Abstract
Specific root length (SRL, m g−1) is probably the most frequently measured morphological parameter of fine roots. It is believed to characterize economic aspects of the root system and to be indicative of environmental changes. The main objectives of this paper were to review and summarize the published SRL data for different tree species throughout Europe and to assess SRL under varying environmental conditions. Meta-analysis was used to summarize the response of SRL to the following manipulated environmental conditions: fertilization, irrigation, elevated temperature, elevated CO2, Al-stress, reduced light, heavy metal stress and physical disturbance of soil. SRL was found to be strongly dependent on the fine root classes, i.e. on the ectomycorrhizal short roots (ECM), and on the roots <0.5 mm, <1 mm, <2 mm and 1 – 2 mm in diameter SRL was largest for ECM and decreased with increasing diameter. Changes in soil factors influenced most strongly the SRL of ECM and roots <0.5 mm. The variation in the SRL components, root diameter and root tissue density, and their impact on the SRL value were computed. Meta-analyses showed that SRL decreased significantly under fertilization and Al-stress; it responded negatively to reduced light, elevated temperature and CO2. We suggest that SRL can be used successfully as an indicator of nutrient availability to trees in experimental conditions.
Introduction
Root systems are known to show a high degree of plasticity in their development in response to local heterogeneity of the soil. On the level of the individual root and the entire root system, various morphological parameters, which are influenced by genetic variability and environmental conditions, have been used as potential indicators of the mineral nutrition of trees on forest soils. These parameters include root length density (RLD, cm cm−3 soil; Majdi, Citation2001), branching pattern (Kottke, Citation2002), specific root area (SRA, m2 kg−1) and specific root length (SRL, m g−1) (Leuschner et al., Citation2004; Ostonen et al., 2007). Among these potential indicators, SRL is probably the most commonly measured morphological parameter of fine roots because it is believed to characterize the economic aspects of root systems. SRL is the length-to-mass ratio (L/M) of a root fragment. The root mass can be replaced by the product of the root tissue density (RTD) and the volume (V), and the volume by a function of root length, diameter (D), and π:
Fitter (Citation1976, Citation1985, Citation1991) was one of the first authors to apply SRL and proposed the length/mass ratio as an index of root benefit to root cost. The root length is assumed to be proportional to resource acquisition (benefit) and the root mass to be proportional to construction and maintenance (cost) (Eissenstat & Yanai, Citation1997). Long and thin roots (high SRL) are believed to be the below-ground equivalent of thin leaves, which are less expensive to produce (Withington et al., Citation2006).
The cost/benefit ratio of operating roots is a key factor for forest productivity according to the optimality theory, (Eissenstat & Yanai, Citation1997). Under natural conditions, trees are exposed to multiple stresses. To sustain and improve mineral nutrition, trees must either use an extensive strategy and invest assimilates, which leads to an increase in biomass and length in the fine root system, or an intensive strategy, with morphological adaptations of the fine roots (Lõhmus et al., Citation2006a). Morphological adaptations of the fine roots enable trees to grow even under harsh soil conditions (Ostonen et al., Citation2006). However, different tree species appear to have different adaptation strategies for optimizing the mineral nutrition of the plant (Comas et al., Citation2002; Curt & Prevosto, Citation2003; Comas & Eissenstat, Citation2004). Increasing SRL is one of the possible fine root morphological parameters (intensive strategy), which increases the volume of soil exploited per unit biomass invested in the fine roots.
In this review we analyzed intra- and interspecific variation of SRL and its components, root diameter (D, mm) and root tissue density (RTD, kg m−3), with the intention of assessing the suitability of SRL as an indicator of environmental change. We took published data on SRL in natural forests stands across Europe to obtain the natural variation of SRL and SRL in treated forest stands and in pot-studies worldwide to obtain the variation of SRL under stressed conditions. If sufficient data were available, SRL was differentiated between the various fine root diameter classes (<1 mm, <2 mm, 1 – 2 mm) and the ectomycorrhizal short roots with primary structure (ECM).
Guo et al. (Citation2004) reported that short roots (orders 1 and 2) could account for more than 75% of the total root length and more than 60% of the total root surface area. King et al. (Citation2002) claimed that about 80% of the root length occurred in fine roots with diameters <1 mm. Whereas the ectomycorrhizal and non-ectomycorrhizal short roots of ectomycorrhizal trees are functionally responsible for nutrient and water uptake, the long fine roots <2 mm in diameter are functionally responsible for the spread and stability of the fine root system and for the nutrient transport to the coarse roots (>2 mm in diameter).
The objectives of this paper were: (1) to review and summarize published SRL data on different tree species in a wide range of European forest sites, (2) to assess SRL change under varying environmental conditions such as fertilization (N, liming or ash), elevated CO2, Al-stress, reduced light, heavy metal pollution, and physical disturbance of soil, and varying moisture and temperature levels, (3) to analyze the intra- and interspecific variation of SRL and its components, root diameter and root tissue density, in different tree species, with different fine-root diameters and methods (forest stands versus pots), and (4) to evaluate the use of SRL as an indicator of environmental change.
Materials and methods
The data were collected from a literature search and from the published data of the participants in the COST Action E38 ‘Woody Root Processes’ (Appendixes 1 and 2).
Natural variability of SRL
The material collected to describe the SRL variation in natural forest stands consisted of data from 22 Picea abies stands, 14 Pinus sylvestris, 9 Fagus sylvatica, 8 Betula pendula, 6 Alnus glutinosa and Quercus robur, 5 Pseudotsuga menziesii, 3 Alnus incana, 1 Picea sitchensis, Pinus nigra, and Abies alba distributed across Europe (Appendix 1). The fine root data included four classes: ECM, <1 mm, <2 mm, and 1 – 2 mm. In general, the mean SRL values of the topsoil (most likely O- and/or Ah – horizon) were used in the analyses. It was assumed that this sampling depth characterized the fine roots of the given forest stand.
Roots were collected mostly by soil coring, and to a lesser extent by the ingrowth core method.The length of roots was determined using different scanning methods and image analyses.
We analyzed the variation of D and RTD in both ECM and fine root classes by calculating the coefficients of variation (cv). For ECM we analyzed random samples for Picea abies, Pinus sylvestris, and Betula pendula short roots in three different sites (Kivalo, Voore, Kuusnõmme), using the database of Ostonen et al. (2007) and the means of D and RTD for Picea abies given by Ostonen et al. (2007) and Withington et al. (Citation2006). For the fine root (<2 mm) class we calculated RTD and used the mean D for Picea abies from Borken et al. (Citation2007) and Fagus sylvatica from Curt & Prevosto (Citation2003).
In order to analyze the effects of different factors on the mean SRL, the geographical position (latitude), climate (mean annual precipitation and temperature) and forest stand characteristics (basal area, tree density, height and age) of the data collection sites were included in the statistical analysis if sufficient data were available.
Statistical analysis
For the statistical analysis, multiple comparisons of means were applied using the Tukey test for unequal number (n) with 95% confidence intervals (CI) to test for differences in SRL values between the root classes and tree species. In addition, correlation analyses were performed to detect relations between SRL and the geographical position, climate and forest stand characteristics of the stand. The coefficients of variation (100 × (SD/mean)) were calculated for random samples and sample means of D and RTD of different species to estimate the relation of either D or RTD to SRL samples, for the <1 mm root class collected by two different methods, ingrowth and soil cores, and the coefficients of variation calculated. The interdependence of D and RTD was checked with correlation analysis. t-test for independent samples was used to evaluate the differences in the means between the samples in the <1 mm root class collected using ingrowth or soil cores. Statistical analyses were conducted using software Statistica 7.0.
Meta-analysis of treatment effects on SRL
We used meta-analyses to summarize the responses of SRL to manipulated environmental conditions. The studies included were conducted in woody plant biomes such as forests and shrubland, as well as under controlled conditions both outdoors and indoors. A larger number of species were included in the meta-analysis (Appendix 2) than the range of tree species used for describing SRL variability in natural forest stands (Appendix 1). The roots of the woody plants included in the meta-analyses had a maximum diameter of 2 mm.
Meta-analyses were performed to determine the significance of SRL responses to nutrient enrichment, irrigation, drought, increased temperature, physical disturbance of soil (soil mixing and homogenization for ingrowth cores), Al-stress, elevated CO2, heavy metal stress, and shading.
Consecutive measurements of the SRL values (at times t 0, t 1, …) within a given study produce dependent data, so only the last measurement was included in meta-analyses. This was done to allow the full response of the SRL to a given treatment, and, most important, to guarantee the assumption of meta-analysis that the data must be independent. Different doses of fertilizer applied within one experiment were, however, handled as independent studies because root morphology can be affected differently by low or high doses of a nutrient (e.g. Kakei & Clifford, Citation2002). When several tree species were tested within one study, they were designated as independent studies, assuming that the roots of the different tree species have different adaptation strategies.
The data obtained from the studies consisted of: the mean value of the control group and the mean value of the experimental group, and the respective standard deviations (SD) and replicate numbers (n). Where results are presented graphically, the values were estimated from figures manually digitized. The reported standard errors (SE) were transformed to SD using the equation
When no variability statistic was reported in a given study, SE was calculated as 20% of the mean. The 20% was assumed not to underestimate variability because our calculation showed that the mean proportion of SE for the studies where the variability statistic was presented was 14% of the means.
In order to measure the ‘effect size’ (magnitude of response to an experimental manipulation), the response ratio was calculated by dividing the mean SRL value of the experimental group by that of the control group for each study and forming the natural log (Gurevitch & Hedges, Citation2001). The statistical software MetaWin v. 2.1 was used to perform meta-analyses (Rosenberg et al., Citation2000). Assuming random variation among the studies, the ‘mixed or random effects model’ was chosen for the analyses. Unless the estimate of the pooled transformed variance was less than or equal to zero, the data were analyzed using a fixed effects model (Rosenberg et al., Citation2000; Gurevitch & Hedges, Citation2001).
Effects of categorical variables – types of treatment, group of tree species (deciduous, coniferous), root diameter class (<0.5 mm, <1 mm, 1 – 2 mm, <2 mm), sampling method (ingrowth core, soil coring, pots) – were examined in separate meta-analyses. The results present the weighted mean response ratio (R), the 95% CI for R with lower and upper limits, and the number of observations (n). A mean response ratio of 1.00 indicates no effect. If both limits of the 95% CI are >1.00, then R is considered significantly positive (SRL increase): If both limits of the 95% CI are <1.00, then R is considered significantly negative (SRL decrease). The means of response variables were tested for significant differences based on the model heterogeneity tests (Q-test). The total homogeneity Q T is partitioned into ‘between-class homogeneity’ Q B and ‘within-class homogeneity’ Q W. The Q statistics follows a χ 2 distribution, with the degrees of freedom equal to the total number of studies minus 1, and with the statistical significance evaluated using a standard χ 2 table (Gurevitch & Hedges, Citation2001).
Results
The mean estimate of SRL has been reported to range from 1.4 to 196 m g−1 in different parts of the fine root system in different tree species (Appendix 1). The SRL is given most often for fine roots (<2 mm in diameter) in the literature, and less frequently also for the finest (<1 mm in diameter) and for the ectomycorrhizal short roots (mostly first and second order, tips regarded as first order). The ECM root class consisted of roots with primary structure. The finest and the fine root fraction consisted of both roots with primary and secondary structure. The variability of SRL values within different root classes and between tree species is described in the following.
SRL in root classes: ECM, <1 mm, <2 mm, and 1 – 2 mm
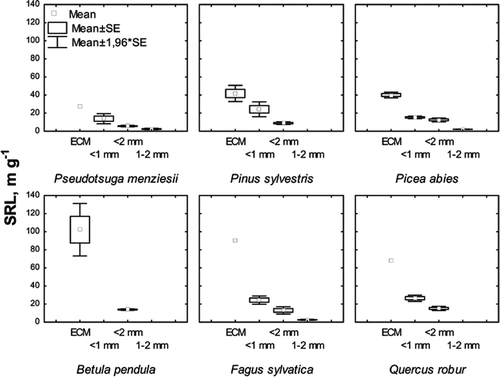
The mean SRL of ECM was more than three times higher than SRL for fine roots (<2 mm in diameter) in different tree species (). SRL decreased in all cases in the following order: (ECM) > (<1 mm) > (<2 mm) > (1 – 2 mm). The extent of SRL variation was very small within the 1 – 2 mm diameter root class.
SRL in different tree species
Fine root SRL depended significantly on tree species. In the whole SRL dataset collected for this review (see Appendix 1), the SRL was significantly higher for deciduous trees than for coniferous trees ( and ) within all root diameter classes except 1 – 2 mm (Tukey test, p < 0.05).
Figure 2. Mean SRL values (±SE) for ECM (A) and fine roots (<2 mm) (B) in coniferous and deciduous tree species. There is a tenfold difference in the scale of the y-axis, between A and B.
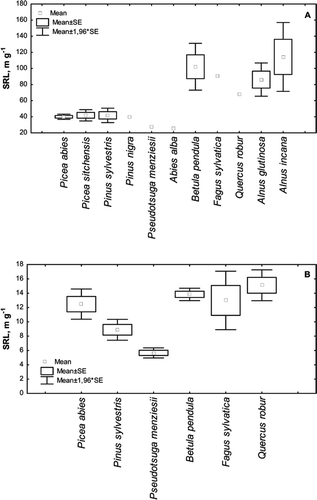
The difference in SRL between tree species appeared mostly in the ECM class (; deciduous > coniferous). The difference in the SRL between different deciduous and coniferous trees was not obvious for the most frequently measured root class <2 mm in diameter (). There was a significant difference between Picea abies and Pseudotsuga menziesii within the coniferous species, but no significant difference was found between deciduous species.
D or RTD: Which determines the value of SRL?
SRL is a complex parameter that includes variations in root diameter and root tissue density, which responded to environmental conditions differently. We have therefore attempted to find out whether D or RTD contributes more to the value of SRL.
For the absolute values of root tissue density and diameter in ectomycorrhizal short roots and fine roots (<2 mm), the RTD was significantly higher for fine roots (<2 mm) but no significant difference for mean D was found between these two root classes.
We verified how strongly D and RTD were correlated. Out of 12 datasets, only three had significant correlations (), although three of the datasets had a low sample number (<10). For our purposes, we concluded that correlations between D and RTD were not a major concern.
Table I. The coefficients of variation (CV) for random samples and sample means of diameter (D) and root tissue density (RTD) of ECM and fine roots (<2 mm) in different species
To compare the variability of both parameters, D and RTD, in the ECM and the fine root (<2 mm) class, we calculated coefficients of variation. The variability of RTD was higher than that of D in all cases ().
SRL in relation to latitudinal location, climate and soil pH
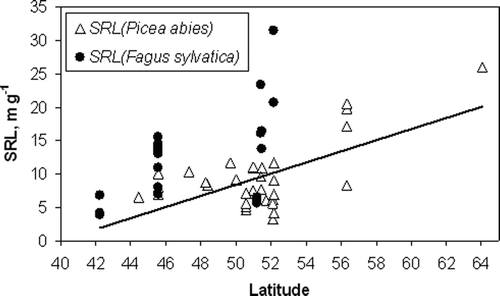
There was significant correlation between the location of forest stands in latitudes and the SRL value of fine roots (<2 mm) in Picea abies (r = 0.80; n = 20; p < 0.001). Even when we omitted the SRL value at the highest latitude in Flakaliden (Sweden), the correlation between the stand location and the SRL value of the fine roots in Picea abies remained significant (r = 0.64; n = 19; p < 0.001; ). No significant correlation for Fagus sylvatica was found. However, the variation of SRL values in beech fine roots at higher latitudes tended to be greater ().
Figure 3. Correlation analysis between SRL and the latitudes of Picea abies and Fagus sylvatica fine roots (<2 mm). Trendline is added only for Picea abies.
We analyzed the impact of the two climate characteristics of sites, mean annual precipitation, and mean annual temperature, separately. The SRL of fine roots (<2 mm) of Fagus sylvatica was positively correlated with the mean annual precipitation (r = 0.72, n = 12; p < 0.01), but no correlation with the mean annual temperature was found. Annual precipitation was negatively correlated (r = −0.58, n = 12; p = 0.05) with latitudes in studies of Fagus sylvatica. No significant correlations between SRL and annual precipitation or mean annual temperature for Picea abies were found.
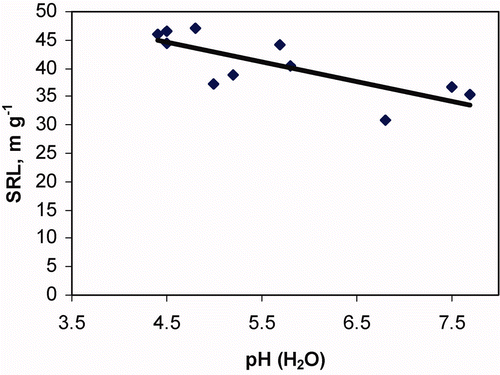
The SRL of ectomycorrhizal short roots in Picea abies was significantly negatively correlated with soil pH (H2O) (r = −0.77; p < 0.01; n = 11) ().
Influence of sampling method on SRL
We used the SRL values reported for Pinus and Picea (n = 35) to compare three different methods: pot-studies, ingrowth cores, and soil cores ().
Figure 5. Influence of the sampling method on the mean SRL values (±SE; ±1.96 × SE) of three fine-root diameter classes.
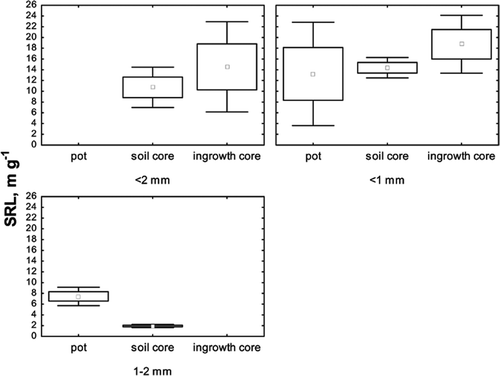
The SRL values increased in the following order: soil cores < ingrowth cores < pot-studies. The variation coefficients of the SRL values for the finest roots (<1 mm) collected using two common methods, soil cores, and ingrowth cores were 18% (n = 6) and 36% (n = 7), respectively. To evaluate the difference in means in the SRL of <1 mm roots collected by ingrowth cores and soil cores, we used a t-test for independent samples. Mean SRL values were significantly higher for the finest roots (<1 mm) collected by ingrowth cores (p < 0.05).
The SRL values of 1 – 2 mm roots were significantly higher for pot-studies than for the same root class collected by soil cores.
SRL as an indicator of environmental change
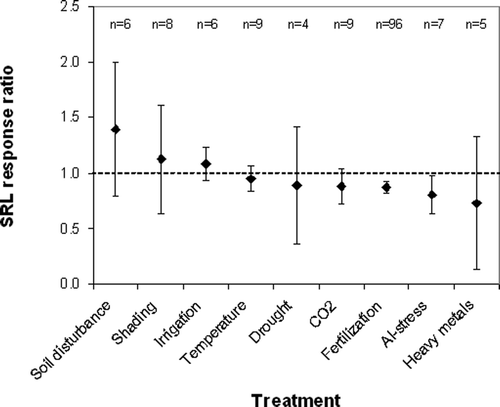
The suitability of SRL as an indicator of environmental change was analyzed using meta-analysis. The mean response ratios of SRL in relation to several environmental factors are presented in . Fertilization (including all fertilization types) and Al-stress/acidification (low Ca/Al molar ratio in soil or nutrient solution) reduced SRL significantly by 13% and 20%, respectively. SRL was affected not significantly by all the other treatments: soil disturbance by homogenization for ingrowth cores (39%), shading (12%), irrigation with water (8%), elevated temperature (−5%), drought (−11%), elevated CO2 (−12%), and heavy metal stress (−27%, to Cu, Cd, Zn, Pb). The treatment groups, estimated by Q T values (not shown), were homogeneous (p > 0.05).
Figure 6. Meta-analysis of treatment effects on the SRL response ratio: mean values and 95% CI. The analyses summarize data from deciduous and coniferous trees, all sampling methods in pots and in the field, and root diameter classes <0.5, <1, 1 – 2, and <2 mm.
To explore how fertilization type, root diameter, group of tree species, sampling method and duration influence SRL responded to treatments, the data were further partitioned into classes (). There was a significant difference in SRL responses between different fertilization types (p = 0.005). Mineral N additions reduced SRL significantly by 19%. Other fertilization treatments, such as liming (14%), organic N (13%), P (24%), elements applied in optimal ratio (−17%), and wood ash fertilization (−4%) did not affect SRL responses significantly.
Table II. Meta-analyses of the treatment effects on SRL, partitioned into classes by type of treatment, group of tree species (coniferous, deciduous), root diameter, length of study, and sampling method.a
There was no significant difference in SRL responses between coniferous and deciduous trees, either to optimal and mineral N fertilization or to liming. However, the SRL of coniferous tree roots after liming was conspicuously positive (32%), while the deciduous group showed hardly any response (−1%).
Partitioning on a root-diameter class basis indicated that, with optimal fertilization, the responses of root classes <0.5 mm and <1 mm diameter were significantly different (p = 0.035). The mean SRL response was more negative (−34%) in <0.5 mm than in the <1 mm class (−2%). The 95% confidence intervals of the groups overlapped, which was not surprising because the <1 mm group contains <0.5 mm roots, although these are not distinguished. There were highly significant differences between the diameter classes in terms of SRL responses to all fertilizers (p < 0.001). The responses were significantly different between the 1 – 2 mm and the <2 mm diameter root classes, between the <1 mm coniferous and the <1 mm deciduous, the 1 – 2 mm coniferous and the <2 mm deciduous root-diameter classes, and between the <1 mm deciduous, the 1 – 2 mm coniferous and the <2 mm deciduous root- diameter classes, as the 95% CI between them did not overlap. The fertilization treatments overall significantly reduced the SRL responses of the root classes <1 mm and 1 – 2 mm by 14% and 28%, respectively. In particular, there were clear significant reductions of SRL in deciduous tree roots <1 mm (23%) as well as in coniferous 1 – 2 mm tree roots (28%).
The length of the studies ranged from 4 weeks to 9 years. The duration of fertilization experiments was significant for the SRL response ratio if the length of the study was either <0.5 or 0.5 – 1.0 year (−23% both), but not when the experiments lasted >1 year.
Sampling methods had a significant effect on the SRL response ratio (Q B = 27.26, p < 0.001) depending on whether SRL was estimated from pot-grown roots (−22%) or from soil cores from the field or ingrowth (4%). Sampling from ingrowth cores and from field soil cores also differed significantly, with either a 6% increase or an 8% decrease in the SRL response to fertilization, respectively.
Mycorrhizal type affected the SRL response to fertilizations significantly only in ectomycorrhizal trees (−15%).
The responses of SRL to the treatments irrigation and combined treatments irrigation, elevated temperature and fertilization varied significantly (p < 0.001 and p < 0.002) ().
Temperature effects on the SRL response ratio were not significant () unless the temperature treatment was combined with fertilization (−28%, ), or with a combined treatment of fertilization, irrigation, and CO2 enrichment (−29%). Responses of SRL to CO2 enrichment varied significantly among root diameter classes (p = 0.001), coniferous and deciduous trees (p = 0.001), and a combined treatment of CO2 and fertilization (p = 0.017). CO2 enrichment combined with elevated temperature and fertilization reduced SRL (−53%, p < 0.001, ).
Discussion
Specific root length within the fine root system
Our analysis of the SRL values of different tree species revealed considerable variation within the fine root system (<2 mm). There was a strong correlation between the root (diameter) class and the SRL value in the fine-root system of different tree species (). SRL increased with decreasing root diameter: (<2 mm) < (<1 mm) < (ECM), which indicates that the economics in the root classes differ. This is in good agreement with Pregitzer et al. (Citation2002), who reported that the roots in an arbitrary fine-root size-class do not function the same way, and that their C cost for both construction and maintenance could be different (Pregitzer et al., Citation2002). King et al. (Citation2002) suggested that the term ‘fine roots’ is a mix of static and dynamic root fractions and that the size class below which root properties and function shift from those of perennial to ephemeral is much smaller than the commonly used 2.0 mm diameter class. ECM short roots include mostly first (tips are considered as first order) and second order short roots with primary structure, so that the results of King et al. (Citation2002) can be compared with those of Pregitzer et al. (Citation2002) and Guo et al. (Citation2004). Both studies showed that there is a decrease in SRL values with increasing root order and that SRL is smaller for roots with secondary structure. Root diameter class <2 mm may involve roots of at least orders 1 – 5, as exemplified with Pinus palustris on sandy soil, where SRL was 47 times smaller in order 5 roots than in order 1 roots. The orders 1 – 4 contained roots with diameters 0.35 – 0.79 mm, and the mean diameter in order 5 was 1.56 mm (Guo et al., Citation2004).
The SRL of fine roots (<2 mm) represents a mean of all root classes and its value varies proportionally with root class. For example, the proportions of roots with diameters 0 – 1 mm of total fine root (<2 mm) biomass have been shown to vary from 62% to 72% for Picea abies (Claus & George, Citation2005; Ostonen et al., Citation2005) and from 60% to 63% for Quercus cerris (Claus & George, Citation2005). The proportion of ECM has been calculated to reach around 39% of the finest root (<1 mm) biomass in Picea abies (Ostonen et al., Citation2005), but empirical data showing the proportions of different root diameter classes in a fine root system are lacking.
The more stable (or static) parts in fine root systems are, irrespective of tree species, the woody fine roots (1 – 2 mm), which are responsible for nutrient transport, spreading and stability. The SRL for fine roots 1 – 2 mm in diameter varied in natural forest stands from 1.4 to 2.8 m g−1 for conifers (Hendriks & Bianchi, Citation1995; Majdi & Viebke, Citation2004; Püttsepp et al., Citation2006) and from 2.3 to 2.8 for beech (Hendriks & Bianchi, Citation1995).
Specific root length across tree species
Differences in the SRL values of different tree species were especially clear for ectomycorrhizal short roots, which were about 2.7 times higher for deciduous tree species than for coniferous species (). This accords well with Pregitzer et al. (Citation2002), who reported that SRL was more than twice as high for Acer saccharum, Populus balsamifera and Quercus alba as for conifers. Ectomycorrhizal short roots are the most variable part of the fine root system. Their morphology is greatly influenced by both biotic and abiotic factors, including associated fungi (Ostonen & Lõhmus, Citation2003), rhizosphere microbial communities (Lõhmus et al., Citation2006b), moisture and nutrient content (Robinson & Rorison, Citation1983). There may be some basic differences in the root systems of gymnosperms and angiosperms. Evergreen conifers typically have thicker roots and lower SRL than coexisting deciduous angiosperms (Reich et al., Citation1998; Bauhus & Messier, Citation1999).
The differences between the SRL values of fine roots (<2 mm) in hardwoods (Fagus sylvatica, Betula pendula, Quercus robur) and conifers (Picea abies, Pinus sylvestris, Pseudotsuga menziesii) were tested for significance. Pinus sylvestris and Pseudotsuga menziesii were found to have significantly lower SRL values (), but the other four species showed no significant differences in their SRL values. When coniferous and deciduous trees were compared in the meta-analysis (<2 mm) in our study, there was no significant group difference between the SRL responses to either optimal, mineral N fertilization or liming ().
Specific root length, root tissue density, and diameter
The response of mean D and mean RTD to different site conditions and environmental stress factors may have contrary effects on SRL. Ryser (Citation1998) has demonstrated the effect of variation in RTD and D on SRL for grass species. The RTD of ECM has been shown to range from 113 to 196 kg m−3 for Picea abies, from 127 to 248 kg m−3 for Pinus sylvestris, and from 59 to 156 kg m−3 for Betula pendula. The mean D for short roots has been found to vary between 0.40 and 0.53 for Picea abies, 0.40 and 0.48 for Pinus sylvestris, and 0.35 and 0.46 mm for Betula pendula (Withington et al., Citation2006; Ostonen et al., 2007).
The RTD of Picea abies fine roots was calculated from Borken et al. (Citation2007) to vary between 169 and 569 kg m−3 and the mean diameter from 0.44 to 1.00 mm in different sites. The RTD for the fine roots of beech was calculated from Curt & Prevosto (Citation2003) to vary between 161 and 494 kg m−3 and the D between 0.28 and 0.41 mm.
It has been hypothesized that most of the differences observed in the SRL values of different tree species are due to differences in the mean root diameter (Pregitzer et al., Citation2002) because SRL is inversely dependent on the square of root diameter (see EquationEquation 1). In our study, the variation of RTD was higher than the variation of D in all cases () but the contribution of root diameter to SRL value is squared, which means the impact of root diameter is more essential for SRL. However, variation in the RTD within fine roots and its impact on the value of SRL has hardly been investigated. A few studies note the importance of further research to determine the RTD of fine roots (Eissenstat & Yanai, Citation1997; Ostonen et al., Citation1999; Bernier et al., Citation2005; Withington et al., Citation2006).
The correlation analysis did not reveal a significant relationship between D and RTD. Hence, although the D and RTD of ECM and/or fine roots were correlated in three forest stands, the parameters seem to be mainly independent (). The independence of the RTD and D of ECM is probably associated with the diversity of ectomycorrhizal fungi in different forest stands. The impact of fungal symbiont on ECM morphological characteristics is significant (Ostonen et al., Citation1999; Ostonen & Lõhmus, Citation2003), and this may well have an effect on SRL. In our study, the SRL of ECM trees decreased significantly in response to fertilizations by 15% (), which can be related to changes in ECM communities with different morphologies. Withington et al. (Citation2006) found RTD to be correlated with the life span of short roots, but mean D as well as SRL were not correlated with the life span of short roots in the 11 tree species in their study.
The significant difference in the RTD, but insignificant difference in mean D, between ECM and the fine roots could probably be partly explained by the sloughing off of the cortex, when transition from primary growth to secondary growth occurs. The D decreases and RTD increases at the same time. The significant difference in the RTD between ECM and the fine roots also indicates the effect of the woody root morphology on the SRL value within each fine-root size-class.
Specific root length as a stress indicator
Our expectations that we would be able to make generalizations about SRL in the context of different soil conditions in natural forests were only fulfilled in a few cases. One reason for this was the lack of data in different fine-root size-classes. The extent of the SRL response depends on the root function within the fine root system. SRL response to all fertilizations was significantly different across root diameter classes ().
The effect of pH was significant for the ECM in Picea abies, and SRL decreased with increasing pH. It has been hypothesized that SRL is linked with root-nutrient uptake efficiency (Eissenstat, Citation1992; Eissenstat et al., Citation2000), so we can presume that lower SRL in soils with high pH indicate decreased nutrient uptake per root length. In manipulated studies, the effect of liming on SRL was not significant across all root diameter classes <2 mm and different tree species ().
Leuschner et al. (Citation2004) found no differences in the specific root area (cm2 g−1) of fine roots along a pH(KCl) gradient (from 2.9 to 6.7) in six Fagus sylvatica stands. Ryser (Citation2006) provides a short overview of the sometimes contradictory responses of SRL (and in root morphology in general) to soil conditions. For instance, the SRL of fine roots has been shown to increase, decrease, or stay constant in response to nutrient limitation (Ryser, Citation1998). In manipulated studies fertilization and Al stress that can occur in acidic soils, both resulted in significantly decreased SRL (). Enhanced nutrient availability under fertilization reduces the need for explorative fine root length growth and thus account for a decrease in SRL. The results presented in show that the SRL decreased (−19%) significantly at increased concentrations of fertilizer N. All other fertilizations did not affect SRL response significantly. However, for some fertilizer types, the number of observations was low. Compared with other mineral nutrients, the concentration of N in tree fine roots is relatively high, around 0.9 – 2.0% of dry weight (e.g. Genenger et al., Citation2003), and due to its physiological importance, changes in the availability of N may readily affect root morphology. Olsthoorn et al. (Citation1991) found that N supply affected SRL response; and that high levels of NH4 were linked with a decrease in SRL of Pseudotsuga meziesii seedlings.
Soil acidity affects root morphology, production, and turnover (Jentschke et al., Citation2001). Vanguelova et al. (Citation2007) found that increased acidity, together with decreased cations in the forest floor and the top mineral layer of a podzol, decreased significantly (p < 0.01) the SRL of Pinus sylvestris fine roots (<2 mm). Godbold et al. (Citation2003) suggested that, under acidic, adverse conditions, the fine roots have to be renewed more often to maintain their resource exploiting function. Higher turnover rates, and therefore shorter life spans, may result in smaller SRL in forest stands. Under controlled conditions, high Al concentrations may hamper root length growth and cause thickening of roots, which is expressed by a decrease in SRL (McQuattie & Schier, Citation1990; Vanguelova, Citation2002).
Leuschner & Hertel (Citation2002) found a highly significant positive relationship between fine root biomass and annual precipitation, but in our analysis of root data from nine Fagus sylvatica temperate forest stands, fine root SRL and biomass were not significantly correlated. Perhaps, with more data in our analysis, the relationship between fine root biomass and SRL would be different. This needs further study. Ostonen et al. (2007) reported weakly significant negative correlations of RTD and significantly positive correlations of the D of ECM with annual precipitation and annual mean temperature in three high fertility Picea abies stands. The responses of D and RTD cancel each other out, so there was no correlation between SRL and climate factors.
Drought is a composite stress affecting, in the first place, the functioning of thinner roots (Davies and Bacon, Citation2003; Trubat et al., Citation2006). Water deficiency, often coinciding with nutrient deficiency, can result in increased RTD in some species (Trubat et al., Citation2006). Mechanical impedance on soils increases with drying, and in some species, D may increase (e.g. Quercus frainetto, Q. ilex in controlled environments; Manes et al., Citation2006), stay constant or decrease (Picea abies and Pinus sylvestris seedlings Bartsch, 1987). Stimulated ethylene production may play a key role in mediating an increased root D under mechanical stress (Clark et al., Citation2003). Under drought stress both root volume and length have been observed to decrease, and roots experience reduced ability to sustain adequate turgor pressure and maintain growth (Davies & Bacon, Citation2003; Manes et al., Citation2006). The mean SRL in this summary decreased by 11% as a result of soil dehydratation, but the result was nonsignificant. As soil moisture reaches wilting point, root growth would, however, be inhibited till cessation.
Soil temperature is important for ecosystem functioning and production since chemical, physical, and biological processes are all sensitive to temperature. Warmer temperatures enhance transpiration stream or water loss via evaporation and photosynhtesis, and thus influencing source – sink relationships between above- and below-ground plant parts (Pregitzer & King, Citation2005). Teskey & Hinckley (Citation1981) observed in oak roots that temperature was the dominant factor for root growth at lower soil temperatures, but soil water potential was the most important factor when temperatures increased above 17°C. Bowen (Citation1991) suggested that root length is a more sensitive indicator of the effects of root temperature than root weight, and D is inversely related to soil temperature. However, the response of SRL (−5%) to increased temperature in our study was not significant.
Vegetation exposed to atmospheric CO2 enrichment responds by substantially increasing belowground C allocation, directed to the root biomass, particularly to the fine roots (King et al., Citation1996; Berntson et al., Citation1997; Crookshanks et al., Citation1998, Janssens et al., Citation1998, Matamala & Schlesinger, Citation2000; Jastrow et al., Citation2005). The within-root biomass allocation, however, varies from species to species. It depends on growth strategies, root order, and the location of the root (upper or lower parts of root system or soil profile), and is related to environmental factors such as N availability and temperature (Larigauderie et al., Citation1994; King et al., Citation1996; Crookshanks et al., Citation1998). Our results showed that SRL decreased by 12% under CO2 enrichment. But when the effects of elevated temperature and fertilization were included, SRL did yield a significant 53% decrease.
The SRL response of the <0.5 mm diameter roots to CO2 enrichment was unexpectedly smaller (−3%) than that of 1 – 2 mm roots (−33%). The trees with the finest roots <0.5 mm in diameter had performed also increased ectomycorrhizal abundance (Berntson et al., Citation1997; Berntson & Bazzaz, Citation1998) that might influence root architecture. In numerous experiments, CO2 enrichment elicited an increase in the degree of ectomycorrhizal colonization as well as changes in the morphotype assemblages (Godbold & Berntson, Citation1997; Treseder, Citation2004; Alberton et al., Citation2005). Increased root length and thickening of diameter (Janssens et al., Citation1998, Pritchard et al., Citation2001) along with enhanced storage of starch in fine roots (Janssens et al., Citation1998) has been observed under CO2 enrichment. Whether the reduced SRL in secondary roots is related to an increased accumulation of structural carbohydrates at elevated CO2 still needs to be demonstrated experimentally.
SRL responded negatively to treatments with heavy metals (−27%), although the variation was high. Metal stress inhibits root growth and may induce lateral branching, as well as a decrease in the capacity of roots to explore soil reserves, particularly in dry soils (Davies, Citation1991; Arduini et al., Citation1994). Shoot biomass, photosynthesis, and transpiration may be reduced, although tree species may respond at different rates, e.g. Populus tremula was sensitive to metal contamination while Picea abies was not (Hermle et al., Citation2006). Induced short-term metal stress is used in nursery practice for designing more horizontally branched root systems to promote root regeneration once the seedlings are transplanted (Tsakaldini & Ganatsas, 2006). Such superficially distributed root systems could well suffer greatly from drought in natural ecosystems. The reduction of SRL under metal stress can be explained by the fact that root dry weights increase relatively more than the total root length. This is at the expense of increased lateral branching, which was demonstrated for Pinus halepensis after treatment with copper in nursery containers (Tsakaldini & Ganatsas, 2006). Davies (Citation1991) emphasizes that the extent of the inhibitory effect of metals on root growth depends on the chemical environment in which the metal is supplied.
Specific root length and sampling methods
The soil coring and ingrowth core methods have been commonly used to determine fine root biomass and length in the field. According to our analysis, the root collection method (soil coring and ingrowth cores) in the field has a significant effect on the SRL value (), which is significantly higher for roots grown in ingrowth cores. The higher variability and the value of SRL in the finest roots (<1 mm) reflects the effects of a root-free soil and changed soil properties. The dynamics of fine root morphology in ingrowth cores need to be studied more in the future.
Roots growing in undisturbed soil sampled by soil coring, and roots growing in ingrowth cores, displayed contrasting responses to fertilization with different nutrients (). Increased SRL in ingrowth cores (6%) possibly indicated a response to fertilization in a soil matrix which was initially root-free, homogenized, with lowered mechanical impedence for root growth, and altered water flow through the soil (Vogt et al., Citation1998). The tendency for increased SRL response to ingrowth core conditions (using undisturbed soil as a control) is also shown in . It is possible, therefore, that root sampling method may interact with the treatment factor and influence the results.
The SRL response to fertilization was significantly negative when measured in pot-grown roots (−22%) and compared with the responses measured in field-grown roots (nonsignificant, 4%). Pot studies differ from studies conducted in natural ecosystems in several ways. For ‘practical’ reasons, pot studies are mostly carried out on young seedlings in a relatively short time, whereas field studies provide better opportunities for long-term observations and usually use older, mature trees. In our compiled database for meta-analysis, all the studies, which were conducted under field conditions, lasted longer than 1 year, whereas only 1% of studies conducted in pots lasted longer than 1 year. The significantly negative response of SRL (−23%) in short-term studies (<1 year) thus coincides with the SRL responses obtained in pot studies. However, we do not know how many of the differential responses were due to age-related changes in the resource economy or ‘pot artefacts’. Major growth-limiting factors may differ greatly for seedlings and large trees, even when they are growing in the same environment. In pots, plants are grown individually or at extremely low densities. Thus pot studies provide insights into the potential responses of plants to manipulations of environmental factors. Interactions with neighbors in close proximity in natural ecosystems may change the response, which can be smaller than in individually grown plants (Berntson et al., Citation1997).
Conclusions
-
An overall estimate of SRL for whole fine root systems (<2 mm in diameter) is not as informative as it is for just the ECM. SRL was strongly dependent on the fine root classes. Its value for the six species considered was always higher for ECM, decreasing with increasing diameter. Soil factors (pH in natural forest stands and optimal fertilization under manipulated conditions) had the most influence on ECM or roots <0.5 mm in diameter.
-
The lack of any clear ecological generalizations about variation in SRL may have various explanations. Firstly, the dataset of SRL for different tree species was rather varied. The SRL value has been reported in a number of studies for different root classes and different soil and stand characteristics. Secondly, generalizations about SRL are difficult because its components, D and RTD, both vary. In our analysis D and RTD contributed equally to the value of SRL. There was no correlation between these two parameters in most cases. The responses of D and RTD to environmental conditions may cancel each other out.
-
Under natural conditions, SRL values of both the finest (<1 mm) and fine roots (<2 mm) do not differ according to the root sampling method used (soil cores or ingrowth cores). In the manipulated studies, the effect, of the sampling method was established. Soil coring and pots after fertilization treatments resulted in a significantly negative SRL response ratio, and ingrowth cores in a positive SRL response ratio. The duration of the experiment in the manipulated studies was significant: in fertilization studies <1 year it resulted in a significant decrease of the SRL response ratio.
-
Among treatments, only fertilizations and Al-stress (soil acidity) affected SRL significantly negatively.
-
We suggest that SRL can be used as indicator of environmental change in experimental conditions, when nutrient availability is manipulated. Other factors influence SRL but involve often composite effects. Further research on the mechanisms of how SRL and its components respond to a changing environment is recommended.
Acknowledgements
Financial support for this work by European COST Action E38 ‘Woody Root Processes’ is gratefully acknowledged. We thank Thomas Kuyper, Wageningen University, for valuable comments on the manuscript, and for introducing Ülle Püttsepp to meta-analysis. COST E38 supported Ü. Püttsepp for a STSM to Wageningen University. Spruce, pine and birch short-root morphology studies were supported by grant nos. 6472 and 6011 from the Estonian Science Foundation. We thank Silvia Dingwall from WSL for improving the readability of the manuscript.
References
- Alberton , O , Kuyper , T W and Gorissen , A . 2005 . Taking mycocentrism seriously: Mycorrhizal fungal and plant responses to elevated CO2 . New Phytol , 167 : 859 – 868 .
- Arduini , I , Godbold , D L and Onnis , A . 1994 . Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pinaster seedlings . Physiologia Plantarum , 92 : 675 – 680 .
- Bakker , M R . 1998 . Fine roots of pedunculate oak (Quercus robur L.) in the Netherlands seven years after liming . Netherlands J Agric Sci , 46 : 209 – 222 .
- Bartsch , N . 1987 . Responses of root systems of young Pinus sylvestris and Picea abies plants to water deficits and soil acidity . Can J Forest Res , 17 : 805 – 812 .
- Bauhus , J and Messier , C . 1999 . Soil exploitation strategies of fine roots in different tree species of the southern boreal forest of eastern Canada . Can J Forest Res , 29 : 260 – 273 .
- Bernier , P Y , Robitaille , G and Rioux , D . 2005 . Estimating the mass density of fine roots of trees for minirhizotron-based estimates of productivity . Can J Forest Res , 35 : 1708 – 1713 .
- Berntson , G M , Wayne , P M and Bazzaz , F A . 1997 . Below-ground architectural and mycorrhizal responses to elevated CO2 in Betula alleghaniensis populations . Funct Ecol , 11 : 684 – 695 .
- Berntson , G M and Bazzaz , F A . 1998 . Regenerating temperate forest mesocosms in elevated CO2: Belowground growth and nitrogen cycling . Oecologia , 113 : 115 – 125 .
- Bolte , A and Villanueva , I . 2006 . Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.) . Eur J Forest Res , 125 : 15 – 26 .
- Borken , W , Kossmann , G and Matzner , E . 2007 . Biomass, morphology and nutrient contents of fine roots in four Norway spruce stands . Plant Soil , 292 : 79 – 93 .
- Bowen , G D . 1991 . “ Soil temperature, root growth, and plant function ” . In Plant roots, the hidden half , Edited by: Waisel , Y , Eshel , A and Kafkafi , U . 309 – 330 . New York : Marcel Dekker .
- Clark , L J , Whalley , W R and Barraclough , P B . 2003 . How do roots penetrate strong soil? . Plant Soil , 255 : 93 – 104 .
- Claus , A and George , E . 2005 . Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences . Can J Forest Res , 35 : 1617 – 1625 .
- Comas , L H , Bouma , T J and Eissenstat , D M . 2002 . Linking root traits to potential growth rate in six temperate tree species . Oecologia , 132 : 34 – 43 .
- Comas , L H and Eissenstat , D M . 2004 . Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species . Funct Ecol , 18 : 388 – 397 .
- Crookshanks , M , Taylor , G and Broadmeadow , M . 1998 . Elevated CO2 and tree root growth: Contrasting responses in Fraxinus excelsior, Quercus petraea and Pinus sylvestris . New Phytol , 138 : 241 – 250 .
- Curt , Th and Prevosto , B . 2003 . Rooting strategy of naturally regenerated beech in Silver birch and Scots pine woodlands . Plant Soil , 255 : 265 – 279 .
- Davies , M S . 1991 . “ Effects of toxic concentrations of metals on root growth and development ” . In Plant root growth: An ecological perspective , Edited by: Atkinson , D . 211 – 227 . Oxford, , UK : Blackwell Scientific .
- Davies , W J and Bacon , M A . 2003 . “ Adaptation of roots to drought ” . In Root ecology. Ecological studies, vol. 168 , Edited by: de Kroon , H and Wisser , E JW . 173 – 192 . New York : Springer .
- Eissenstat , D M . 1992 . Costs and benefits of constructing roots of small diameter . J Plant Nutr , 15 : 763 – 782 .
- Eissenstat , D M and Yanai , R D . 1997 . The ecology of root lifespan . Adv Ecol Res , 27 : 1 – 62 .
- Eissenstat , D M , Wells , C E , Yanai , R D and Whitbeck , J L . 2000 . Building roots in a changing environment: Implications for root longevity . N Phytol , 147 : 33 – 42 .
- Fitter , A H . 1976 . Effects of nutrient supply and competition from other species on root growth of Lolium perenne in soil . Plant Soil , 45 : 177 – 189 .
- Fitter , A H . 1985 . “ Functional significance of root morphology and root system architecture ” . In Ecologcial interactions in soil , Edited by: Fitter , A H , Atkinson , D , Read , D J and Usher , M B . 87 – 106 . Oxford, , UK : Blackwell Scientific .
- Fitter , A H . 1991 . “ Characteristics and functions of root systems ” . In Plant roots: The hidden half , Edited by: Waisel , Y , Eshel , A and Kafkafi , U . 3 – 25 . New York : Marcel Dekker .
- Ford , E D and Deans , J D . 1977 . Growth of Sitka spruce plantation: Spatial distribution and seasonal fluctuations of lengths, weights and carbohydrate concentrations of fine roots . Plant Soil , 47 : 463 – 485 .
- Genenger , M , Zimmermann , S , Hallenbarter , D , Landolt , W , Frossard , E and Brunner , I . 2003 . Fine root growth and element concentrations of Norway spruce as affected by wood ash and liquid fertilisation . Plant Soil , 255 : 253 – 264 .
- Godbold , D L , Fritz , H-W , Jentschke , G , Meesenburg , H and Rademacher , P . 2003 . Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity . Tree Physiol , 23 : 915 – 921 .
- Godbold , D L and Berntson , G M . 1997 . Elevated atmospheric CO2 concentration leads to changes in ectomycorrhizal morphotype assemblage in Betula papyrifera . Tree Physiol , 17 : 347 – 350 .
- Göransson , H , Wallander , H , Ingerslev , M and Rosengren , U . 2006 . Estimating the relative nutrient uptake from different soil depths in Quercus robur, Fagus sylvatica and Picea abies . Plant Soil , 286 : 87 – 97 .
- Guo , D L , Mitchell , R J and Hendricks , J J . 2004 . Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest . Oecologia , 140 : 450 – 457 .
- Gurevitch , J and Hedges , L V . 2001 . “ Meta-analysis. Combining the results of independent experiments ” . In Design and analysis of ecological experiments , 2nd ed , Edited by: Scheiner , S M and Gurevitch , J . 347 – 369 . Oxford : Oxford University Press .
- Hendriks , C MA and Bianchi , F JJA . 1995 . Root density and root biomass in pure and mixed forest stands of Douglas fir and Beech . NJAS Wageningen J Life Sci , 43 : 321 – 331 .
- Hermle , S , Günthardt-Goerg , M S and Schulin , R . 2006 . Effects of metal-contaminated soil on the performance of young trees growing in model ecosystems under field conditions . Environ Pollut , 144 : 703 – 714 .
- Janssens , I A , Crookshanks , M , Taylor , G and Ceulemans , R . 1998 . Elevated atmospheric CO2 increases fine root production, respiration, rhizosphere respiration and soil CO2 efflux in Scots pine seedlings . Global Change Biol , 4 : 871 – 878 .
- Jastrow , J D , Miller , R M , Matamala , R , Norby , R J , Boutton , T W , Rice , C W and Owensby , C E . 2005 . Elevated atmospheric carbon dioxide increases soil carbon . Global Change Biol , 11 : 2057 – 2064 .
- Jentschke , G , Drexhage , M , Fritz , H-W , Fritz , E , Schella , B Lee , D-H . 2001 . Does soil acidity reduce subsoil rooting in Norway spruce (Picea abies)? . Plant Soil , 237 : 91 – 108 .
- Kakei , M and Clifford , P E . 2002 . Short-term effects of lime application on soil properties and fine-root characteristics for a 9-year-old Sitka spruce plantation growing on a deep peat soil . Forestry , 75 : 37 – 50 .
- King , J S , Thomas , R B and Strain , B R . 1996 . Growth and carbon accumulation in root systems of Pinus taeda and Pinus ponderosa seedlings as affected by varying CO2, temperature, and nitrogen . Tree Physiol , 16 : 635 – 642 .
- King , J S , Albaugh , T J , Allen , H L , Buford , M , Strain , B R and Dougherry , P . 2002 . Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine . New Phytol , 154 : 389 – 398 .
- Kottke , I . 2002 . “ Mycorrhizae – rhizosphere determinants of plant communities ” . In Plant roots: The hidden half , 3rd ed , Edited by: Waisel , Y , Eshel , A and Kafkafi , U . 919 – 932 . New York : Marcel Dekker .
- Larigauderie , A , Reynolds , J F and Strain , B R . 1994 . Root response to CO2 enrichment and nitrogen supply in loblolly pine . Plant Soil , 165 : 21 – 32 .
- Leuschner , C and Hertel , D . 2002 . Fine root biomass of temperate forests in relation to soil acidity and fertility, climate, age and species . Prog Bot , 64 : 405 – 438 .
- Leuschner , C , Hertel , D , Schmid , I , Koch , O , Muhs , A and Hölscher , D . 2004 . Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility . Plant Soil , 258 : 43 – 56 .
- Lõhmus , K , Truu , J , Truu , M , Kaar , E , Ostonen , I Alama , S . 2006a . “ Black alder as a perspective deciduous species for reclaiming of oil shale mining areas ” . In Brownfields III. Prevention, assessment, rehabilitation and development of Brownfield sites , Edited by: Brebbia , C A and Mander , Ü . 87 – 97 . Southampton : WIT Press .
- Lõhmus , K , Truu , M , Truu , J , Ostonen , I , Vares , A Uri , V . 2006b . Functional diversity of culturable bacterial communities in the rhizosphere in relation to fine-root and soil parameters in alder stands on forest, abandoned agricultural, and oil-shale mining areas . Plant Soil , 283 : 1 – 10 .
- Majdi , H and Persson , H . 1995 . Effects of ammonium sulphate application on the chemistry of bulk soil, rhizosphere, fine roots and fine-root distribution in a Picea abies (L.) Karst. Stand . Plant Soil , 168 – 169 : 151 – 160 .
- Majdi , H . 2001 . Changes in fine root production and longevity in relation to water and nutrient availability in a Norway spruce stand in northern Sweden . Tree Physiol , 21 : 1057 – 1061 .
- Majdi , H and Viebke , C G . 2004 . Effects of fertilization with dolomite lime + PK or wood ash on root distribution and morphology in a Norway spruce stand in Southwest Sweden . Forest Sci , 50 : 802 – 809 .
- Manes , F , Vitale , M , Donato , E , Giannini , M and Puppi , G . 2006 . Different ability of three Mediterranean oak species to tolerate progressive water stress . Photosynthetics , 44 : 387 – 393 .
- Matamala , R and Schlesinger , W H . 2000 . Effects of elevated atmospheric CO2 on fine root production and activity in an intact temperate forest ecosystem . Global Change Biol , 6 : 967 – 979 .
- McQuattie , C J and Schier , G A . 1990 . Response of red spruce seedlings to aluminum toxicity in nutrient solution: alterations in root anatomy . Can J Forest Res , 20 : 1001 – 1011 .
- Olsthoorn , A FM , Keltjens , W G , Van Baren , B and Hopman , M CG . 1991 . Influence of ammonium on fine root development and rhizosphere pH of Douglas-fir seedlings in sand . Plant Soil , 133 : 75 – 81 .
- Ostonen , I , Lõhmus , K and Lasn , R . 1999 . The role of soil conditions in fine root ecomorphology in Norway spruce (Picea abies (L.) Karst.) . Plant Soil , 208 : 283 – 292 .
- Ostonen , I and Lõhmus , K . 2003 . Proportion of fungal mantle, cortex and stele of ectomycorrhizas in Picea abies (L.) Karst. in different soils and site conditions . Plant Soil , 257 : 435 – 442 .
- Ostonen , I , Lõhmus , K and Pajuste , K . 2005 . Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce stand: Comparison of soil core and ingrowth core methods . Forest Ecol Manag , 212 : 264 – 277 .
- Ostonen , I , Lõhmus , K , Alama , S , Truu , J , Kaar , E Vares , A . 2006 . Morphological adaptations of fine roots in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula Roth.) and black alder (Alnus glutinosa (L.) Gaertn.) stands in recultivated oil shale mining and semi-coke areas . Oil Shale , 23 : 187 – 202 .
- Ostonen , I , Lõhmus , K , Helmisaari , H-S , Truu , J and Meel , S . Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests . Tree Physiol , 27 1627 – 1634 .
- Persson , H and Ahlström , K . 1990/91 . The effects of forest liming on fertilization on fine-root growth . Water Air Soil Pollut , 54 : 365 – 375 .
- Persson , H , Ahlström , K and Clemensson-Lindell , A . 1998 . Nitrogen addition and removal at Gårdsjön – Effects on fine-root growth and fine-root chemistry . Forest Ecol Manag , 101 : 199 – 205 .
- Persson , H and Ahlström , K . 2002 . Fine-root response to nitrogen supply in nitrogen manipulated Norway spruce catchment areas . Forest Ecol Manag , 168 : 29 – 41 .
- Pregitzer , K S , DeForest , J L , Burton , A J , Allen , M F , Ruess , R W and Hendrick , R L . 2002 . Fine root architecture of nine North American trees . Ecol Monogr , 72 : 93 – 309 .
- Pregitzer , K S and King , J S . 2005 . “ Effects of soil temperature on nutrient uptake ” . In Nutrient acquisition by plants: An ecological perspective , Edited by: BassiriRad , H . 277 – 310 . New York : Springer . Ecological studies, vol. 181
- Pritchard , S G , Rogers , H H , Davis , M A , Van Santen , E , Prior , S A and Schlesinger , W H . 2001 . The influence of elevated atmospheric CO2 on fine root dynamics in an intact temperate forest . Global Change Biol , 7 : 829 – 837 .
- Püttsepp , Ü , Lõhmus , K , Persson , HÅ and Ahlström , K . 2006 . Fine-root distribution and morphology in an acidic Norway spruce (Picea abies (L.) Karst.) stand in SW Sweden in relation to granulated wood ash application . Forest Ecol Manag , 221 : 291 – 298 .
- Rosenberg , M S , Adams , D C and Gurevitch , J . 2000 . MetaWin, version 2.1 , Sunderland, MA : Sinauer Associates .
- Reich , P B , Tjoelker , M G , Walters , M B , Vanderklein , D W and Buschena , C . 1998 . Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light . Funct Ecol , 12 : 327 – 338 .
- Robinson , D and Rorison , I H . 1983 . Relationships between root morphology and nitrogen availability in a recent theoretical model describing nitrogen uptake from soil . Plant Cell Environ , 6 : 641 – 647 .
- Ryser , P . 1998 . “ Intra- and interspecific variation in root length, root turnover and the underlying parameters ” . In Inherent variation in plant growth: Physiological mechanisms and ecological consequences , Edited by: Lambers , H , Poorter , H and VanVuuren , M MI . 441 – 465 . Leiden, , Netherlands : Backhuys .
- Ryser , P . 2006 . The mysterious root length . Plant Soil , 286 : 1 – 6 .
- Teskey , R O and Hinckley , T M . 1981 . Influence of temperature and water potential on root-growth of white oak . Physiol Plant , 52 : 363 – 369 .
- Treseder , K K . 2004 . A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies . N Phytologist , 164 : 347 – 355 .
- Trubat , R , Cortina , J and Vilagrosa , A . 2006 . Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.) . Trees , 20 : 334 – 339 .
- Tsakaldimi , M N and Ganatsas , P P . 2006 . Effect of chemical root pruning on stem growth, root morphology and field performance of the Mediterranean pine Pinus halepensis Mill . Scientia Horticulturae , 109 : 183 – 189 .
- Uri , V , Lõhmus , K , Ostonen , I , Tullus , H , Lastik , R and Vildo , M . 2007 . Biomass production, foliar and root characteristics and nutrient accumulation in young silver birch (Betula pendula Roth.) stand growing on abandoned agricultural land . Eur J For Res , : 1 – 12 .
- Vanguelova , E I . 2002 . Soil acidification and fine root response of Scots pine , Berkshire, , UK : The University of Reading . PhD thesis
- Vanguelova , E , Nortcliff , S , MoVat , A J and Kennedy , F . 2007 . Short-term eVects of manipulated increase in acid deposition on soil, soil solution chemistry and fine roots in Scots pine (Pinus sylvestris) stand on a podzol . Plant Soil , 294 : 41 – 54 .
- Vogt , K A , Vogt , D J and Bloomfield , J . 1998 . Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level . Plant Soil , 200 : 71 – 89 .
- Withington , J M , Reich , P B , Oleksyn , J and Eissenstat , D M . 2006 . Comparison of structure and life span in roots and leaves among temperate trees . Ecol Monogr , 76 : 381 – 397 .
Appendix 1. Ranges of mean SRL of different species and different diameter classes used in Tukey's test, the correlation analyses, and the calculation of variation coefficients
Appendix 2. References on SRL used in the meta-analyses
Bakker MR. 1998. Fine roots of pedunculate oak (Quercus robur L.) in the Netherlands seven years after liming. Netherlands J Agric Sci 46:209 – 222.
Bakker MR, Kerisit R, Verbist K, Nys C. 1999. Effects of liming on rhizosphere chemistry and growth of fine roots and of shoots of sessile oak (Quercus petraea). Plant Soil 217:243 – 255.
Bakker MR, Augusto L, Achat DL. 2006. Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 286:37 – 51.
Bauhus J, Messier C. 1999. Soil exploitation strategies of fine roots in different tree species of the southern boreal forest of eastern Canada. Can J Forest Res 29:260 – 273.
Berntson GM, Bazzaz FA. 1998. Regenerating temperate forest mesocosms in elevated CO2: Belowground growth and nitrogen cycling. Oecologia 113:115 – 125.
Berntson GM, Wayne PM, Bazzaz FA. 1997. Below-ground architectural and mycorrhizal responses to elevated CO2 in Betula alleghaniensis populations. Funct Ecol 11:684 – 695.
Brunner I. Unpublished submitted data, 2007.
Crookshanks M, Taylor G, Broadmeadow M. 1998. Elevated CO2 and tree root growth: Contrasting responses in Fraxinus excelsior, Quercus petraea and Pinus sylvestris. N Phytologist 138:241 – 250.
Espeleta JF, Donovan LA. 2002. Fine root demography and morphology in response to soil resources availability among xeric and mesic sandhill tree species. Funct Ecol 16:113 – 121.
Fujimaki R, McGonigle TP, Takeda H. 2004. Soil micro-habitat effects on fine roots of Chamaecyparis obtusa Endl.: A field experiment using root ingrowth cores. Plant Soil 266:325 – 332.
Genenger M, Zimmermann S, Hallenbarter D, Landolt W, Frossard E, Brunner I. 2003. Fine root growth and element concentrations of Norway spruce as affected by wood ash and liquid fertilisation. Plant Soil 255:253 – 264.
George E, Seith B, Schaeffer C, Marschner H. 1997. Responses of Picea, Pinus and Pseudotsuga roots to heterogeneous nutrient distribution in soil. Tree Physiol 17:39 – 45.
George E, Seith B. 1998. Long-term effects of a high nitrogen supply to soil on the growth and nutritional status of young Norway spruce trees. Environ Pollut 102:301 – 306.
Godbold DL, Fritz H-W, Jentschke G, Meesenburg H, Rademacher P. 2003. Root turnover and root necromass accumulation of Norway spruce (Picea abies) are affected by soil acidity. Tree Physiol 23:915 – 921.
King JS, Thomas RB, Strain BR. 1997. Morphology and tissue quality of seedling root systems of Pinus taeda and Pinus ponderosa as affected by varying CO2, temperature, and nitrogen. Plant Soil 195:107 – 119.
Kunstler G, Curt T, Bouchaud M, Lepart J. 2006. Indirect facilitation and competition in tree species colonization of sub-Mediterranean grasslands. J Vegetation Sci 17:379 – 388.
Majdi H, Viebke C-G. 2004. Effects of fertilization with dolomite lime + PK or wood ash on root distribution and morphology in a Norway spruce stand in Southwest Sweden. Forest Sci 50:802 – 809.
Metcalfe DB. 2006. Understanding the effects of drought upon carbon allocation and cycling in an Amazonian rain forest. PhD Thesis. University of Edinburgh, UK.
Mou P, Mitchell RJ, Jones RH. 1997. Root distribution of two tree species under a heterogeneous nutrient environment. J Appl Ecol 34:645 – 656.
Olsthoorn AFM, 1991. Fine root density and root biomass of two Douglas-fir stands on sandy soils in The Netherlands: 2. Periodicity of fine root growth and estimation of belowground carbon allocation. Neth J Aric Sci 39, 1:61 – 77.
Olsthoorn AFM, Keltjens WG, van Baren B, Hopman MCG. 1991. Influence of ammonium on fine root development and rhizosphere pH of Douglas-fir seedlings in sand. Plant Soil 133:75 – 81.
Ostertag R. 2001. Effects of nitrogen and phosphorus availability on fine-root dynamics in Hawaiian mountane forests. Ecology 82:485 – 499.
Persson H, Ahlström K. 1990/91. The effects of forest liming on fertilization on fine-root growth. Water Air Soil Pollut 54:365 – 375.
Persson H, Ahlström K. 2002. Fine-root response to nitrogen supply in nitrogen manipulated Norway spruce catchment areas. Forest Ecol Manag 168:29 – 41.
Persson H, Ahlström K, Clemensson-Lindell A. 1998. Nitrogen addition and removal at Gårdsjön – Effects on fine-root growth and fine-root chemistry. Forest Ecol Manag 101:199 – 205.
Prior SA, Rogers HH, Hendrey GR. 1994. Free-air CO2 enrichment of cotton: Vertical and lateral root distribution patterns. Plant Soil 165:33 – 44.
Pronk AA., De Willigen P, Heuvelink E, Challa H. 2002. Development of fine and coarse roots of Thuja occidentalis ‘Brabant’ in non-irrigated and drip irrigated field plots. Plant Soil 243:161 – 171.
Püttsepp Ü, Lõhmus K, Persson HÅ, Ahlström K. 2006. Fine-root distribution and morphology in an acidic Norway spruce (Picea abies (L.) Karst.) stand in SW Sweden in relation to granulated wood ash application. Forest Ecol Manag 221:291 – 298.
Treseder KK, Vitousek PM. 2001. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946 – 954.
Trubat R, Cortina J, Vilagrosa A. 2006. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees 20:334 – 339.
Tsakaldimi MN, Ganatsas PP. 2006. Effect of chemical root pruning on stem growth, root morphology and field performance of the Mediterranean pine Pinus halepensis Mill. Scientia Horticulturae 109:183 – 189.
Vanguelova EI. 2002. Soil acidification and fine root response of Scots pine. PhD thesis, The University of Reading, UK, 231 p.
Vanguelova EI, Nortcliff S, Moffat AJ, Kennedy F. 2005. Morphology, biomass and nutrient status of fine roots of Scots pine (Pinus sylvestris) as influenced by fluctuations in soil moisture and soil solution chemistry. Plant Soil 270:233 – 247.
Vanguelova EI, Nortcliff S, Moffat AJ, Kennedy F. 2007 Short-term effects of manipulated increase in acid deposition on soil, soil solution chemistry and fine roots in Scots pine (Pinus sylvestris) stand on a podzol. Plant Soil 294:41 – 54.
Van Hees AFM, Clerkx APPM. 2003. Shading and root-shoot relations in saplings of silver birch, pedunculate oak and beech. Forest Ecol Manag 176:439 – 448.
Van Kanten R, Schroth G, Beer J, Jiménez F. 2005. Fine-root dynamics of coffee in association with two shade trees in Costa Rica. Agroforestry Forum 63:247 – 261.
Weih M. 2001. Evidence for increased sensitivity to nutrient and water stress in a fast-growing hybrid willow compared with a natural willow clone. Tree Physiol 21:1141 – 1148.
Weih M, Karlsson PS, Skre O. 1998. Intra-specific variation in nitrogen economy among three mountain birch provenances. Écoscience 5:108 – 116.
Weih M, Karlsson PS. 1999. Growth response of altitudinal ecotypes of mountain birch to temperature and fertilization. Oecologia 119:16 – 23.
Woolfolk WTM., Friend AL. 2003. Growth response of cottonwood roots to varied NH4:NO3 ratios in enriched patches. Tree Physiol 23:427 – 432.
Železnik P, Hrenko M, Then C, Koch N, Grebenc T, Levanič T, et al. 2007. CASIROZ: Root parameters and types of ectomycorrhiza of young beech plants exposed to different ozone and light regimes. Plant Biol 9:298 – 308.