Abstract
Five garfish Belone belone (Linnaeus, 1761) were sampled to study the morphology of the digestive system. Hematoxylin-eosin staining as well as Alcian blue/PAS (Periodic Acid Schiff) were used in order to elucidate the histological features of the digestive organs in garfish. Garfish B. belone is a stomachless fish whose digestive system is a hollow tube made of oesophagus and intestines. The oesophagus consists of three distinctive layers: the mucosa, the muscular layer and the outer layer. The oesophageal epithelium contains abundant goblet cells. All three observed parts (anterior, middle and posterior) of the intestines consisted of four-layered wall containing muscosa, submucosa, muscular layer and outer layer. The liver as an associated digestive gland also contains some pancreatic tissue spread out through the liver parenchyma. The results show that the histology of the digestive system in garfish is congruent with its feeding habits.
Introduction
The digestive system of fishes shows remarkable diversity in its morphology and function, related to both taxonomy and different feeding habits (Abdulhadi Citation2005). In fishes, as in other vertebrates, the digestive system is made up of the alimentary canal, varying in diameter and longitudinally divided into the oesophagus, the stomach, the intestines and the rectum (Treer et al. Citation1995). Some organs such as the tongue and the teeth in the oral cavity, and the extramural digestive glands, specifically the liver, gall bladder, and exocrine pancreas, are associated to the alimentary tube; while the salivary glands are usually missing in the oral cavity of fish (Treer et al. Citation1995). Histologically, from the cranial end to the caudal end of digestive tract, the wall of the alimentary canal is formed by four distinctive layers. Starting at the lumen, these layers are: mucosa, submucosa, muscularis externa, and serosa or adventitia (Kierszenbaum & Tres Citation2012).
The garfish, Belone belone, is a pelagic fish widely spread in the north-eastern Atlantic, Mediterranean (Collette & Parin Citation1970; Zorica et al. Citation2011; Zorica & Čikeš Keč Citation2012). In the Black Sea, the Sea of Azov and the Sea of Marmara, Belone euxini (Günther, 1866) was reported (Bilgin et al. Citation2014). This fish species is usually found in offshore areas, except during spawning period when it migrates into coastal regions (Zorica et al. Citation2011; Zorica & Čikeš Keč Citation2012). Although it is not known as a fish of major commercial and scientific importance, some interesting data considering its biology have been published (Dorman Citation1989, Citation1991; Sever et al. Citation2009; Zorica et al. Citation2011; Zorica & Čikeš Keč Citation2012). In spite of all these data on garfish biology, including its size, maturity, fecundity, reproductive cycle and feeding habits, the data on histological features of its digestive tract seem to be lacking. Some studies concerning enzymatic digestion in stomachless herbivorous and carnivorous fish (Day et al. Citation2011) were performed on Belonidae family members, but with no data on histology of the digestive tract organs. It seems that the present study is the first record on the morphology of the digestive system in garfish B. belone, and since some morphological and functional differences of the digestive system in fishes depend usually on whether they are herbivorous or carnivorous, it is important to elucidate whether the morphology of the garfish digestive system is in accordance to its feeding habits.
Materials and methods
Five garfish Belone belone were sampled to study morphology of the digestive system. The specimens were captured in the middle Eastern Adriatic in June 2013 by seine net. The average body length of the samples was 28 cm. The parts of the digestive system, from the cranial end of the oesophagus to the caudal end of the rectum, were fixed in 10% formalin immediately after collection. The tissues were first dehydrated in an ascending series of ethanol and cleared with xylene, and then embedded in paraffin. Tissues sections were cut transversally at 6 μm and mounted on glass slides. The sections were then deparaffinised with xylene and stained with hematoxylin–eosin staining to present the basic morphology of the digestive organs (Sheehan & Hrapchak Citation1980). The sections were observed using an Olympus BX51 light microscope (Alcian blue / Periodic Acid Schiff [PAS] staining).
The sections through the mucosal layer of the oesophagus and the intestines were treated with Alcian blue/PAS staining in order to elucidate the type of the mucous cells and the nature of the produced mucus. The sections were deparaffinised and stained with Alcian blue for 15 minutes. After washing in distilled water, the sections were treated with periodic acid for 10 minutes and then with Schiff’s reagent for 10 minutes. The sections were then dehydrated, cleared and mounted on glass slides (Sheehan & Hrapchak Citation1980).
Results
The mean body length of the five examined garfish was 28 cm, with a standard deviation of 0.5. The mean weight was 32.478 g with a standard deviation of 0.21358. The digestive system of the garfish B. belone consists of the alimentary canal with associated liver containing some pancreatic tissue. The alimentary canal is a hollow tube distinguished into the oesophagus and the intestine, respectively. The stomach as a digestive organ could not be distinguished down the length of the garfish alimentary canal.
The oesophagus
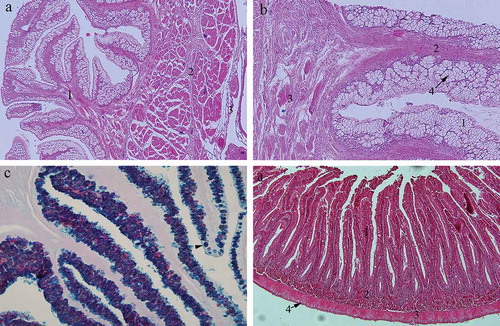
Three basic layers can be distinguished in the oesophageal wall of the garfish Belone belone: mucosa (tunica mucosa), the muscular layer (tunica muscularis) and the outer layer (tunica adventitia) (). Mucosa is the innermost layer, which forms the leaf-like folds deeply protruding into the oesophageal lumen. It consists of three different concentric layers: epithelium, lamina propria and muscularis mucosae. The oesophageal epithelium contains abundant goblet cells. The goblet cells are well organised, mostly forming the acini (). The nuclei of the goblet cells are usually flattened at the basal domain of the cell. Although some goblet cells stained red when treated with Alcian blue/PAS staining, oesophageal goblet cells predominantly stained blue indicating the presence of the acid mucopolysaccharides (). In the basal part of the epithelium some cube-shaped cells as well as the round ones can be seen. Lamina propria is a layer of dense connective tissue centrally positioned in the mucosal fold. It usually contains blood vessels. The muscularis mucosa is the deepest layer of the mucosa, consisting of a thin layer of circular striated muscle fibres, which cannot be clearly distinguished from the underlaying muscular layer. The muscular layer of the oesophageal wall contains two layers consisting of longitudinal bundles of striated muscle fibres. The muscular layers are separated by loose connective tissue containing blood vessels. The outermost layer (tunica adventitia) of the oesophageal wall is thin and made of loose connective tissue.
Figure 1. (a) Cross section through the oesophagus of the garfish Belone belone: 1 – tunica mucosa; 2 – tunica muscularis; 3 – tunica adventitia. Hematoxylin-eosin, ×4; (b) cross section through the oesophageal mucosa: 1 – epithelium; 2 – lamina propria; 3 – lamina muscularis mucosae; 4 – acini with goblet cells. Hematoxylin-eosin, ×10; (c) oesophageal mucosa: Goblet cells predominantly stained blue indicating the presence of the acid mucopolysaccharides (arrowhead). Alcian blue/PAS, ×20; (d) cross section through the intestines: 1 – tunica mucosa; 2 – tunica submucosa; 3 – tunica muscularis; 4 – tunica serosa. Hematoxylin-eosin, ×10.
The intestines
Since the stomach as a separate sac-like organ down the length of the digestive tract of the garfish could not be distinguished, it is obvious that the oesophagus in the garfish opens directly to the intestines. The intestines spread straight down the length of the garfish body, having no curves. We observed the three parts of the garfish intestines (anterior, middle and posterior) in order to determine whether there are any histological differences in their structure.
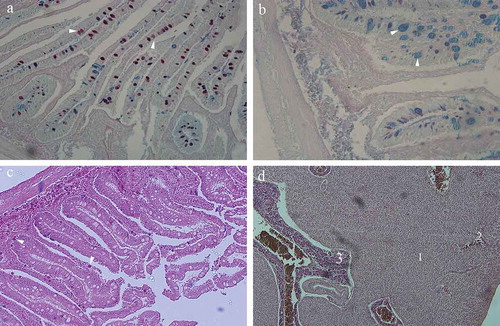
All three parts of the intestines showed the same histological features. They all consisted of a four-layered wall containing muscosa, submucosa, muscular layer and outer layer (). The intestinal mucosa is a three-layered structure containing epithelium, lamina propria and muscularis mucosae, all included in deep finger-like folds entering the lumen. The intestinal epithelium is a simple columnar with microvilli. Some goblet cells are disposed among columnar cells. The columnar epithelial cells usually contained one nucleus placed at the basal domain of the cell. The goblets cells are big, blister-like cells containing mucus. Each goblet cell opens into the intestinal lumen. When we used Alcian-blue/PAS method, the goblet cells of the mucosa in the upper parts of the intestines stained predominantly red, indicating neutral polysaccharides (); while in the lower parts of the intestines, goblet cells stained predominantly blue, indicating acid mucopolysaccharides (). A narrow layer of the connective tissue containing blood vessels enters the mucosal folds, thus forming the lamina propria of the intestinal mucosa. Occasionally, some cells containing acidophilic granules (stained distinctively red with hematoxylin–eosin dye), could be seen in the lamina propria (). These cells usually contained one eccentric nucleus. Some smooth muscle cells belonging to the muscularis mucosae separate lamina propria and submucosa. The main feature of the submucosal layer is an abundance of the granulated acidophilic cells previously described in the lamina propria. Numerous blood vessels were spread through the submucosal dense connective tissue.
Figure 2. (a) Cross section through the upper intestines of the garfish Belone belone: Mucosal goblet cells stained predominantly red indicating neutral polysaccharides (arrowhead). Alcian blue/PAS, ×20; (b) cross section through the lower intestines: Mucosal goblet cells stained predominantly blue, indicating acid mucopolysaccharides (arrowhead). Alcian blue/PAS, ×40; (c) intestinal mucosa: cells in the lamina propria containing acidophilic granules, stained distinctively red (arrowhead). Hematoxylin-eosin, ×10; (d) cross section through the liver parenchyma: 1 – plates of hepatocytes; 2 – sinusoides separating plates of hepatocytes; 3 – pancreatic acini incorporated into liver parenchyma. Hematoxylin-eosin, ×10.
The muscular layer of the intestinal wall consists of two distinctive layers of smooth muscles. The inner layer contains circularly placed smooth muscle cells with a centrally located nucleus. The nucleus has a typical corkscrew appearance in longitudinal section. The outer muscular layer consists of longitudinally arranged smooth muscle cells. Some sections through the nerve fibres could be seen among the muscle cells. The only structural difference between the three parts of the intestines is that the wall of the posterior intestine is slightly thinner than that of the anterior intestine.
The liver and the pancreas
The liver in garfish is a simple unilobular organ that surrounds the upper part of the intestines. Liver parenchyma consists of the anastomosing plates of hepatocytes separated by sinusoidal capillaries. The sinusoidal capillaries open into the central vein. Hepatocytes are round in shape, with usually one nucleus and lipid droplets in the cytoplasm. Pancreatic tissue is dispersed down the length of the intestines and the liver parenchyma. It consists of numerous serous acini, blood vessels and a system of ducts. The acinar cells are characterised by the presence of acidophilic zymogen granules in the apical cytoplasm ().
Discussion
It is known that the morphology of the digestive tract in fishes is in accordance with differences in their feeding habits, as was studied in numerous fish species (Al-Hussaini Citation1949; Bishop & Odense Citation1966; Bucke Citation1971; Clarke & Witcomb Citation1980; Cataldi et al. Citation1987; Grau et al. Citation1992; Albrecht et al. Citation2001; Nazlić et al. Citation2014; Bočina et al. Citation2016). In vertebrates, the digestive system consists of the alimentary canal and ancillary organs such as liver, gallbladder and pancreas (Stevens & Hume Citation1995). The morphology of the intestines is usually conservative among vertebrates, but stomach morphology shows remarkable diversity (Smith et al. Citation2000). In a number of freshwater and marine fishes such as the Cyprinidae, Labridae and Gobiidae, the stomach has been evolutionarily lost (Al-Hussaini Citation1949; Barton & Bond 2007). It is difficult to understand how stomachless fish digest food, especially the carnivorous ones with great protein uptake in their diet (Day et al. Citation2011), but it seems that this loss does not influence the digestion, as the stomachless fish can be either herbivorous or carnivorous. The herbivorous fishes usually have relatively long guts, whereas in carnivorous fishes the guts are generally shorter than their body length (Buddington et al. Citation1996; Horn et al. Citation2006; German et al. Citation2010).
The present study has shown that B. belone is a stomachless fish with relatively short guts. This is in accordance with its dietary habits since the garfish is a carnivorous fish. When analysing guts content of B. belone, Zorica and Čikeš Keč (Citation2012) found that 94% of prey items were animals, mostly euphasiids and copepods. The kind and size of the prey are usually related to the morphology and the size of the fish (Ross Citation1978; Stagioni et al. Citation2011). Generally, the jaw size, gut length, reproduction and spreading are related to trophic changes and to intraspecific resource distribution (Ross Citation1978). All beloniforms have large jaw apparatus; some functional differences depend usually on whether they are herbivorous or carnivorous (Day et al. Citation2011). In vertebrates, the stomach is a sac-like muscular organ separated from the oesophagus and intestines by sphincters, and is characterised by specialised cells that produce hydrochloric acid and pepsin (Stevens & Hume Citation1995; Smith et al. Citation2000). It seems that, due to its lack of a stomach, the oesophagus in B. belone is a well-developed glandular organ with mucosa deeply protruding into the lumen and containing abundant goblet cells, mostly organised in glandular acini and containing neutral polysaccharides. The main function of the oesophagus of different teleost fishes, as well as of most vertebrates, is transporting food bolus, so it is usually lined by stratified squamous epithelium and contains cells secreting mucus, thus enabling lubrication and extension of the organ when large prey is passed through (Cataldi et al. Citation1987; Abdulhadi Citation2005; Nazlić et al. Citation2014; Bočina et al. Citation2016). In some fish such as freshwater stingray Himantura signifer, the oesophagus is covered by stratified columnar epithelium (Chatchavalvanich et al. Citation2006). The oesophageal mucosa of the common eel Anguilla anguilla consists of stratified epithelium, columnar epithelium and goblet cells (Clarke & Witcomb Citation1980). Besides epithelium, a typical oesophageal mucosa contains lamina propria and lamina muscularis mucosae, although the latter is usually missing in fish (Kozarić et al. Citation2004; Nazlić et al. Citation2014). Although lamina propria could be clearly distinguished in the oesophageal mucosa of the B. belone; it is hard to differentiate circular striated muscle fibres of lamina muscularis mucosae from those of the underlaying muscular layer. Since the submucosal layer separating mucosa from the muscular layer is lacking in the oesophagus of B. belone, it is hard to say whether lamina muscularis mucosae really exist or not.
Morphology of the intestines varies among carnivorous fishes (Buddington et al. Citation1996). According to published data, intestine is usually divided into anterior and posterior parts (Abdulhadi Citation2005; Chatchavalvanich et al. Citation2006; Dai et al. Citation2007; Bočina et al. Citation2016). Via evolution the guts of carnivorous fishes have been adapted to a diet high in protein and low in carbohydrate (Buddington et al. Citation1996). The absorptive processes generally take place in the intestinal parts containing complex mucosa, and the absorption area of the intestine is increased by intestinal caeca of pyloric region (Canan et al. Citation2012). Generally, in fishes, the intestine wall is a three-layered structure containing mucosa, muscularis externa and serosa (Nazlić et al. Citation2014; Bočina et al. Citation2016). In garfish B. belone, all portions of the intestine show the same histological features. The only structural difference between analysed portions of the intestines is that the wall of the distal portion is slightly thinner than that of the proximal ones. They all consist of four-layered wall containing muscosa, submucosa, muscular layer and outer layer. The intestinal mucosa seemed to be a three-layered structure containing simple columnar epithelium, lamina propria and muscularis mucosae, all included in deep finger-like folds entering the lumen. Some goblet cells were found to be disposed among columnar cells. When treated with Alcian-blue/PAS, a significant difference among the intestinal portions was noticed: goblet cells of the upper intestines stained predominantly red, while those of the lower intestines stained predominantly blue. This indicates the different contents of mucopolysaccharides inside the goblet cells of upper and lower parts of the intestines. Goblet cells of the upper intestines contained neutral mucopolysaccharides while those of lower intestines contained acid mucopolysaccharides. So, we could say that, histologically, the intestines of the garfish are divided into two parts: anterior and posterior intestines. The intestinal simple columnar epithelium has been previously described in Scorpaena porcus (Nazlić et al. Citation2014) and Merluccius merluccius (Bočina et al. Citation2016), and goblet cells are usually disposed among epithelial cells of the fish gut (Abdulhadi Citation2005; Nazlić et al. Citation2014; Bočina et al. Citation2016). Abundant granulated acidophilic cells were found in the mucosal lamina propria as well as in the intestinal submucosa of B. belone. Intestinal goblet cells of Mylio cuvieri (Abdulhadi Citation2005) and Merluccius merluccius (Bočina et al. Citation2016) contain acid mucopolysaccharides, while neutral mucopolysaccharides were found in Tilapia spilurus (Abdulhadi Citation2005). It seems that the quality and quantity of the mucus from the intestinal goblet cells is related to environmental pollution (Ferrando et al. Citation2006). Environmental pollution can also influence the enzymatic activity of the liver (Fasulo et al. Citation2010). The liver in fish is usually a unilobular organ with fat storage as one of its many functions (Bucke Citation1971; Clarke & Witcomb Citation1980). The liver of Scorpaena porcus consists of two lobes, while that of Merluccius merluccius has three lobes (Nazlić et al. Citation2014; Bočina et al. Citation2016). In garfish, the liver consists of one simple lobe containing some pancreatic tissues spread out through the liver parenchyma. The pancreas in fish is usually spread out along the intestine or pyloric caeca or infiltrated in liver parenchyma (Cataldi et al. Citation1987; Bočina et al. Citation2016); or unusually compact (Bucke Citation1971; Clarke & Witcomb Citation1980). In some fishes, the pancreas contains islets of Langerhans (Bishop & Odense Citation1966; Clarke & Witcomb Citation1980).
In conclusion, the present study is the first record on garfish B. belone digestive system histology, suggesting that its histological features are mostly similar to those of other carnivorous fishes, and congruent with its feeding habits.
References
- Abdulhadi H. 2005. Some comparative histological studies on alimentary tract of tilapia fish (Tilapia spilurus) and sea bream (Mylio cuvieri). Egyptian Journal of Aquatic Research 31:387–397.
- Albrecht MP, Ferreira MFN, Caramaschi EP. 2001. Anatomical features and histology of the digestive tract of two related neotropical omnivorous fishes (Characiformes; Anostomidae). Journal of Fish Biology 58:419–430. doi:10.1111/jfb.2001.58.issue-2
- Al-Hussaini A. 1949. On the functional morphology of the alimentary tract of some fish in relation to differences in their feeding habits: Anatomy and histology. Journal of Cell Science 3:109–139.
- Barton M, Bond CE. 2006. Bond’s biology of fishes. Thomson. 3rd Edition. Thomson Brooks Cole. California, USA.
- Bilgin S, Taşçi B, Bal H. 2014. Population dynamics of the garfish, Belone euxini (Belonidae: Belone) from the south-east Black Sea. Journal of the Marine Biological Association of the United Kingdom 94:1687–1700. doi:10.1017/S0025315414000769
- Bishop C, Odense P. 1966. Morphology of the digestive tract of the cod, Gadus morhua. Journal of the Fisheries Research Board of Canada 23:1607–1615. doi:10.1139/f66-149
- Bočina I, Ružić S, Restović I, Paladin A. 2016. Histological features of the digestive tract of the adult European hake Merluccius merluccius (Pisces: Merlucciidae). Italian Journal of Zoology 83:26–33. doi:10.1080/11250003.2015.1113311
- Bucke D. 1971. The anatomy and histology of the alimentary tract of the carnivorous fish the pike Esox lucius L. Journal of Fish Biology 3:421–431. doi:10.1111/jfb.1971.3.issue-4
- Buddington RK, Krogdahl A, Bakke-McKellep AM. 1996. The intestines of carnivorous fish: Structure and functions and the relations with diet. Acta Physiologica Scandinavica. Supplementum 638:67–80.
- Canan B, Nascimento WSD, Silva NBD, Chellappa S. 2012. Morphohistology of the digestive tract of the damsel fish Stegastes fuscus (Osteichthyes: Pomacentridae). The Scientific World Journal 2012:1–9. doi:10.1100/2012/787316
- Cataldi E, Cataudella S, Monaco G, Rossi A, Tancioni L. 1987. A study of the histology and morphology of the digestive tract of the sea‐bream, Sparus aurata. Journal of Fish Biology 30:135–145. doi:10.1111/jfb.1987.30.issue-2
- Chatchavalvanich K, Marcos R, Poonpirom J, Thongpan A, Rocha E. 2006. Histology of the digestive tract of the freshwater stingray Himantura signifer Compagno and Roberts, 1982 (Elasmobranchii, Dasyatidae). Anatomy and Embryology 211:507–518. doi:10.1007/s00429-006-0103-3
- Clarke A, Witcomb D. 1980. A study of the histology and morphology of the digestive tract of the common eel (Anguilla anguilla). Journal of Fish Biology 16:159–170. doi:10.1111/jfb.1980.16.issue-2
- Collette B, Parin N. 1970. Needlefishes (Belonidae) of the eastern Atlantic Ocean. Atlantide Report 11:7–60.
- Dai X, Shu M, Fang W. 2007. Histological and ultrastructural study of the digestive tract of rice field eel, Monopterus albus. Journal of Applied Ichthyology 23:177–183. doi:10.1111/jai.2007.23.issue-2
- Day RD, German DP, Manjakasy JM, Farr I, Hansen MJ, Tibbetts IR. 2011. Enzymatic digestion in stomachless fishes: How a simple gut accommodates both herbivory and carnivory. Journal of Comparative Physiology B 181:603–613. doi:10.1007/s00360-010-0546-y
- Dorman J. 1989. Some aspects of the biology of the garfish Belone belone (L.) from southern Ireland. Journal of Fish Biology 35:621–629. doi:10.1111/jfb.1989.35.issue-5
- Dorman J. 1991. Investigations into the biology of the garfish, Belone belone (L.), in Swedish waters. Journal of Fish Biology 39:59–69. doi:10.1111/jfb.1991.39.issue-1
- Fasulo S, Marino S, Mauceri A, Maisano M, Giannetto A, D’Agata A, Parrino V, Minutoli R, De Domenico E. 2010. A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicology and Environmental Safety 73:1565–1573. doi:10.1016/j.ecoenv.2010.01.008
- Ferrando S, Maisano M, Parrino V, Ferrando T, Girosi L, Tagliafierro G. 2006. Gut morphology and metallothionein immunoreactivity in Liza aurata from different heavy metal polluted environments. Italian Journal of Zoology 73:7–14. doi:10.1080/11250000500502228
- German DP, Nagle BC, Villeda JM, Ruiz AM, Thomson AW, Balderas SC, Evans DH. 2010. Evolution of herbivory in a carnivorous clade of minnows (Teleostei: Cyprinidae): Effects on gut size and digestive physiology. Physiological and Biochemical Zoology 83:1–18. doi:10.1086/648510
- Grau A, Crespo S, Sarasquete MC, González de Canales ML. 1992. The digestive tract of the amberjack Seriola dumerili, Risso: A light and scanning electron microscope study. Journal of Fish Biology 41:287–303. doi:10.1111/jfb.1992.41.issue-2
- Horn M, Gawlicka A, German D, Logothetis E, Cavanagh J, Boyle K. 2006. Structure and function of the stomachless digestive system in three related species of New World silverside fishes (Atherinopsidae) representing herbivory, omnivory, and carnivory. Marine Biology 149:1237–1245. doi:10.1007/s00227-006-0281-9
- Kierszenbaum AL, Tres LL. 2012. Histology and cell biology. An introduction to pathology. Philadelphia: Elsevier. pp. 701.
- Kozarić Z, Kužir S, Nejedli S, Petrinec Z, Srebočan E. 2004. Histochemical distribution of digestive enzymes in hake, Merluccius merluccius L. 1758. Veterinarski Arhiv 74:299–308.
- Nazlić M, Paladin A, Bočina I. 2014. Histology of the digestive system of the black scorpionfish Scorpaena porcus L. Acta Adriatica 55:65–74.
- Ross S. 1978. Trophic ontogeny of the Leopard searobim, Prionotus scitulus (Pisces: Triglidae). Fishery Bulletin 76:225–234.
- Sever T, Bayhan B, Bilge G, Taå Kavak E. 2009. Diet composition of Belone belone (Linnaeus, 1761) (Pisces: Belonidae) in the Aegean Sea. Journal of Applied Ichthyology 25:702–706. doi:10.1111/jai.2009.25.issue-6
- Sheehan DC, Hrapchak BB. 1980. Theory and practice of histotechnology. Battelle Press, Ohio, USA.
- Smith DM, Grasty RC, Theodosiou NA, Tabin CJ, Nascone‐Yoder NM. 2000. Evolutionary relationships between the amphibian, avian, and mammalian stomachs. Evolution & Development 2:348–359. doi:10.1046/j.1525-142x.2000.00076.x
- Stagioni M, Montanini S, Vallisneri M. 2011. Feeding habits of European Hake, Merluccius merluccius (Actinopterygii: Gadiformes: Merlucciidae), from the Northeastern Mediterranean Sea. Acta Ichthyologica Et Piscatoria 41:277–284. doi:10.3750/AIP2011.41.4.03
- Stevens C, Hume I. 1995. Microbial fermentation and synthesis of nutrients and the absorption of end products. Comparative physiology of the vertebrate digestive system. New York: Press Syndicate. pp. 188–228.
- Treer T, Safner R, Aničić I, Lovrinov M. 1995. Ribarstvo. Zagreb: Nakladni zavod Globus. p. 463.
- Zorica B, Čikeš Keč V. 2012. Preliminary observations on feeding habits of garfish Belone belone (L., 1761) in the Adriatic Sea. Croatian Journal of Fisheries 70:53–60.
- Zorica B, Sinovčić G, Čikeš Keč V. 2011. The reproductive cycle, size at maturity and fecundity of garfish (Belone belone, L. 1761) in the eastern Adriatic Sea. Helgoland Marine Research 65:435–444. doi:10.1007/s10152-010-0233-0
