechanisms of active hypo-osmotic salt regulation in the body fluids, keeping a lower concentration of salts in these fluids than in the environment. Osmo-conforming animals do not have mechanisms for salt regulation in body fluids; osmo-adaptation is carried out at the cellular level by the synthesis of compatible osmolytes and/or by obtaining them from the outside with accumulation in cells. Compatible osmolytes are low-molecular-weight organic compounds (polyols, some amino acids and methylamines, etc.); they protect proteins under conditions of osmotic stress and do not impact on the normal course of metabolic processes (Yancey Citation2001). Among the Diptera, including Chironomidae, there are species that use one or the other of these strategies (Sutcliffe Citation1960; Neumann Citation1961; Bradley Citation1987; Herbst & Bradley Citation1988; Patrick & Bradley Citation2000; Renault et al. Citation2016). Some species can use both osmo-adaptation mechanisms together; Culex tarsalis Coquillett, 1896 can accumulate various compatible osmolytes not only in the cells but also in the body fluids (Patrick & Bradley Citation2000). Osmo-adaptations of the Chironomidae species found during this study have not been studied yet; however, some assumptions can be made. Using data available in literature, primarily Kokkinn (Citation1986), it can be assumed that the species found during the studies, especially B. noctivagus, have quite effective osmo-regulation mechanisms that can ensure the existence of larvae at salinities up to 90–120 g/L. At higher salinities these mechanisms become insufficient; the animals also begin to accumulate osmolytes – osmo-protectants in cells and body fluids. Osmolytes can be accumulated in the body due to the breakdown of proteins (amino acids–alanine, proline, etc.), or come from food (glycerol, betaine, etc.); this was indicated for several Diptera species (Patrick & Bradley Citation2000; Yoder et al. Citation2006; Renault et al. Citation2016). Mechanisms of osmo-regulation, as well as the synthesis of osmolytes, require a lot of energy, so obtaining osmolytes from the external environment (Yoder et al. Citation2006) can significantly reduce the energy costs for osmo-adaptation processes. It is likely that the smaller average body size and mass of individuals in the water bodies with higher salinity () are due to higher energy costs for osmo-adaptation processes which reduce the efficiency of use of assimilated energy for the growth of larvae. It was noted previously (Kokkinn Citation1986) that T. barbitarsis in Australia, at salinity between 100 and 177 g/L, was found only in highly productive waters where blooms of algae were observed. In Crimea, the authors found chironomids at the highest salinity also only in waters where microalgal blooming was observed. Previously, this phenomenon was noted for the copepods in the hypersaline lakes of Crimea (Shadrin & Anufriieva Citation2013b; Anufriieva Citation2015). The presence of microalgal blooms may ensure the presence of chironomids not only by providing necessary energy, but also by providing osmolytes. In Crimea, blooming of the green unicellular alga Dunaliella salina (Dunal) Teodoresco, 1905 in water bodies with salinity of more than 200 g/L is a common phenomenon; the concentration of glycerol (osmolyte) in their biomass can reach 80% of organic matter at that salinity (Shadrin & Anufriieva Citation2013b). All this leads to the conclusion that the physiological capabilities do not define the upper level of salinity at which Chironomidae naturally inhabit water bodies, and the biotic environment (algal concentration and composition) is just as important. Both elements – salinity and microalgae development – are changing in the water bodies and determine the site-to-site and temporal variability of chironomid larva composition and density in hypersaline waters, as was shown for brackish waters (Cañedo-Argüelles & Rieradevall Citation2009).
Hypersaline water bodies in Crimea are characterized by high variability; many of them are partially temporal or dry completely. The successful existence of animal species in these water bodies is ensured by the ability to endure, in a resting state, conditions which are incompatible with active life; the existence of dormant stages has been demonstrated for almost all crustaceans living in hypersaline lakes in Crimea (Moscatello & Belmonte Citation2009; Anufriieva & Shadrin Citation2014; Shadrin et al. Citation2015). Chironomid larvae Polypedilum vanderplanki Hinton, 1951 are able to remain in anhydrobiosis for up to 17 years, and to restore normal activity when released into the water (Cornette & Kikawada Citation2011). The ability of the larvae to be in an inactive state (anhydrobiosis) for quite a long time has been revealed in some other сhironomid species (Suemoto et al. Citation2004; Jones Citation2009). With respect to B. noctivagus this issue has not been studied, but it can be assumed that the larvae of this species are also capable of long-term anhydrobiosis because they are present in significant numbers in ephemeral ponds also.
The study results clearly show that dependence of the length of the body on body weight varies depending on environmental factors, so averaging may lead to significant distortion of the actual situation. The following generalized equation was calculated for all of our samples:
Using Equation (5), the larva mass was calculated for individuals of 6 mm in length – 0.752 mg – but the average weight of larvae of such length ranged from 0.567 mg to 1.044 mg in the samples. When calculating (Equation 5) the mass of 6-mm larvae, it was overestimated or underestimated by nearly 30% compared with the real values. In the case of 8-mm larvae, the calculation gave 1.468 mg, but the average actual weight of this size larva ranged from 0.750 to 2.203 mg in the samples. Overestimation or underestimation of the average actual weight can exceed 40%. The issue requires more detailed study and discussion in future. In this study, biomass and production were calculated using actual average individual mass. According to the data above, biomass and production are dependent on salinity but other factors such as food are likely to play an equally important role. Salinity determines the maximum possible performance. The question of interaction of factors in determining the structural and functional characteristics of larvae is interesting, but there is not enough data on the state of the environment and Chironomidae biology for an in-depth discussion. However, some things may be assumed. Calculations of chironomid production are most likely overestimating the actual values because a generalized Equation (2) of chironomid growth was used. It was shown for some Chironomidae species that the growth rate decreases and development duration increases with a salinity increase (Kokkinn Citation1990; Cartier et al. Citation2011). On this basis, it can be assumed that calculated production values are being used which may be 30–60% higher than the real values in the hypersaline lakes of Crimea. Further research is needed in this direction.
Conclusions
Chironomid larvae are a common and abundant component of the Crimean hypersaline waters, playing an important role in the functioning of the ecosystems. B. noctivagus is the most common and abundant species among them. It is likely that it is the most halotolerant Chironomidae species in the world. Despite this, the adaptation mechanisms providing for its existence in the harsh conditions of hypersaline waters are not known. Further physiological, biochemical, genetic and ecological research is needed in this direction.
Acknowledgements
The authors thank Mr. Oleg Eremin, who helped to organize and conduct the expeditions. We are also very grateful to Dr. Bindy Datson (Australia) for her selfless work on improving our English of the manuscript.
Additional information
Funding
References
- Anufriieva E, Hołyńska M, Shadrin N. 2014. Current invasions of Asian Cyclopid species (Copepoda: Cyclopidae) in Crimea, with taxonomical and zoogeographical remarks on the hypersaline and freshwater fauna. Annales Zoologici 64:109–130. DOI:10.3161/000345414X680636.
- Anufriieva E, Shadrin N. 2014. Resting stages of crustaceans in the Crimean hypersaline lakes (Ukraine) and their ecological role. Acta Geologica Sinica (English Edition) 88(s1):46−49. DOI:10.1111/1755-6724.12266_3.
- Anufriieva EV. 2015. Do copepods inhabit hypersaline waters worldwide? A short review and discussion. Chinese Journal of Oceanology and Limnology 33:1354–1361. DOI:10.1007/s00343-014-4385-7.
- Armitage PD, Pinder LC, Cranston P. 1995. The Chironomidae: Biology and ecology of non-biting midges. London: Chapman & Hall.
- Balushkina EV. 1987. The functional significance of chironomid larvae in inland water bodies. Leningrad: Nauka. (in Russian).
- Balushkina EV, Golubkov SM, Golubkov MS, Litvinchuk LF, Shadrin NV. 2009. Effect of abiotic and biotic factors on the structural and functional organization of the saline lake ecosystems. Zhurnal Obshchei Biologii 70:504–514. (in Russian).
- Balushkina EV, Petrova NP. 1989. Functioning of chironomid populations in hypersaline lakes of Crimea. Proceedings of the Zoological Institute of Academy of Sciences of USSR 205:129–140 (in Russian).
- Bayly IAE. 1972. Salinity tolerance and osmotic behavior of animals in athalassic saline and marine hypersaline waters. Annual Review of Ecology and Systematics 3:233–268. DOI:10.1146/annurev.es.03.110172.001313.
- Beadle LC. 1969. Osmotic regulation and the adaptation of freshwater animals to inland saline waters. Verhandlungen des Internationalen Verein Limnologie 17:421–429.
- Belmonte G, Moscatello S, Batogova EA, Pavlovskaya T, Shadrin NV, Litvinchuk LF. 2012. Fauna of hypersaline lakes of the Crimea (Ukraine). Thalassia Salentina 34:11–24.
- Belyanina SI, Voronin M, Belonogova Y. 2014. Chironomids (Diptera, Chironomidae) in the hypersaline water bodies of the Bogdinsko-Baskunchak nature reserve. In: Anikin VV, Zolotukhin VV, editors. Biodiversity of arid ecosystems. Moscow: Planeta. pp. 22–23. (in Russian).
- Bradley TJ. 1987. Physiology of osmoregulation in mosquitoes. Annual Review of Entomology 32:439–462. DOI:10.1146/annurev.en.32.010187.002255.
- Brock MA, Shiel RJ. 1983. The composition of aquatic communities in saline wetlands in Western Australia. Hydrobiologia 105:77–84. DOI:10.1007/BF00025178.
- Broza M, Gancz H, Halpern M, Kashi Y. 2005. Adult non-biting midges: Possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environmental Microbiology 7:576–585. DOI:10.1111/emi.2005.7.issue-4.
- Cañedo-Argüelles M, Rieradevall M. 2009. Quantification of environment-driven changes in epiphytic macroinvertebrate communities associated to Phragmites australis. Journal of Limnology 68:229–241. DOI:10.4081/jlimnol.2009.229.
- Cartier V, Claret C, Garnier R, Franquet E. 2011. How salinity affects life cycle of a brackish water species, Chironomus salinarius Kieffer (Diptera: Chironomidae). Journal of Experimental Marine Biology and Ecology 405:93–98. DOI:10.1016/j.jembe.2011.05.019.
- Cornette R, Kikawada T. 2011. The induction of anhydrobiosis in the sleeping chironomid: Current status of our knowledge. IUBMB Life 63:419–429. DOI:10.1002/iub.463.
- Drake P, Arias AM. 1995. Distribution and production of Chironomus salinarius (Diptera, Chironomidae) in a shallow coastal lagoon in the Bay of Cadiz. Hydrobiologia 299:195–206. DOI:10.1007/BF00767326.
- El-Shabrawy GM, El Sayed TR. 2005. Long-term changes and community structure of macrobenthic Arthropoda and Mollusca in Bardawill lagoon. Thalassia Salentina 28:17–30.
- Herbst DB, Bradley TJ. 1988. Osmoregulation in dolichopodid larvae (Hydrophorus plumbeus) from a saline lake. Journal of Insect Physiology 34:369–372. DOI:10.1016/0022-1910(88)90105-9.
- Hirvenoja M. 1973. Revision der Gattung Cricotopus van der Wulp und ihrer Verwandten (Diptera, Chironomidae). Annales Zoologici Fennici 10:1–363.
- Huang D, Cheng L. 2011. The flightless marine midge Pontomyia (Diptera: Chironomidae): Ecology, distribution, and molecular phylogeny. Zoological Journal of the Linnean Society 162:443–456. DOI:10.1111/zoj.2011.162.issue-2.
- Ivanova MB, Balushkina EV, Basova SL. 1994. Structural-functional reorganization of ecosystem of hyperhaline Lake Saki (Crimea) at increased salinity. Russian Journal of Aquatic Ecology 3:111–126.
- Jones RE. 2009. Dehydration in an Australian rock pool chironomid larva, (Paraborniella tonnoiri). Journal of Entomology Series A, General Entomology 49:111–119. DOI:10.1111/phen.1975.49.issue-2.
- Khlebovich VV, Aladin NV. 2010. The salinity factor in animal life. Herald of the Russian Academy of Sciences 80:299–304. DOI:10.1134/S1019331610030172.
- Kohshima S. 1984. A novel cold-tolerant insect found in a Himalayan glacier. Nature 310:225–227. DOI:10.1038/310225a0.
- Kokkinn MJ. 1986. Osmoregulation, salinity tolerance and the site of ion excretion in the halobiont chironomid, Tanytarsus barbitarsis Freeman. Australian Journal of Marine & Freshwater Research 37:243–250. DOI:10.1071/MF9860243.
- Kokkinn MJ. 1990. Is the rate of Embryonic development a predictor of overall development rate in Tanytarsus barbitarsis Freeman (Diptera: Chironomidae)? Australian Journal of Marine & Freshwater Research 41:575–579. DOI:10.1071/MF9900575.
- Kokkinn MJ, Williams WD. 1988. Adaptations to life in a hypersaline water-body: Adaptations at the egg and early embryonic stage of Tanytarsus barbitarsis Freeman (Diptera, Chironomidae). Aquatic Insects 10:205–214. DOI:10.1080/01650428809361331.
- Korelyakova IL. 1977. Vegetation of the Kremenchug reservoir. Kiev: Naukova Dumka (in Russian.
- Kurnakov NS, Kuznetsov VG, Dzens-Lytovsky AI, Ravich MI. 1936. The Crimean salt lakes. Moscow: AN USSR Publ. (in Russian).
- Laville H, Tourenq JN. 1967. Contribution à la connaissance de trois chironomides de camargue et des marismas du Guadalquivir [Diptères]. Annales de Limnologie 3:185–204. DOI:10.1051/limn/1967009.
- Litvinenko NM, Shlyakhov VA. 2011. The state of Chironmidae larvae resources in the Crimean inland water bodies. In: Petrenko OA, editor. Main results of complex research in the Azov-Black Sea basin and the World Ocean. Kerch: YugNIRO Publishers’. pp. 84–90. (in Russian).
- Makarchenko EA, Makarchenko MA. 1999. Chironomidae. Non-biting midges. In: Tsalolikhin SJ editor. Key to freshwater Invertebrates of Russia and Adjacent lands. V.4. Higher Insects. Diptera. St. Petersburg: Zoological Institute RAS. pp. 210–295. (in Russian).
- Mirabdullayev IM, Joldasova IM, Mustafaeva ZA, Kazakhbaev S, Lyubimova SA, Tashmukhamedov BA. 2004. Succession of the ecosystems of the Aral Sea during its transition from oligohaline to polyhaline water body. Journal of Marine Systems 47:101–107. DOI:10.1016/j.jmarsys.2003.12.012.
- Moscatello S, Belmonte G. 2009. Egg banks in hypersaline lakes of the South-East Europe. Saline Systems 5:1. DOI:10.1186/1746-1448-5-3.
- Müller PH, Neuman P, Storm R. 1979. Tafeln der mathematischen Statistik. Leipzig: VEB Fachbuchverlag.
- Neumann D. 1961. Osmotische resistenz und osmoregulation aquatischer Chironomidenlarven. Biologisches Zentrablatt 80:693–715.
- Palmégn E, Lindeberg B. 1959. The marine midge, Clunio marinus Hal. (Dipt., Chironomidae), found in brackish water in the northern Baltic. Internationale Revue der gesamten Hydrobiologie und Hydrographie 44:383–394. DOI:10.1002/(ISSN)1522-2632.
- Pankratova V. 1970. Larvae and pupae of mosquitoes of the subfamily Orthocladiinae in the fauna of the USSR (Diptera, Chironomidae = Tendipedidae). Leningrad: Nauka. (in Russian).
- Pankratova V. 1983. Larvae and pupae of mosquitoes of the subfamily Chironominae in the fauna of the USSR (Diptera, Chironomidae = Tendipedidae). Leningrad: Nauka. (in Russian).
- Patrick ML, Bradley TJ. 2000. Regulation of compatible solute accumulation in larvae of the mosquito Culex tarsalis: Osmolarity versus salinity. Journal of Experimental Biology 203:831–839.
- Por FD, Ben-Tuvia A. 1981. The Bardawil lagoon (Sirbonian lagoon) of north Sinai-a summing up. Rapports et procès-verbaux des réunions, Commission internationale pour l’Exploration scientifique de la mer Méditerranée 27:101–107.
- Przhiboro A. 2014. Diversity and adaptations of immature Diptera in semiaquatic habitats at shorelines of hypersaline lakes in the Crimea, with a brief review of Diptera in mineralized bodies of water. Acta Geologica Sinica (English Edition) 88(s1):98–100. DOI:10.1111/1755-6724.12266_22.
- Przhiboro A, Shadrin N. 2012. Mass occurrence of flies of the genus Ephydra (Diptera: Ephydridae) at the coastline zone of hypersaline coastal lagoons in the Eastern Crimea. Marine Ekological Journal 11:24. (in Russian).
- Renault D, Lombard M, Vingère J, Laparie M. 2016. Comparative salinity tolerance in native flies from the subantarctic Kerguelen Islands: A metabolomic approach. Polar Biology 39:47–56. DOI:10.1007/s00300-014-1605-8.
- Sæther OA, Willassen E. 1987. Four new species of Diamesa Meigen, 1835 (Diptera, Chironomidae) from the glaciers of Nepal. Entomologica Scandinavica 29:189–203.
- Shadrin N, А K, Batogova Е. 2010. Zooplankton structure and dynamics of the hypersaline Tobechik Lake in 2007-2009 (Crimea, Kerch Peninsula). Current problems of the Azov-Black Sea Region ecology. Materials of V International Conference, Kerch, 8-9 October, 2009, Kerch: YugNIRO Publishers’. pp. 50–56 (in Russian).
- Shadrin NV. 2009. The Crimean hypersaline lakes: Towards development of scientific basis of integrated sustainable management. Proceedings of 13th World Lake Conference, 1–5 November, 2009, Wuhan, China. Available: http://wldb.ilec.or.jp/data/ilec/WLC13_Papers/S12/s12-1.pdf. Accessed Dec 2016 01.
- Shadrin NV, Anufriieva E. 2013b. Dependence of Arctodiaptomus salinus (Calanoida, Copepoda) halotolerance on exoosmolytes: New data and a hypothesis. Journal of Mediterranean Ecology 12:21–26.
- Shadrin NV, Anufriieva EV. 2013a. Climate change impact on the marine lakes and their Crustaceans: The case of marine hypersaline Lake Bakalskoye (Ukraine). Turkish Journal of Fisheries and Aquatic Sciences 13:603–611. DOI:10.4194/1303-2712-v13_4_05.
- Shadrin NV, Anufriieva EV, Amat F, Eremin OY. 2015. Dormant stages of crustaceans as a mechanism of propagation in the extreme and unpredictable environment in the Crimean hypersaline lakes. Chinese Journal of Oceanology and Limnology 33:1362–1367. DOI:10.1007/s00343-015-4363-8.
- Shadrin NV, Sergeeva NG, Latushkin AA, Kolesnikova EA, Kipriyanova LM, Anufriieva EV, Chepyzhenko AA. 2016. Transformation of Gulf Sivash (the Sea of Azov) in conditions of growing salinity: Changes of meiobenthos and other ecosystem components (2013-2015). Journal of Siberian Federal University. Biology 9:452–466. (in Russian).
- Suemoto T, Kawai K, Imabayashi H. 2004. A comparison of desiccation tolerance among 12 species of chironomid larvae. Hydrobiologia 515:107–114. DOI:10.1023/B:HYDR.0000027322.11005.20.
- Sutcliffe DW. 1960. Osmotic regulation in the larvae of some euryhaline Diptera. Nature 187:331–332. DOI:10.1038/187331a0.
- Suworow EK. 1908. Zur Beurteilung der lebenserscheinungen in gestatigten salzseen. Zoologischer Anzeiger 32:647–674.
- Szadziewski R, Hirvenoja M. 1981. Cricotopus zavreli sp. n. (Diptera, Chironomidae), a halobiontic non-biting midge from Poland. Annales Entomologici Fennici 47:111–118.
- Varesсhi E. 1987. Saline lake ecosystems. Ecological Studies 61:347–364.
- Velasco J, Millán A, Hernández J, Gutiérrez C, Abellán P, Sánchez D, Ruiz M. 2006. Response of biotic communities to salinity changes in a Mediterranean hypersaline stream. Saline Systems 2:1. DOI:10.1186/1746-1448-2-12.
- Wharton DA. 2002. Life at the limits: Organisms in extreme environments. Cambridge: Cambridge University Press.
- Wiederholm Т. 1983. Chironomidae of the Holarctic Region: Keys and diagnoses. Part 1. Larvae. Entomologica Scandinavica 19:457.
- Williams WD. 1998. Salinity as a determinant of the structure of biological communities in the salt lakes. Hydrobiologia 381:191–201. DOI:10.1023/A:1003287826503.
- Yancey PH. 2001. Water stress, osmolytes and proteins. American Zoologist 41:699–709.
- Yoder JA, Benoit JB, Denlinger DL, Rivers DB. 2006. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. Journal of Insect Physiology 52:202–214. DOI:10.1016/j.jinsphys.2005.10.005.
- Zagorodnyaya Y, Batogova EA, Shadrin NV. 2008. Long-term transformation of zooplankton in the hypersaline lake Bakalskoe (Crimea) under salinity fluctuations. Marine Ecological Journal 7:41–50. (in Russian).
- Zerguine K. 2014. Chironomidae (Diptera: Insecta) of temporary salt lakes in the eastern Hauts Plateaux of Algeria. The Experiment 25:1704–1710.