Abstract
High soil acidity and elevated soil Al concentrations limit plant growth in many terrestrial ecosystems. Aluminium toxicity can be ameliorated by Ca. Thus, Ca/Al molar ratios in soil solution and in plant tissues have been proposed as superior indicators than Al concentration itself for evaluating the Al toxicity stress to trees (Cronan & Grigal, J Environ Qual Citation1995;24:209 – 226). This article presents an overview of publications since 1995 where the reduced Ca/Al ratio in fine tree roots has been used as an indicator of stress for Al and/or soil acidity. The main aim of this review was to evaluate the use and the critical threshold of the fine root Ca/Al ratio as a potential indicator for Al toxicity stress to trees in acid soils. Based on the reviewed literature, the fine root Ca/Al molar ratio was strongly negatively related to Al stress in small tree seedlings in controlled environments, whereas the response was not clear under field conditions where other environmental factors interact. Fine root Ca/Al ranged from 0.03 to 17 in tree seedlings and from 0.1 to 18 in mature trees depending on experimental and site conditions, as well as the tolerance and uptake mechanisms of the different tree species. Fine root Ca/Al was positively related to the soil solution Ca/Al molar ratio. Fine root Ca/Al ratios were related positively to fine root length, growth, specific root length, and biomass, and negatively to root diameter, callose formation, respiration chain activity, starch concentration, and root necromass. A number of relationships have been also found between the fine root Ca/Al and above-ground seedling and/or mature tree growth and nutrient uptake. The critical thresholds for the Ca/Al fine root ratio of 0.2 suggested by Cronan and Grigal (Citation1995) is estimated to represent 90% risk of inverse impact on root and above-ground tree growth. Values of Ca/Al molar ratio in the fine roots of mature trees were only rarely determined below the critical 0.2. The caveats for the use and the interpretation of Ca/Al ratio in fine roots have been addressed in detail. A protocol for root processing and elemental analysis to obtain reliable and comparable results of Ca and Al concentrations in roots is also provided. The article concludes with recommendations for a wider use of the Ca/Al ratio in roots as a bioindicator of Al toxicity to trees in acid soils.
Introduction
Since the tree root system links the soil environment to the tree canopy, changes in any of these compartments can lead to a growth response in the total tree (Persson, Citation1988; Vogt et al., Citation1993). In forest soils, fine roots are exposed to various stresses influencing root growth and metabolic processes, and fine roots of trees have been suggested to be sensitive indicators of changes in the soil environment (Vogt et al., Citation1993; Persson et al., Citation1995; Bakker, Citation1999a; Puhe, Citation2003; Hirano et al., Citation2007). Uncertainty remains over the following issues: (1) which root parameters are the most sensitive indicators of a specific stress, (2) whether below ground root response can be linked to above-ground tree responses (e.g. can root chemistry be linked to foliar chemistry) and (3) how can a short term (acute) response be distinguished from long term (chronic) response/damage, taking into account recovery and plasticity of the root system.
The effects of atmospheric pollution on soil acidification have been identified and studied since the 1970s. Soil acidification demonstrably causes soil nutrient depletion, nutrient imbalances, and increased concentrations of free aluminium species in soil solutions (Driscoll et al., 2001; de Vries et al., Citation2003) which can result in toxicity to fine roots of trees (Godbold et al., Citation1988; Lõhmus & Lasn, Citation1990). Different tree species have different sensitivity to soil acidity and Al toxicity (Schaedle et al., Citation1989). Within a given tree species, different tree rootstock types may also differ in their sensitivity to Al (Sas Paszt & Mercik, Citation2004). Two soil surveys of the European Level I forest condition monitoring plots found no link between soil acidity and above-ground forest conditions and growth, and work by Freer-Smith and Read (Citation1995) failed to link the crown condition with aluminium or base cations in soil solution. In contrast, field research has established relationships between soil acidification and root functioning (Lõhmus & Lasn, Citation1990; Hahn & Marschner, Citation1998a, Citation1998b; Jentschke et al., Citation2001; Sas & Mercik, Citation2001; Vanguelova et al., Citation2005, Citation2007). These studies show that tree roots are more sensitive to changes in soil acidity than above-ground tree parameters.
The idea of using the Ca/Al ratio in soil solution as an indicator of potential stress from Al toxicity, first advocated by Ulrich et al. (Citation1978), is rooted in the idea of Al toxicity as a predisposing factor for fine roots damage, and thus, tree damage and deteriorating forest health. The rationale behind the use of the Ca/Al ratio is that acid precipitation leads to increased concentrations of dissolved Al in the soil solution when base cations and Al ions are displaced by H+ from soil exchange sites (Tomlinson, Citation2003). This may in turn lead to Ca and Mg leaching and to Al replacing Ca and Mg on root binding sites, as the cell walls and plasma membranes have up to 700 times higher affinity for Al than for Ca (Kinraide, Citation2003). Al is strongly adsorbed by roots either by exchange processes or formation of insoluble organo-Al complexes. However, Al concentration is much higher in the roots than in the above-ground plant, which implies that plants actively or passively exclude Al from the uptake stream (Taylor, Citation1991). This exclusion mechanism is suggested to occur between root apoplast and symplast (Taylor, Citation1987). If Al enters the symplast, then it can inhibit (i) cell division (by binding to nucleotides and nucleic acids); (ii) energy transport (by binding to ATP, ADP, or membrane-bound ATPases); (iii) cellular control (by binding to calmodium – a protein found in the cytosol of plant cells (Cronan & Grigal, Citation1995; Taiz & Zeiger, Citation1998). Calcium is important in cell physiology due to its role in the synthesis of new cell walls by stabilising pectate, and as a messenger in transport processes (McLaughlin & Wimmer, Citation1999; Rengel & Zhang, Citation2003). Calcium is also used in the mitotic spindle during cell division, it bridges phospholipids and proteins in the plasma-lemma and it is of crucial importance for the functioning of calmodium, which plays a major role in membrane bound enzyme functions and phytohormone control. Thus, it is reasonable that a decrease in root Ca/Al ratio, due to decreased Ca and increased Al, will rapidly influence root physiological functions and root morphology as a consequence of unbalanced nutrient uptake and tree nutrient status.
Cronan and Grigal (Citation1995) wrote an authoritative review paper on the subject concluding that percent base saturation and the molar Ca:Al or base cation:Al ratio (BC/Al) in the soil solution are acceptable indicators of soil acidification. Cronan and Grigal (Citation1995) suggested potential stress thresholds of <0.2 Ca/Al in fine roots and <12.5 Ca/Al in leaves when the soil solution Ca/Al ratio is less than 1. Since the review of Cronan and Grigal (Citation1995), ecological indicators (or bioindicators) for acidity stress have mainly focused on the use of the Ca/Al molar ratios of fine roots. Bioindicators are, according to Fränzle (Citation2006), organelles, organs, organisms, or ecosystems that respond to early stages of environmental changes that puts a stress on the system in question. An “accumulation indicator”, such as the Ca/Al molar ratio of fine roots has a high resistance to change and accumulates possibly toxic substances over time. In contrast, an “effect indicator” responds quickly and measurably to the stress; it has low resistance and low adaptive potential.
The objectives of this paper were to (i) collate published results of Ca/Al molar ratio in fine roots of trees for the last decade, (ii) review the variability of the ratio in response to Al and Ca supply and soil acidity, (iii) review the relationships between this ratio and other root, soil, and plant related parameters, (iv) evaluate the critical threshold and the use of fine root Ca/Al ratio as a potential indicator for Al toxicity stress to fine roots of trees in acid soils, and (v) highlight the research and practical issues (caveats) of using and interpreting the ratio.
Fine root Ca/Al molar ratio as indicator of Al and soil acidity stress
Data collection, analysis and presentation
summarises results from experiments on the effects of Al in soil or culture solutions on fine root Ca/Al ratios of seedlings and mature trees. The data were collected from participants in COST Action E38 “Woody roots under changing environment” and additional literature. The Ca/Al ratio is expressed as stoichiometric atomic ratio (molCa/molAl), which facilitates the comparison with the Ca/Al molar ratios in soil solution and above-ground plant ratios. The data in are presented in accordance to the experimental conditions: (A) in solution cultures, (B) sand cultures, (C) in soil mixture, and (D) natural and manipulated field conditions. Changes in the fine root Ca/Al ratio are given as percentages of reduction between the control and the highest stress treatment. The ranges of the fine root Ca/Al ratio in the different experimental conditions and also in control and in natural field conditions are presented. For some experiments, threshold values are presented below which marked changes occur in the fine root morphology and nutrient status or in tree nutrient uptake and growth. Related changes in root or above-ground tree parameters are also given. In addition, an overall estimate of the responsive value of the indicator and its consistency based on each experiment is given.
Table I. Effect of aluminium concentrations and soil acidity on fine root Ca/Al molar ratio of different tree species observed in tree seedlings and mature trees under different treatments/manipulated conditions. Fine root diameter is <2 mm unless otherwise indicated
Changes and variability of fine root Ca/Al ratio in response to Al and soil acidity stress
Concentrations of Al and Ca in the fine root are determined by (i) soil solution concentrations of Al and Ca, (ii) soil acidity, (iii) soil exchangeable Al and Ca concentrations, (iv) root uptake capabilities, and (v) translocation to above-ground plant parts. Additional factors that may be species specific include plant tolerances to Al, internal detoxification mechanisms, and exclusion of Al from the symplast (Marschner, Citation1991; Barceló & Poschenrieder, Citation2002; Kochian et al., Citation2005).
In solution culture experiments, the highest Al treatments (225 – 5000 μM) with Ca/Al ratios between 0.16 and 0.8 caused fine root Ca/Al ratios to decrease by 56 – 97% compared to control (). The range of decrease was dependent on the duration of exposure to Al, Al concentrations, and tree species (, A and B). Across all experiments where seedlings were exposed to Al in solution (20 – 5000 μM; Ca/Al 0.16 – 7.5), fine root Ca/Al ratios ranged from 0.03 to 5.8 (, A and B). Fine root Ca/Al was considerably higher in controls (no Al in soil solution; Ca/Al > 5); for example, in Picea abies (13 – 19) and Pinus sylvestris (6 – 9) seedlings. When the same species were exposed to Al treatments of 20 – 560 μM (Ca/Al 0.16 – 0.2), fine root Ca/Al ranged from 0.46 – 0.63 (Picea abies), and 0.59 – 5.82 (Pinus sylvestris). Fine root Ca/Al ratio as low as 0.03 was observed in Cryptomeria japonica when Al application was as high as 5000 μM and soil solution Ca/Al ratio 0.16 in comparison to fine root Ca/Al of 6.9 in the control, suggesting very quick response and high sensitivity of this species to Al.
In experiments with soil mixture and under field conditions, the negative rate of change of the root Ca/Al ratio is much more variable and lower, between 1 and 46% (, C and D), in comparison to solution and sand culture experiments (, A and B). A negative change of 82% was observed in roots of Pinus sylvestris in the soil organic layer. This was not due to Al stress but to drought stress (Vanguelova et al., Citation2005). It is also notable that where field manipulations like liming and ash applications were carried out with an increase in Ca content of the soil and soil solution, this resulted in a very distinct positive effect on the fine root Ca/Al ratio with an increase of between 1 and 260%. In most cases, the improvement of soil nutrient status resulted in a positive response in fine root Ca/Al ratio and fine root and tree growth (Bakker, Citation1999b; Bakker et al., Citation1999, Genenger et al., Citation2003). However, other studies have reported lower fine root Ca/Al in response to increased pH and added salt concentrations (Püttsepp, Citation2004) or no effect on root Ca/Al with liming or elevated exchangeable Ca concentrations in the subsoil (Borken et al., Citation2007). Differences in the response of the ratio to experimental manipulations in the different soil layers is notable in almost all field experiments, which depends on the bio-physico-chemical properties in different soil horizons such as soil pH, availability of nutrients, as well as toxic Al species and dissolved organic carbon.
The range of fine root Ca/Al ratio found in the field experiments is between 0.1 and 10.5 with exceptionally low values of 0.11 in subsoil Quercus robur roots (Bakker, Citation1999a) and 0.005 to 0.011 of apple tree roots (Sas Paszt & Mercik, Citation2004). Low fine root Ca/Al ratios have also been reported for Picea abies growing in Scandinavia (0.05 – 0.38), at Solling, Germany (0.11 – 0.63) and at a site in Canada (0.09 – 0.81) dominated by Picea glauca-Abies lasiocarpa (Persson et al., Citation1995). These differences may be partly due to the general recovery from acidifying pollution across Europe but also may be a reflection of the differences in methodology used to study roots in the past and now.
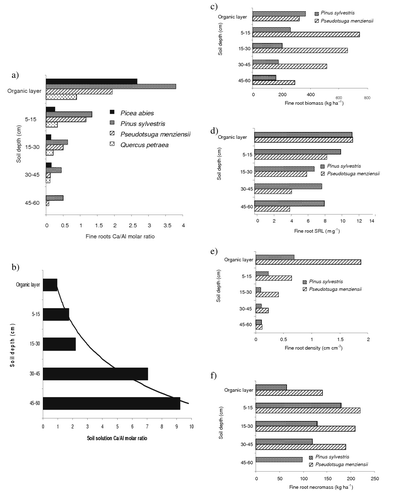
There is a distinct difference in the Ca/Al ratio in fine roots taken from different soil horizons, with higher values in the upper soil horizons in comparisons to subsoils ( and ). In five long-term forest ecosystem forest sites in Switzerland, Ca/Al molar ratio in fine roots Picea abies and Pinus sp. was found to be between 0.8 and 3.6 for roots distributed mainly in the mineral soil and a ratio of 19.4 of roots mainly distributed in the organic layer (Brunner et al., Citation2002). In four Picea abies stands in Germany, the highest Ca/Al ratios of 4.8 – 8.5 were found in the humus layer and lowest ratios in the mineral soil at 20 – 40 cm depth (0.5 – 1.1) mainly due to increasing Al concentrations and to a lesser extent by decreasing Ca concentrations (Borken et al., Citation2007). Root diameter also influences the Ca/Al ratio, being lower (0.3 – 6.4) in thinner roots (0 – 1 mm) compared to 1 – 10.6 in thicker roots (1 – 2 mm) of mature Picea abies (Püttsepp, Citation2004) and also in agreement to previous findings (Persson et al., Citation1995; Zysset et al., Citation1996).
Figure 1. Vertical gradients in stands of Pinus sylvestris (Vanguelova et al., Citation2005, Citation2007), Pseudotsuga menziensii (Olsthoorn, Citation1991), Picea abies (Nygaard & de Wit, Citation2004), and Quercus petraea (Bakker, Citation1999a). (a) Fine root Ca/Al molar ratio; (b) mineral soil solution Ca/Al molar ratio; (c) fine root biomass; (d) specific root length (SRL); (e) root density; and (f) necromass.
The extent to which fine root Ca/Al ratios change in response to Al exposure varies with tree species. For example, fine root Ca/Al can change by a factor of thirty in Picea abies (; Eldhuset & Nygaard, Citation1997; Nygaard, Citation1999) compared to a factor of 10 in Pinus sylvestris (; Vanguelova, Citation2002) on exposure to similar concentrations of Al (and Ca/Al ratios) in soil solutions. Another example is the decrease of fine root Ca/Al molar ratio up to a factor of 230 in Cryptomeria japonica compared to factor 30 in Chamaecyparis obtusa both under the same experimental conditions and at the same amount of Al applied and Ca/Al molar ratio in the nutrient solution (Hirano, Citation2001). These comparisons suggest the differences in the plasticity of the species response to Al, which is most likely related to the sensitivity of these species to Al. Seasonal fluctuations of soil water and nutrients results in high variations in the fine root Ca/Al ratio. For example, the ratio was reduced by a factor three in a Scots pine stand on a podzol, reflecting natural fluctuations of soil moisture and chemistry (Vanguelova et al., Citation2007). This highlights the importance of taking into account seasonal fluctuations of the indicator when investigating cause-effect relationships.
Relationships of fine root and soil solution Ca/Al ratio
The key indicator of the soil acidity risk in forest ecosystems perhaps will always be the soil solution Ca/Al molar ratio (Cronan & Grigal, Citation1995), since this is the preconditioning indication of whether the soil is acid and high in Al and low in base cations. However, soil water content may vary considerably throughout the season and nutrient concentrations show strong seasonal fluctuations as well. Moreover, this ratio in the soil solution cannot be the only true indication of the risk of acidity and Al because this risk also depends on the tree sensitivity to Al and acidity (e.g. roots of some trees excrete more organic exudates, have different ectomycorrhizal infection, and higher tolerance mechanisms to exclude Al from the uptake stream). classifies tree species according to their sensitivities to different ranges of Al concentrations in soil solution. In trees with intermediate and high tolerance, nutrient effects become visible at lower Al concentrations than biomass effects. The lower Al concentration inducing changes in nutrient status is probably also the level at which changes in root morphology start (see ). Adverse effects on biomass growth start at higher Al concentrations, particularly in the tolerant species ( and ). Concentrations of Al in fine roots do not necessarily indicate risk of Al toxicity as some woody plants accumulate Al in roots or leaves, for example Melastoma malabathricum (Watanabe et al., Citation2005). In addition, the levels of Al in roots are strongly dependent on apoplast pH (Godbold & Jentschke, Citation1998). Although fine root Ca/Al molar ratio reflects the relative availability of Ca and Al in the soil solution, the correspondence is not exact because of homeostatic regulation by plants. For example, in Scots pine seedlings exposed to a 30-fold increase in soil solution Ca/Al (20 – 560 μM) the response was a 10-fold decrease (from 5.8 to 0.6) in fine root Ca/Al ratio (, ).
Figure 2. Relationships between soil solution and fine root Ca/Al molar ratio in (a) laboratory and (b) field experiments. Lines are used to highlight trends in the data.
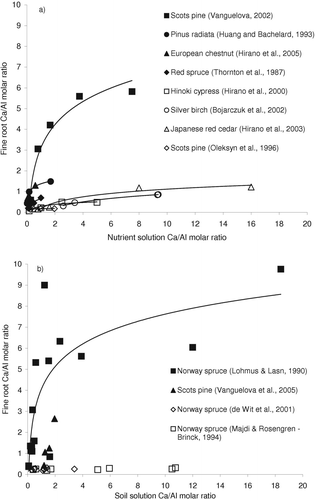
Table II. Response of tree seedlings, grouped according their sensitivities to aluminium in soil or nutrient solution. The response is expressed either by changes in nutrient status or growth inhibition
Highly significant relationships between soil and fine root Ca/Al molar ratios were found in 10 stands of Picea abies and Pinus sylvestris grown on different soil types in Estonia (Lõhmus & Lasn, Citation1990), suggesting that fine root uptake reflects soil conditions. Nevertheless, fine roots and tree uptake of different tree species will respond differently to a same Ca/Al molar ratio in soil solution. The importance of tree species is highlighted in the positive relationships found between soil solution Ca/Al and Ca/Al molar ratio in fine roots of several tree species (). Stronger relationships are often observed in experiments carried out under controlled compared with field conditions (). The slopes of the relationships and positions of the y-intercept in indicate that sensitivity to Al and acidity is species-specific, resulting in different effective uptake of Al into the root. For some species, fine root Ca/Al ratios do not reflect soil chemistry and soil solution Ca/Al. For example, fine root Ca/Al did not differ among four stands of Picea abies on soils of contrasting base and acidity status nor were they correlated with soil solution Ca/Al (Borken et al., Citation2007). This highlights the need for alternative indicators of environmental quality; the Ca/Al ratio in fine roots may be a better indicator of the risk of Al toxicity to tree species than is soil or solution chemistry.
Relationships between Ca/Al molar ratio of fine roots and root morphological and physiological parameters
Morphological and physiological responses of fine roots to Al stress can be a faster and more sensitive indicator than changes in root biomass (Schaedle et al., Citation1989; Hirano et al., Citation2007). Cronan and Grigal (Citation1995) reviewed relationships between soil and soil solution chemistry with root parameters such as biomass, growth, and nutrients and proposed Ca/Al molar ratios in soil solution, in fine roots or in foliage as potential indicators for acidification. Indeed, seedling roots of American, European, and Japanese trees had reduced Ca/Al molar ratios, reduced concentrations of Ca and Mg, and increased concentrations of Al after exposure to Al and low Ca/Al in the nutrient solution under laboratory conditions (see reviews by Andersson, Citation1988; Schaedle et al., Citation1989; Hirano et al., Citation2007). In a number of German sites, Jentschke et al. (Citation2001) reported a decrease in root biomass in the mineral soil (20 – 40 cm soil depth) with decreasing Ca/Al ratios (). Decreased fine root Ca/Al (<0.5) ratio in Pinus sylvestris was associated with significantly increased root necromass (Vanguelova et al., Citation2005). These results clearly encourage the use of root Ca/Al molar ratio as an indicator of Al toxicity. The first symptom of Al stress is inhibition of root growth (Matsumoto, Citation2000). In wheat (Triticum aestivum) roots, Al markedly decreased the cell length and increased the diameter of cells (Sasaki et al., Citation1996). This was also shown in tree seedling roots exposed to Al solutions (Hirano & Hijii, Citation1998). In experiments where Al was added, typical root responses were: decreased specific root length (SRL m g−1 DM), increased root diameter, and decreased root length; for many tree species, these responses correlated significantly with decreased fine root Ca/Al molar ratios (; Hirano, Citation2001; Vanguelova, Citation2002; Hirano et al., Citation2003; Sas Paszt & Mercik, Citation2004). Aluminium rapidly binds to binding sites in the apoplast. Replacements of Ca by Al occurs in the cell wall and alters structural and mechanical properties, making it more rigid, leading to a decrease in the mechanical extensibility (Horst, Citation1995; Kochian et al., Citation2005). In turn, root apices and laterals, which become thick, stubby, and brown in appearance, provide limited water and nutrient uptake.
Fine-root Ca/Al ratios are considered as accumulation indicators, while plants physiological reactions are considered as effect indicators, as described by Fränzle (Citation2006). Fine roots of European chestnut (Castania sativa) seedlings decreased significantly their Ca/Al molar ratios after an exposure of 168 μM Al for 28 days but did not after 7 days exposure (Hirano et al., Citation2006), indicating an accumulative effect. The defence-related cell wall polysaccharide callose in root tips of forest trees is one prospective physiological parameter of Al toxicity (Hirano et al., Citation2006). In crop plants such as maize (Zea mays), callose formation in root tips has been considered as a powerful tool in screening for Al-sensitive or resistant cultivars (Horst et al., Citation1997; Eticha et al., Citation2005). In the chestnut seedlings, the concentrations of callose increased significantly in root tips after one-day exposure of 168 μM Al (Hirano et al., Citation2006). Callose has also been shown to form in root tips of Norway spruce (Picea abies) seedlings exposed to Al after 6 h (with 280 μM: Hirano et al., Citation2004; with 5 mM: Nagy et al., Citation2004). Under forest field conditions, callose concentrations in short roots of Norway spruce was positively correlated with Al concentration but not with Ca/Al molar ratios in the soil solution (Wissemeier et al., Citation1998). Callose synthesis is dependent on both depolarization of the plasma membrane and an increase in intracellular Ca2+ concentrations in root cells (Bhuja et al., Citation2004; Sivaguru et al., Citation2005). As an initial response of Al treatment, Ca2+ is released from the exchange sites, thus increasing the free Ca2+ concentration in the apoplast and Ca2+ efflux, which leads to an increase in the cytosolic Ca2+ activity and triggering callose synthesis (Horst, Citation1995). This is a reasonable explanation of the negative relationships found between root Ca/Al molar ratio and callose formation in root tips of the seedlings of European chestnut grown in forest-field soils which has low base saturation (, Hirano et al., Citation2006).
Table III. Relationships found between fine root Ca/Al molar ratio and other root characteristics in the papers published since 1995
Some other physiological parameters such as the respiratory activity with triphenyltetrazolium chloride (TTC) and starch concentrations in Norway spruce roots in Swiss forests were measured with root Ca/Al molar ratios (, Brunner et al., Citation2002). However, both physiological parameters were found negatively correlated with the Ca/Al ratios, indicating that fine roots with lower Ca/Al ratios have higher vitality evaluated by the amount of TTC reductions. These results cannot be rationalised in this study, which suggest that respiratory activity and starch concentrations might not be suitable as indicators of Al stress (Brunner et al., Citation2002). Recently, Richter et al. (Citation2007) found that polyphenols in roots can mask changes in the amount of TTC reductions and therefore this test should not be applied to the fine roots of adult trees. This is partly why the lower Ca/Al ratio in roots has high TTC reductions in Norway spruce (Brunner et al., Citation2002).
Relationships between the Ca/Al ratio in fine roots and above-ground tree response
Cronan and Grigal (Citation1995) showed that molar Ca/Al ratios in foliar and root tissues are positively correlated with the solution Ca/Al molar ratios under controlled conditions. In general, foliar Al concentrations are far lower than root Al concentrations. This suggests that there is a root barrier to Al translocation at lower external Al concentrations (Cronan & Grigal, Citation1995). Increased Al concentration in roots is manifested both as decreased Ca/Al ratio in roots and as decreased translocation of different nutrient elements to shoots. So far, little information is available on the relationships between the root Ca/Al ratios and the parameters of above-ground parts.
In Pinus sylvestris seedlings grown in sand, a low fine root Ca/Al molar ratio was related to P uptake with increased retention of P due to Al-P precipitation in the roots (Vanguelova, Citation2002). In addition, fine root Ca/Al molar ratio was negatively related to Ca/Al molar ratio in new needles, old needles, new shoots, and stems. In Castanea sativa seedlings grown in quartz sand, the foliar and root Ca/Al molar ratios decreased simultaneously after exposure to 10 mM Al (Zysset et al., Citation1996). Under field conditions, foliar Ca/Al ratios of Castanea sativa seedlings responded more to soil base saturation than did fine root Ca/Al ratios (Hirano et al., Citation2006). Tree growth, however, correlated well with fine root Ca/Al ratio in eight Quercus petraea stands in France (Bakker, Citation1999b). Low Ca/Al ratios in rootstocks of apple trees were related to low Ca/Al ratios of soils and foliage, inducing a reduction in shoot and root growth (Sas Paszt & Mercik, Citation2004).
The overall relationships between soil, fine roots, and above-ground nutrient uptake and growth suggest that trees either below- or above-ground exhibit relatively consistent trends in response to changing Al and Ca/Al molar ratio in the soil solution. Further studies are needed to clarify the relationship between root Ca/Al ratio and e.g. photosynthesis and respiration, as the existing studies in this respect have taken solution or needle Ca/Al but not root Ca/Al into account (Oleksyn et al., Citation1996; Akaya & Takenaka, Citation2001).
Critical thresholds of fine root Ca/Al ratios as indicators of Al stress
Critical Ca/Al ratios typically have been derived from treatment experiments where the level of Al or the level of Ca and or acidity has been varied and the response of fine root Ca/Al evaluated. Critical Ca/Al ratio in roots is the value below which drastic/significant changes occur in root morphology and physiology as well as in seedling growth and uptake of related nutrients. For example, at Ca/Al ratio of 0.5 of Chamaecyparis obtusa roots, significant decrease in length of branching roots was observed; at Ca/Al ratio of 0.68 of Pinus sylvestris seedlings roots, significant changes in root morphology, chemistry, and reduced P uptake were observed (). Fine root Ca/Al below 0.2 and foliar Ca/Al below 12.5 (when soil solution Ca/Al is below 1) were suggested by Cronan and Grigal (Citation1995) to be associated with risk of Al toxicity. In the experiments reviewed here fine root ratios below 0.2 were observed in a few laboratory experiments, but the ratio was mainly above 0.2 in field conditions ().
However, thresholds for root and foliar Ca/Al may not coincide in individual tree species exposed to Al. For example, Vanguelova (Citation2002) reports that for Pinus sylvestris seedlings exposed to solution Ca/Al <1 with root Ca/Al <4.2, the foliage Ca/Al was already far below the critical value (12.5) proposed by Cronan and Grigal (Citation1995). When the root Ca/Al ratio dropped to 0.5, the Ca/Al ratio in new needles was already 5.5 (Vanguelova, Citation2002). Foliar Ca/Al ratios below 6.5 but root Ca/Al more than 0.4 were also reported for mature Quercus robur, Pinus radiata, and Eucalyptus nitens, Spain (Álvarez et al., Citation2005). Fine roots Ca/Al molar ratio of below 0.5 of mature Pices abies fine roots was associated with foliar Ca/Al molar ratio of 10.5 (Brunner et al., Citation2002), which is below the critical 12.5 while in all other cases where root ratio was more than 2.99, the foliar ratio was at least 20 or more. In other studies, Ca/Al ratios less than 12.5 in current and last year needles in Picea abies stands (Frey & Frey, Citation1993) had Ca/Al molar ratios in fine roots higher than the threshold level 0.2 (; Lõhmus & Lasn, Citation1990). Hence it is not excluded that the fine root Ca/Al threshold level should be higher for such a sensitive tree species as Picea abies.
The fine roots of apple trees with Ca/Al molar ratio of 0.005 was associated with shoots Ca/Al molar ratio of 0.20 and leaves Ca/Al molar ratio of 0.21 (Sas Paszt & Mercik, Citation2004) suggesting that fruit trees have much lower Ca/Al molar ratios not only in their roots but also in their above-ground parts.
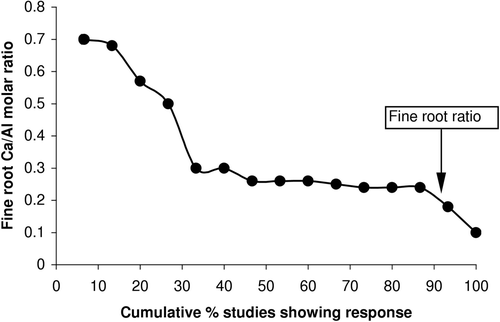
The threshold of root Ca/Al ratio in solution cultures and in sand and soil mixtures seems variable, and it is difficult to determine whether this is due to tree species or to experimental conditions (). Apart from four experiments, it has been difficult to establish thresholds in field experiments. Although these thresholds are only valid for the experimental conditions used, they give a general indication of the sensitivity of tree species and also the usefulness of the fine root Ca/Al as indicator for Al stress. To aid extrapolation from individual studies to general prediction about forest ecosystems, the data from were used to develop a cumulative probability plot in . Although based only on 15 datasets, shows that with the decline of fine root Ca/Al molar ratio, the probability of adverse impact on root and/or tree growth and nutrition increases. Forty percent of the studies reported a critical threshold between 0.3 and 0.7, 50% of between 0.2 and 0.3, and 10% of below 0.2. The ratio above and equal to 0.2 is associated with 90% of the studies exhibiting significant negative response which is higher in comparison to 60% for 0.2 and 80% for a root ratio of 0.1 estimated by Cronan and Grigal (Citation1995) based on 17 studies. The differences between this review and that of Cronan and Grigal (Citation1995) are due to the inclusion of above- and below-ground responses here, whereas in their review, Cronan and Grigal (Citation1995) considered only above-ground growth and nutrient response. This again confirms that roots are more sensitive and quickly reacting indicators to environmental stress compared to above-ground tree health indicators.
Figure 3. Cumulative probability distribution showing the studies listed in ranged sequentially according to the fine root Ca/Al molar ratio at which significant impacts on root/tree growth, morphology, physiology, or tissue nutrient concentrations were observed. As indicated, 90% of the studies reported thresholds at Ca/Al molar ratios above or equal to 0.2, which is the threshold suggested by Cronan and Grigal (Citation1995).
Despite the criticism (Högberg & Jensén, Citation1994; van Schöll et al., Citation2004) directed towards the use of Ca/Al thresholds in soil solution, foliage, and roots, they are potentially useful assessment tools for evaluating the risks of Al and acidity stress to ecosystems. However, research needs to continue if Ca/Al ratios are to be used with confidence as criteria in calculating critical loads and subsequently influencing political decisions (de Vries et al., Citation2003). Most importantly, there is a need to decrease the uncertainty associated with predicting the risk of ecosystem damage from environmental change.
Caveats for the use of Ca/Al ratio in fine roots
Growth in soil versus growth in culture solution
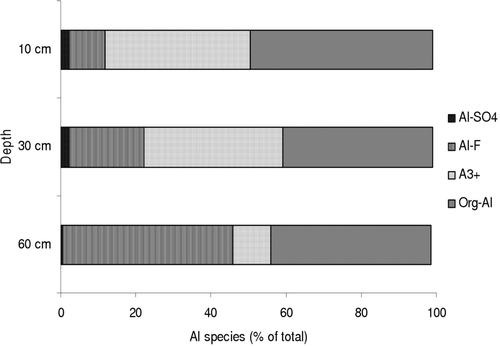
The literature shows that it is simpler to find a direct relationship between the Ca/Al ratio in roots as well as other root and plant parameters when the plants are grown in culture solution than when they are grown in soil ( and ). The reason for this is the complex matrix of soils, which constantly adsorb, exchange, and release elements from and into the soil solution depending on external and internal environmental factors and root/soil interactions. In addition, concentrations of available or potentially toxic Al could be quite different from total concentrations in soil solution due to the presence of organic substances in the soil solution (Göttlein, Citation1998). For example, free Al3+ in the mineral soil of a podzol was 10 – 20% of the total Al at 60-cm depth (B horizon) compared to 30 – 40% at 10 and 30 cm (A and E horizons) () and may constitute >80% of total Al in some mineral soils (Mulder & Stein, Citation1994). Consequently, relationships between Ca/Al ratios in soil solutions and roots may only be valid for mineral soils that have low concentrations of organically bound Al. Wargo et al. (Citation2003) found that in Picea rubens stands with a range of different Ca/Al ratios in the Oa horizon, there were no consistent correlations of root vitality variables with O horizon root chemistry or of O horizon root variables with soil solution elements. The reason is the high content of organic compounds in both solid and dissolved form in the O horizon which complex Al (Hue et al., Citation1986; Dijkstra & Fitzhugh, Citation2003). In sugar maple stands at the Hubbard Brook Experimental Forest, USA, there was a strong relationship between organically bound Al in the rhizosphere soils and fine root (Ca + Mg)/Al molar ratios after AlCl3 was added to the soil for three years (Phillips & Yanai, Citation2004). For eight Picea abies stands on acid soils in Estonia, stronger relationships were observed between soil and fine root Ca/Al when data for highly organic layers were excluded from the analyses (Lohmus & Lasn, Citation1990). Still the relationship in the subsoil soil (r = 0.93, P < 0.001) was stronger compared to one in the upper soil (r = 0.88, P < 0.01) again highlighting the importance of organo-mineral complexes in the organic topsoil.
Figure 4. Al speciation in the soil solution of a podzol modelled by MINEQL+ (data taken from Vanguelova, Citation2002).
Interhorizon differences of root Ca/Al ratio in the soil is another caveat for the interpretation of the root tissue ratios. There are strong vertical gradients in the fine root Ca/Al ratio independent of soil type and species, which is high in the organic layer and decreases with soil depth. An example is shown in . Root biomass, specific root length (SRL), and root density also show a decline with depth which is similar to this vertical decrease of root Ca/Al ratio (). This is, however, contradicting with the vertical gradient of Ca/Al ratio in the soil solution which is usually lower in the organic and top layer and increases with soil depth () which also coincides with higher root necromass in the top mineral soils compared with lower root necromass in the subsoil ().
One of the tolerance mechanisms of Al includes the exudation of chelating substances such as low molecular weight organic acids that form stable non-toxic complexes with Al (Jones, Citation1998). The role of organic acids in forming stable complexes with Al species is well documented in agricultural species (Jones, Citation1998; Barceló & Poschenrieder, Citation2002; Kochian et al., Citation2005). For tree species, the formation of such complexes has been shown for Picea rubens cell cultures (Minocha & Long, Citation2004) and for seedlings of Picea abies and Populus tremula (Heim et al., Citation2001; Qin et al., Citation2007). In eucalypt species, Al treatment induced the secretion of citric and malic acid in root exudates (Nguyen et al., Citation2003; Silva et al., Citation2004). Fine roots of trees in forest soils are almost entirely mycorrhizal (Jentschke & Godbold, Citation2000). The ectomycorrhizal fungus can moblilize P, K, Ca, and Mg from solid mineral substrates through organic acid excretion (Landeweert et al., Citation2001), inducing changes of Ca/Al ratios in soil solutions. The presence of ectomycorrhizal mycelia decreased leaching of base cations and Al from the soil (Ahonen-Jonnarth et al., Citation2003). Exposure to Al enhanced the secretion of oxalic and citrate acid from ectomycorrhizal roots of Pinus sylvestris and Pinus desiflora seedlings, respectively (Ahonen-Jonnarth et al., Citation2000; Tahara et al., Citation2005). The fungal mantle and the Hartig net may bind Al so that it does not reach the roots (Moyer-Henry et al., Citation2005). Arbuscular mycorrhizal fungi may have similar abilities (Lux & Cumming, Citation2001). However, the effects of mycorrhizal associations on tree response to acidification depend on the species both of the fungi and host trees (Finlay, Citation1995; Jentschke & Godbold, Citation2000; van Schöll et al., Citation2005).
Root tips of several crop plants alkalinize their surroundings due to different uptake and exchange mechanisms (Hinsinger et al., Citation2003), thus lowering the concentration of toxic Al species, so that the concentration of toxic Al species experienced by the roots may be lower than the concentration measured in bulk solution (Degenhardt et al., Citation1998). Greenhouse experiments using rhizoboxes revealed that rhizosphere pH in strawberry plants was dependent on root activity and the concentrations of exchangeable Al in the soil (Sas Paszt et al., Citation2002). In tree species, the concentrations of Al3+ ions significantly increased in soil solution in the rhizosphere of Quercus robur seedlings growing in rhizoboxes (Göttlein et al., Citation1999) and of mycorrhizal roots of mature P. abies (Dieffenbach & Matzner, Citation2000). This was partly due to proton release for maintenance of the cation-anion balance during nutrient uptake (Göttlein et al., Citation1999). The root uptake creates concentration gradients around the roots, whether they are grown in soil or in well-stirred culture solution. The preference of tree uptake of NH4 over NO3, which is the case with most coniferous species, acidifies the rhizosphere soil, which may increase the free Al concentration around the root.
pH considerations
A decrease in pH of “only” 0.3 units means a doubling of the H+ concentration in a solution. There is a complex interaction between pH and Al, and the effects of pH and Al are difficult to separate. Kinraide (Citation2003), using wheat as a model plant, calculated the ion activities and the electrical potential at the plasma membrane. He concluded that in acid soils, Al3+ may prevent intrinsic toxicity of H+, but Al3+ may at the same time be an extrinsic toxicant and thus induces Ca and Mg deficiency. On the other hand, Kinraide (Citation2003) suggested that Al3+ toxicity is likely to be more severe in soils at pH 4.1 than in more acid ones. This is due to the complex activity between Al and H (Kinraide et al., Citation1992). In natural soils, Al activity increases as pH declines to pH 4.1, but it decreases further as pH goes below 4.1, and this occurs not only in the bulk phase Al activity but also at root cell plasma membrane levels. Therefore, it is possible that with the acidification of the soil solution, the Al activity at the root plasma membrane also declines, which may determine plant response to ions more directly than bulk-phase activities. For example, in an acid irrigated field experiment with Picea abies, pH of mineral soil (0 – 20 cm depth) was 4.1, Ca and Mg concentrations in roots had decreased, while the Al contents in roots had not increased one year after the irrigation stopped; root growth declined only in the topsoil, below 5 cm depth root growth was unaffected (Hahn & Marschner, Citation1998a,Citationb). In another field based manipulated study, acid irrigation affected fine root growth in the organic layer and the top 5 cm of the mineral soil, which was not due to a direct Al effect but more likely due to high H+ and reduced base cations concentrations in soil and roots (Vanguelova et al., Citation2007). In Picea abies seedlings grown for 7 weeks in nutrient solutions of pH 3.2, 4.0, or 5.0 and with 0, 100, or 400 μM Al, Godbold and Jentschke (Citation1998) found that low pH reduced the inhibiting effect of Al on root and shoot growth, and Al in roots decreased as pH decreased. The Mg uptake was strongly affected by Al at pH 3.2 but not at pH 5.0, as discussed earlier.
Root Al fractions
In the root, Al can be bound at several places and in different forms, and all of these are not available for interactions with other elements, e.g. Ca in the root. Due to higher pH in the cytoplasm and vacuole than outside the plasma membrane, Al that has been transported across the plasma membrane may, to a great extent, be complexed to carboxylate groups or precipitated as Al(OH)3. It is assumed that it is only the Al fraction bound in some way to the cell wall that is available for interactions with Ca. There are both exchangeable and non-exchangeable Al in the cell wall. In some studies (Dahlgren et al., Citation1991), the exchangeable fraction has been exchanged (by rinsing the root in a salt solution) before elemental analysis to determine the Al that is really “inside” the root. The resulting Ca/Al ratio will thus be higher than in cases where such ion exchange has not been performed. Much of the Al taken up by the roots will be confined to the cell wall (Godbold & Jentschke, Citation1998). Ca/Al ratios were measured in both the total roots and in cortical cell walls using X-ray microanalysis in a study by Jentschke et al. (Citation2001). These two measures showed similar relationships, although using X-ray microanalysis the amplitude of the changes was much greater. This difference in Ca/Al ratios between total roots and in cortical cell walls using X-ray microanalysis reflects the importance of cell walls as sites of Al accumulation and provides the basis of the explanation of differences in Ca/Al ratios of roots of different diameters shown by Püttsepp (Citation2004). Thus, the absolute Ca/Al ratio is affected by differences in the relative amounts of cell walls active in Al binding to root dry weight.
The Al bound to or precipitated in the cell wall is excluded from transport to the shoot. For example, Nowak and Friend (Citation2005) used 5 mm long root tips of different pine species grown in nutrient solution with Al and treated them with 0.5 mM citric acid. They found that 55% of the root Al was cell-wall-labile (exchangeable), 33% was cell-wall-bound, and only 12% was found in the symplasm. On the other hand, Camps et al. (Citation2004), in roots of Quercus robur, Pinus radiata, and Eucalyptus globulus grown in two different types of acid soil found that of the Al in the cell wall less than 14% was easily exchangeable (extractable by 1 M NH4Cl) and up to 92% was in the form of phosphate and organic precipitates (soluble in 1 and 10 mM HCl). They also found that the total Al concentration in the roots was lowest in the soil type with lowest pH and highest contents of Al-undersaturated organic matter, probably due to Al solubility being controlled mainly by complexation with organic matter (Gustafsson et al., Citation2001). A caveat in Camps et al. (Citation2004) is that they did not dry ash the roots, and so part of the Al measured may have come from adhering soil particles (see next section).
Jentschke et al. (Citation2001) showed for Picea abies that Ca/Al ratios below 0.2 in the roots (determined by element analysis) coincided with Ca/Al ratios below 0.01 in the cell walls (determined by X-ray microanalysis) and with low fine-root length density in the field.
In general, Al toxicity is accompanied by the accumulation of Al in the meristematic zone of root apices. Therefore, to evaluate the degree of the Al toxicity, Al contents and Ca/Al ratios in root tips should be more accurate criteria than those in whole roots. However, such a procedure is very laborious to be applied for field-grown roots.
When the exchangeable Al is removed, then its place is taken by other ions, which may interfere with the normal exchange on the root surface. Thus, it is important to differentiate at least between the two Al fractions: exchangeable (labile) extracted with salts and not exchangeable (non-labile) extracted by acid digestion. Overall, the measurement of total Al in roots without any pre-treatment is the most practical, especially for field grown fine roots, but if the focus is on the mechanisms of Al toxicity in fine roots, the Al fractions in fine roots as mentioned above need to be considered.
Root sampling, processing and analysis
Cronan and Grigal (Citation1995) suggested that fine roots of trees be sampled one month after tree flush. However, the seasonal changes in soil solution chemistry could influence significantly the chemistry of fine roots (Vanguelova et al., Citation2005). Therefore, samples should be taken a minimum of twice a year, once after leaf flush and once at the end of the growing season, just before leaf senescence. Soil coring is the method most used for sampling fine roots, with core sampler diameter of 6 – 10 cm and length of 10 – 15 cm, with sharp serrated edges to cut small roots. Ten to twelve samples for an area of 10 × 10 m in an average aged tree stands are the statistically minimum sample size estimated to represent reliable data on root biomass and length density (Vogt et al., Citation1993; Olsthoorn, Citation1991; Vanguelova et al., Citation2005), and this sample size is recommended for root chemistry. Root samples need to be transported and sorted out as soon as possible or stored for some days in a temperature not higher than 4°C before sorting.
The suggested protocol for sorting, processing, and analysing fine roots to determine chemical contents is shown in . This protocol is well established (e.g. Majdi & Persson, Citation1995; Jentschke et al., Citation2001; Vanguelova et al., Citation2005, Citation2007) but still overlooked by some researchers. Soil grown roots should be dry ashed before being analyzed, so that calculations can be done regarding how much of the analyzed elements stems from the soil adhering to the roots. Soil contamination of fine roots in some cases may be negligible; for example, ash content remained under 5% in fine roots studied by Lõhmus and Lasn (Citation1990). However in other cases, mineral soil adherent to the roots can overestimate root biomass content with up to 19% (Vanguelova, Citation2002), which will reduce the Ca/Al molar ratio measured in fine roots. In cases where this has not been done, one cannot reliably compare the Ca/Al results with those from dry ashed roots. It should be noted that even with the procedures shown in , there may be caveats (Misra, Citation1994; Hunt et al., Citation1998).
Table IV. Suggested protocol for fine root processing and chemical analysis
Overall evaluation of the use of Ca/Al in fine roots as indicator for Al and acidity stress
It has to be admitted that using roots as an indicator is a cumbersome method due to the fact that studies of soil-grown roots is tedious and time consuming. This is not the easy and quick way it should be if indicators are meant to act as “environmental fever thermometers”, to borrow the expression from Fränzle (Citation2006). Using tree foliar chemistry as environmental indicator, which needs climbing trees, or shooting entire branches out of the tree to sample from the tree-top layers, however, is probably as tedious and time and resource consuming as to dig up tree fine roots, especially if the emphasis is on the fine roots within the top 20 cm in the soil profile.
The difficulties of working with root indicators to give meaningful indicators of tree health have been discussed earlier. Foliar analysis could be used as a surrogate for such indicators in an interim period. The link between below- and above-ground responses in terms of tree health is only possible if both are monitored. This is where the potential of long term forest monitoring (e.g. European Level I, Level II Forest Monitoring networks) comes in answering some of the fundamental questions about tree plasticity and its response, damage, C allocation, and adaptation to a changing environment. Distinguishing between interactive and influencing factors is only possible if most of these are monitored as in an intensive monitoring programme such as the European Level II Forest Monitoring Network.
Fine root Ca/Al molar ratio can be used as a parameter in forest monitoring in two ways. First, a decreasing Ca/Al ratio in the roots is a specific response to Al stress and/or soil acidification. In contrast, changes in root biomass and morphology can occur under different stresses. These parameters are regarded as indicators of root damage in general but are not suitable to detect the responses to a specific stress (Hirano et al., Citation2007). As shown in , the Ca/Al molar ratio in fine roots is related to morphological and physiological changes in fine roots. If we monitor and clarify the trend of the ratio in fine roots at long-term for a specific site, the effects of soil acidification on forest trees can be detected at the early stage.
Secondly, changes in Ca/Al molar ratio in fine roots of a specific tree-species occur within the range of concentrations of Al at forest-site levels. Some root parameters of forest trees such as biomass and morphological changes usually cannot be detected at lower levels of Al found in the field. These results support the use of the root Ca/Al ratio as an indicator of Al stress in the forest-field and in orchard conditions.
Based on the synthesis of the research carried out and the expert judgement of the authors of this review, attempts to evaluate the usefulness of fine root Ca/Al ratio based on general criteria for environmental indicators.
Table V. Criteria for the use of environmental indicators and how Ca/Al molar ratio stands according to the above synthesis
Conclusions
This review of the literature demonstrates that fine root Ca/Al ratio has been used successfully to indicate Al stress to small tree seedlings exposed to Al under controlled conditions.
A number of relationships have been found between the fine root Ca/Al ratio and root morphology and nutrition, as well as with fine root elongation, seedling growth, and nutrient uptake. Fine root Ca/Al ranged from 0.03 to 17 in tree seedlings under controlled experimental conditions with exposure to Al. The Ca/Al molar ratio in fine roots was negatively correlated with callose formation, which is another indicator specific to Al stress. Some of the changes mentioned above have occurred when the fine root Ca/Al molar ratio was below the suggested critical value of 0.2, but others have occurred at higher ratios. This is partly due to differences in the experimental conditions and in the tolerance and uptake mechanisms of the different tree species. Data on fine root Ca/Al molar ratio from field-based experiments or observations suggest that this ratio is responsive to changes in soil and soil solution pH, Ca, Al, and Ca/Al molar ratio. Fine root Ca/Al ratio was found in some cases to be negatively related to root necromass and positively related to root biomass and specific root length. Values between 0.1 and 18 for Ca/Al molar ratio in the fine roots of mature trees were reported, but values are only rarely determined below the critical 0.2 threshold. Differences in tree age, soil vertical chemical gradient, and aluminium speciation in the soil solution play an important role in the comparison of indices for Al stress in laboratory and field based research studies.
Based on the literature reviewed, the critical thresholds for the Ca/Al fine root ratio of 0.2 suggested by Cronan and Grigal (Citation1995) was estimated to represent 90% risk of inverse impact on root and above-ground tree growth. However, there is uncertainty in the derivation of the Ca/Al molar ratio in fine roots including difference in experimental/environmental conditions, tree sensitivities, potential errors in sampling and analysis of plant tissues, and a poor understanding of adsorption phenomenon at the root surface. Thus, improvement and harmonisation of the methodologies used in experimental research either in controlled or field environment is necessary.
The difficulties of working with root to give meaningful indicators of tree health have been discussed in great detail. However, the overall results of this review support strongly the use of root Ca/Al ratio as an indicator of Al stress in the forest-field and in orchard conditions. Based on scientific evidence and expert judgement, the Ca/Al molar ratio in fine roots is proposed as a suitable parameter to be included in long-term forest monitoring networks as a reliable indicator of the risk of Al toxicity and soil acidity stress to the fine root and tree growth.
Acknowledgements
Financial support for this study by COST E38 “Woody root processes under changing environment”, the Norwegian Forest and Landscape Institute and Forestry Commission, UK, are gratefully acknowledged. Yasuhiro Hirano was financially supported by a Grant-in-Aid (No. 18681006) for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Thanks to Dr. Andy Moffat, Dr. Sheila Palmer and the anonymous reviewers for their valuable comments on the manuscript and considerable improvement of the English.
References
- Ahonen-Jonnarth , U , Göransson , A and Finlay , R D . 2003 . Growth and nutrient uptake of ectomycorrhizal Pinus sylvestris seedlings in a natural substrate treated with elevated Al concentrations . Tree Physiol , 23 : 157 – 167 .
- Ahonen-Jonnarth , U , van Hees , P AW , Lundström , U S and Finlay , R D . 2000 . Production of organic acids by mycorrhizal and nonmycorrhizal Pinus sylvestris L. seedlings exposed to elevated concentrations of aluminum and heavy metals . New Phytol , 146 : 557 – 567 .
- Akaya , M and Takenaka , C . 2001 . Effects of aluminum stress on photosynthesis of Quercus glauca Thumb . Plant Soil , 237 : 137 – 146 .
- Álvarez , E , Fernández-Marcos , M L , Monterroso , C and Fernándes-Sanjurjo , M J . 2005 . Application of aluminium toxicity indices to soils under various forest species . For Ecol Manag , 211 : 227 – 239 .
- Andersson , M . 1988 . Toxicity and tolerance of aluminium in vascular plants . Water Air Soil Pollut , 39 : 439 – 462 .
- Bakker , M R . 1999a . The effect of lime and gypsum applications on a sessile oak (Quercus petraea (M.) Liebl.) stand at La Croix Scaille (French Ardennes) II. Fine root dynamics . Plant Soil , 206 : 109 – 121 .
- Bakker , M R . 1999b . Fine-root parameters as indicators of sustainability of forest ecosystems . Forest Ecol Manag , 122 : 7 – 16 .
- Bakker , M R , Kerisit , R , Verbist , K and Nys , C . 1999 . Effects of liming on rhizosphere chemistry and growth of fine roots and of shoots of sessile oak (Quercus petraea) . Plant Soil , 217 : 243 – 255 .
- Bakker , M R and Nys , C . 1999 . Effects of lime-induced differences in site fertility on fine roots of oak . Ann For Sci , 56 : 599 – 606 .
- Barceló , J and Poschenrieder , C . 2002 . Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review . Environ Exp Bot , 48 : 75 – 92 .
- Bhuja , P , McLachlan , K , Stephens , J and Taylor , G . 2004 . Accumulation of 1,3-β-D-glucans in response to aluminum and cytosolic calcium in Triticum aestivum . Plant Cell Physiol , 45 : 543 – 549 .
- Bojarczuk , K , Karolewski , P , Oleksyn , J , Kieliszewska-Rokicka , B , Żytkowiak , R and Tjoelker , M G . 2002 . Effect of polluted soil and fertilisation on growth and physiology of silver birch (Betula pendula Roth.) seedlings . Polish J Environ Studies , 11 : 483 – 492 .
- Borken , W , Kossmann , G and Matzner , E . 2007 . Biomass, morphology and nutrient contents of fine roots in four Norway spruce stands . Plant Soil , 292 : 79 – 93 .
- Brunner , I , Brodbeck , S and Walthert , L . 2002 . Fine root chemistry, starch concentration, and ‘vitality’ of subalpine conifer forests in relation to soil pH . Forest Ecol Manag , 165 : 75 – 84 .
- Brunner , I , Zimmermann , S , Zingg , A and Blaser , P . 2004 . Wood ash recycling affects forest soil and tree fine-root chemistry and reverses soil acidification . Plant Soil , 267 : 61 – 71 .
- Camps , A M , Mourenza , C , Álvarez , E and Macías , F . 2004 . Influence of parent material and soil type on the root chemistry of forest species grown on acid soils . Forest Ecol Manag , 193 : 307 – 320 .
- Cronan , C S and Grigal , D F . 1995 . Use of calcium/aluminum ratios as indicators of stress in forest ecosystems . J Environ Qual , 24 : 209 – 226 .
- Dahlgren , R A , Vogt , K A and Ugolini , F C . 1991 . The influence of soil chemistry on fine root aluminum concentrations and root dynamics in a subalpine Spodosol, Washington State, USA . Plant Soil , 133 : 117 – 129 .
- Degenhardt , J , Larsen , P B , Howell , S H and Kochian , L V . 1998 . Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH . Plant Physiol , 117 : 19 – 27 .
- de Vries , W , Reinds , G J and Vel , E . 2003 . Intensive monitoring of forest ecosystems in Europe 2: Atomospheric deposition and its impacts on soil solution chemistry . For Ecol Manag , 174 : 97 – 115 .
- De Wit , H A , Mulder , J , Nygaard , P H , Aamlid , D , Huse , M , Kortnes , E , Wollebæk , G and Brean , R . 2001 . Aluminium: The need for a re-evaluation of its toxicity and solubility in mature forest stands . Water Air Soil Pollut Focus , 1 : 103 – 118 .
- Dieffenbach , A and Matzner , E . 2000 . In situ soil solution chemistry in the rhizosphere of mature Norway spruce (Picea abies (L.) Karst.) trees . Plant Soil , 222 : 149 – 161 .
- Dijkstra , F A and Fitzhugh , R D . 2003 . Aluminum solubility and mobility in relation to organic carbon in surface soils affected by six tree species of the northern United States . Geoderma , 114 : 33 – 47 .
- Driscoll , C T , Lawrence , G B , Bulger , A J , Butler , T J , Cronan , C S , Eager , C , Lambert , K F , Likens , G E , Stoddard , J L and Weathers , K C . 2001 . Acidic deposition in the northeastern United States: Sources and inputs, ecosystem effects, and management strategies . BioScience , 51 : 180 – 198 .
- Eldhuset , T D , Lange , H and de Wit , H A . 2006 . Fine root biomass, necromass and chemistry during seven years of elevated aluminium concentrations in the soil solution of a middle-aged Picea abies stand . Sci Tot Environ , 369 : 344 – 356 .
- Eldhuset , T D and Nygaard , P H . Effects of aluminium and base cations on nutrient uptake in magnesium deficient Norway spruce . Oral presentation at the Annual Meeting of Soil Science Society of America . 1997 , Anaheim, CA. Agronomy Abstracts Annual Meetings 1997:92
- Eticha , D , Thé , C , Welcker , C , Narro , L , Staß , A and Horst , W J . 2005 . Aluminium-induced callose formation in root apices: Inheritance and selection trait for adaptation of tropical maize to acid soils . Field Crop Res , 93 : 252 – 263 .
- Finlay , R D . 1995 . Interactions between soil acidification, plant growth and nutrient uptake in ectomycorrhizal associations of forest trees . Ecol Bull , 44 : 197 – 214 .
- Fränzle , O . 2006 . Complex bioindication and environmental stress assessment . Ecol Ind , 6 : 114 – 136 .
- Frey , T and Frey , J . 1993 . “ Foliar chemical composition of Norway spruce with and without defoliation ” . In Nutrient uptake and cycling in forest ecosystems. European Commission Ecosystem Research Report No. 21 Edited by: Nilsson , L O , Hüttl , R F , Johansson , U T and Mathy , P . 123 – 129 .
- Freer-Smith , P H and Read , D B . 1995 . The relationship between crown condition and soil solution chemistry in oak and Sitka spruce in England and Wales . For Ecol Manag , 79 : 185 – 196 .
- Genenger , M , Zimmermann , S , Hallenbarter , D , Landolt , W , Frossard , E and Brunner , I . 2003 . Fine root growth and element concentrations of Norway spruce as affected by wood ash and liquid fertilisation . Plant Soil , 255 : 253 – 264 .
- Godbold , D L , Fritz , E and Hüttermann , A . 1988 . Aluminum toxicity and forest decline . Proc Natl Acad Sci USA , 85 : 3888 – 3892 .
- Godbold , D L and Jentschke , G . 1998 . Aluminum accumulation in root cell walls coincides with inhibition of root growth but not with inhibition of magnesium uptake in Norway spruce . Physiol Plant , 102 : 553 – 560 .
- Göransson , A and Eldhuset , T D . 1987 . Effects of aluminium on growth and nutrient uptake of Betula pendula seedlings . Physiol Plantarum , 69 : 193 – 199 .
- Göransson , A and Eldhuset , T D . 1991 . Effects of aluminium on growth and nutrient uptake of small Picea abies and Pinus sylvestris plants . Trees , 5 : 136 – 142 .
- Göttlein , A . 1998 . Determination of free Al3+ in soil solutions by capillary electrophoresis . Eur J Soil Sci , 49 : 107 – 112 .
- Göttlein , A , Heim , A and Matzner , E . 1999 . Mobilization of aluminum in the rhizosphere soil solution of growing tree roots in an acidic soil . Plant Soil , 211 : 41 – 49 .
- Gustafsson , J P , Berggren , D , Simonsson , M , Zysset , M and Mulder , J . 2001 . Aluminium solubility mechanisms in moderately acid Bs horizons of podzolized soils . Eur J Soil Sci , 52 : 655 – 665 .
- Hahn , G and Marschner , H . 1998a . Effect of acid irrigation and liming on root growth of Norway spruce . Plant Soil , 199 : 11 – 22 .
- Hahn , G and Marschner , H . 1998b . Cation concentrations of short roots of Norway spruce as affected by acid irrigation and liming . Plant Soil , 199 : 23 – 27 .
- Heim , A , Brunner , I , Frey , B , Frossard , E and Luster , J . 2001 . Root exudation, organic acids, and element distribution in roots of Norway spruce seedlings treated with aluminium in hydroponics . J Plant Nutr Soil Sci , 164 : 519 – 526 .
- Hinsinger , P , Plassard , C , Tang , C and Jaillard , B . 2003 . Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review . Plant Soil , 248 : 43 – 59 .
- Hirano , Y . 2001 . “ Effects of aluminum on the root morphology of Japanese red cedar and Hinoki cypress seedlings ” . In L'Arbre/The Tree 2000 , Edited by: Labrecque , M . 339 – 344 . Quebec : Isabelle Quentin .
- Hirano , Y , Graf Pannatier , E , Zimmermann , S and Brunner , I . 2004 . Induction of callose in roots of Norway spruce seedlings after short-term exposure to aluminum . Tree Physiol , 24 : 1279 – 1283 .
- Hirano , Y and Hijii , N . 1998 . Effects of low pH and Al on root morphology of Japanese red cedar saplings . Environ Pollut , 101 : 339 – 347 .
- Hirano , Y , Isomura , A and Kaneko , S . 2003 . Root morphology and nutritional status of Japanese red cedar saplings subjected to in situ levels of aluminum in forest soil solution . J Forest Res , 8 : 209 – 214 .
- Hirano , Y , Matsumoto , C and Takenaka , C . 2000 . Root response of Hinoki cypress seedlings to various levels of Ca/Al molar ratios . Environ Sci , 7 : 71 – 82 .
- Hirano , Y , Mizoguchi , T and Brunner , I . 2007 . Root parameters of forest trees as sensitive indicators of acidifying pollutants: A review of research of Japanese forest trees . J Forest Res , 12 : 134 – 142 .
- Hirano , Y , Walthert , L and Brunner , I . 2006 . Callose in root apices of European chestnut seedlings: A physiological indicator of aluminum stress . Tree Physiol , 26 : 431 – 440 .
- Högberg , P and Jensén , P . 1994 . Aluminium and uptake of base cations by tree roots: A critique of the model proposed by Sverdrup et al . Water Air Soil Pollut , 75 : 121 – 125 .
- Horst , W J . 1995 . The role of the apoplast in aluminum toxicity and resistance of higher plants: A review . Z Pflanzernähr Bodenk , 158 : 419 – 428 .
- Horst , W J , Püschel , A K and Schmohl , N . 1997 . Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize . Plant Soil , 192 : 23 – 30 .
- Huang , J and Bachelard , E P . 1993 . Effects of aluminium on growth and cation uptake in seedlings of Eucalyptus manifera and Pinus radiata . Plant Soil , 149 : 121 – 127 .
- Hue , N V , Craddock , G R and Adams , F . 1986 . Effects of organic acids on aluminium toxicity in subsoils . Soil Sci Soc Am J , 50 : 28 – 34 .
- Hunt , H W , Reuss , D E and Elliott , E T . 1998 . Correcting estimates of root chemical composition for soil contamination . Ecology , 80 : 702 – 707 .
- Jentschke , G , Drexhage , M , Fritz , H W , Fritz , E , Schella , B , Lee , D H , Gruber , F , Heinmann , J , Kuhr , M , Schmidt , J , Schmidt , S , Zimmermann , R and Godbold , D L . 2001 . Does soil acidity reduce subsoil rooting in Norway spruce (Picea abies)? . Plant Soil , 237 : 91 – 108 .
- Jentschke , G and Godbold , D L . 2000 . Metal toxicity and ectomycorrhizas . Physiol Plant , 109 : 107 – 116 .
- Jones , D L . 1998 . Organic acids in the rhizosphere – A critical review . Plant Soil , 205 : 25 – 44 .
- Keltjens , W G and Loenen , E V . 1989 . Effects of aluminium and mineral nutrition on growth and chemical composition of hydroponically grown seedlings of five different forest soil species . Plant Soil , 119 : 39 – 50 .
- Kinraide , T B . 2003 . Toxicity factors in acidic forest soils: Attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation . Eur J Soil Sci , 54 : 323 – 333 .
- Kinraide , T B , Ryan , P R and Kochian , L V . 1992 . Interactive effects of Al3+, H+ and other cations on root elongation considered in terms of cell-surface electrical potential . Plant Physiol , 99 : 1461 – 1468 .
- Kochian , L V , Pineros , M A and Hoekenga , O A . 2005 . The physiology, genetics and molecular biology of plant aluminum resistance and toxicity . Plant Soil , 274 : 175 – 195 .
- Landeweert , R , Hoffland , E , Finlay , R D , Kuyper , T W and van Breemen , N . 2001 . Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals . Tree , 16 : 248 – 254 .
- Lõhmus , K and Lasn , R . 1990 . “ Spruce and pine root structures and chemical characteristics in moderate acid soils ” . In Above and below-ground interactions in forest trees in acidified soils Air Pollution Research Report 32
- Lux , H B and Cumming , J R . 2001 . Mycorrhizae confer aluminium resistance to tulip-poplar seedlings . Can J Forest Res , 31 : 694 – 702 .
- Majdi , H and Persson , H . 1995 . Effects of ammonium sulphate application on the chemistry of bulk soil, rhizosphere, fine roots and fine-root distribution in a Picea abies (L.) Karst. stand . Plant Soil , 168/169 : 151 – 160 .
- Majdi , H and Rosengren-Brinck , U . 1994 . Effects of ammonium sulphate application on the rhizosphere, fine root and needle chemistry in a Picea abies (L.) Karst. Stand . Plant Soil , 162 : 71 – 80 .
- Marschner , H . 1991 . Mechanisms of adaptation of plants to acid soils . Plant Soil , 134 : 1 – 20 .
- Matsumoto , H . 2000 . Cell biology of Al tolerance and toxicity in higher plants . Int Rev Cytol , 200 : 1 – 46 .
- McLaughlin , S B and Wimmer , R . 1999 . Calcium physiology and terrestrial ecosystem processes . New Phytol , 142 : 373 – 417 .
- Minocha , R and Long , S . 2004 . Effects of aluminium on organic acid metabolism and secretion by red spruce cell suspension cultures and the reversal of Al effects on growth and polyamine metabolism by exogenous organic acids . Tree Physiol , 24 : 55 – 64 .
- Misra , R K . 1994 . Assessment of errors in nutrient analyses of roots . Austral J Soil Res , 32 : 1275 – 1286 .
- Moyer-Henry , K , Silva , I , Macfall , J , Johannes , E , Allen , N , Goldfarb , B and Rufty , T . 2005 . Accumulation and localization of aluminium in root tips of loblolly pine seedlings and the associated ectomycorrhiza Pisolithus tinctorius . Plant Cell Environ , 28 : 111 – 120 .
- Mulder , J and Stein , A . 1994 . The solubility of aluminum in acidic forest soils: Long-term changes due to acid deposition . Geochim Cosmochim Acta , 58 : 85 – 94 .
- Nagy , N E , Dalen , L S , Jones , D L , Fossdal , C G and Eldhuset , T D . 2004 . Cytological and enzymatic responses to aluminium stress in root tips of Norway spruce seedlings . New Phytol , 163 : 595 – 607 .
- Nguyen , N T , Nakabayashi , K , Thompson , J and Fujita , K . 2003 . Role of exudation of organic acids and phosphate in aluminium tolerance of four tropical woody species . Tree Physiol , 23 : 1041 – 1050 .
- Nowak , J and Friend , A L . 2005 . Aluminium fractions in root tips of slash pine and loblolly pine families differing in Al resistance . Tree Physiol , 25 : 245 – 250 .
- Nygaard , P H . 1999 . Effects of sulphur and nitrogen on boreal forest vegetation, soils and nutrient uptake [thesis] , 99 Ås : Agricultural University of Norway . Dr. scient. theses 1999:3
- Nygaard , P H and de Wit , H A . 2004 . Effects of elevated soil solution Al concentrations on fine roots in a middle aged Norway spruce (Picea abies (L.) Karst.) stand . Plant Soil , 265 : 131 – 140 .
- Oleksyn , J , Karolewski , P , Giertych , M J , Werner , A , Tjoelker , M G and Reich , P B . 1996 . Altered root growth and plant chemistry of Pinus sylvestris seedlings subjected to aluminium in nutrient solution . Trees , 10 : 135 – 144 .
- Olsthoorn , A . 1991 . Fine root density and root biomass of two Douglas-fir stands on sandy soils in The Netherlands. I. Root biomass in early summer . Neth J Agr Sci , 39 : 49 – 60 .
- Persson , H . 1988 . “ Root growth and root processes – Effects of air pollutants in forests stands ” . In Relationships between above and below-ground influences of air pollutants of forests trees Air Pollut Res Rep 16: 180 – 186
- Persson , H , Majdi , H and Clemensson-Lindell , A . 1995 . Effects of acid deposition on tree roots . Ecol Bull , 44 : 158 – 167 .
- Phillips , R P and Yanai , R D . 2004 . The effects of AlCl3 additions on rhizophere soil and fine root chemistry of sugar maple (Acer saccarum) . Water Air Soil Pollut , 159 : 339 – 356 .
- Puhe , J . 2003 . Growth and development of the root system of Norway spruce (Picea abies) in forest stands – A review . For Ecol Manag , 175 : 253 – 273 .
- Püttsepp , Ü . 2004 . Effects of sustainable management practices on fine-root systems in willow (Salix viminalis, S. dasyclados), grey alder (Alnus incana), and Norway spruce (Picea abies) stands [thesis] , Uppsala : Acta Universitatis Agriculturae Sueciae . Silvestria 283
- Püttsepp , Ü , Lõhmus , K , Persson , HÅ and Ahlström , K . 2006 . Fine-root distribution and morphology in an acidic Norway spruce (Picea abies (L.) Karst.) stand in SW Sweden in relation to granulated wood ash application . Forest Ecol Manag , 221 : 291 – 298 .
- Qin , R J , Hirano , Y and Brunner , I . 2007 . Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn . Tree Physiol , 27 : 313 – 320 .
- Rengel , Z and Zhang , W H . 2003 . Role of dynamics of intracellular calcium in aluminum-toxicity syndrome . New Phytol , 159 : 295 – 314 .
- Richter , A K , Frossard , E and Brunner , I . 2007 . Polyphenols in the woody roots of Norway spruce and European beech reduce TTC . Tree Physiol , 27 : 155 – 160 .
- Sas Paszt , L , Marschner , H , Römheld , V and Mercik , S . 2002 . The influence of aluminium on rhizosphere and bulk soil pH and growth of strawberry plants . Zeszyty Problemowe Postępów Nauk Rolniczych z , 482 : 467 – 474 .
- Sas , L and Mercik , S . 2001 . Content of K, Mg and Ca in apple rootstocks, varieties and soil depending on soil pH. Międzynar. Konf. Nauk. . “Potas i magnez w rolnictwie”, Rogów, 4 – 6 września , 2001 : 87 – 88 .
- Sas Paszt , L and Mercik , S . 2004 . The response of Apple Rootstocks P.22, M.9, M.26, and Apple Tree Cultivars ‘Jonagold’ and ‘Gala’ to Soil Acidification . Acta Hortic , 636 : 167 – 172 .
- Sas Paszt , L and Żurawicz , E . 2005 . Studies of the Rhizosphere of Strawberry Plants at the Research Institute of Pomology and Floriculture in Skierniewice, Poland . Int J Fruit Sci , 5 : 115 – 126 .
- Sasaki , M , Yamamoto , Y and Matsumoto , H . 1996 . Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots . Physiol Plant , 96 : 193 – 198 .
- Schaedle , M , Thornton , F C , Raynal , D J and Tepper , H B . 1989 . Response of tree seedlings to aluminum . Tree Physiol , 5 : 337 – 356 .
- Silva , I R , Novais , R F , Jham , G N , Barros , N F , Gebrim , F O , Nunes , F N , JCL and Leite , F P . 2004 . Responses of eucalypt species to aluminium: The possible involvement of low molecular weight organic acids in the Al tolerance mechanism . Tree Physiol , 24 : 1267 – 1277 .
- Sivaguru , M , Yamamoto , Y , Rengel , Z , Ahn , S J and Matsumoto , H . 2005 . Early events responsible for aluminium toxicity symptoms in suspension-cultured tobacco cells . New Phytol , 165 : 99 – 109 .
- Tahara , K , Norisada , M , Tange , T , Yagi , H and Kojima , K . 2005 . Ectomycorrhizal association enhances Al tolerance by inducing citrate secretion in Pinus densiflora . Soil Sci Plant Nutr , 51 : 397 – 403 .
- Taiz , L and Zeiger , E . 1998 . Plant physiology , Sunderland, MA : Sinauer .
- Taylor , G . 1987 . Exclusion of metals from the symplasm: A possible mechanism of metal tolerance in higher plants . J Plant Nutr , 10 : 1213 – 1222 .
- Taylor , G . 1991 . Current views of the aluminium stress response: The physiology basis of tolerance . Curr Top Plant Biochem , 10 : 57 – 93 .
- Thornton , F C , Schaedle , M and Raynal , D L . 1987 . Effects of aluminium on red spruce seedlings in solution culture . Environ Exp Bot , 27 : 489 – 498 .
- Tomlinson , G H . 2003 . Acidic deposition, nutrient leaching and forest growth . Biogeochem , 65 : 51 – 81 .
- Ulrich , B , Mayer , R and Khanna , P . 1978 . Deposition von Luftverunreinigungen und ihre Auswirkungen in Waldökosystemen in Solling . Schr Forstl Fak Univ Gött Vers Anst , 58 : 1 – 29 .
- Vanguelova , E I . 2002 . Soil acidification and fine root response of Scots pine [thesis] , Reading, , UK : Reading University .
- Vanguelova , E I , Nortcliff , S , Moffat , A J and Kennedy , F . 2005 . Morphology, biomass and nutrient status of fine roots of Scots pine (Pinus sylvestris) as influenced by seasonal fluctuations in soil moisture and soil solution chemistry . Plant Soil , 270 : 233 – 247 .
- Vanguelova , E I , Nortcliff , S , Moffat , A J and Kennedy , F . 2007 . Short-term effects of manipulated increase in acid deposition on soil, soil solution chemistry and fine roots of Scots pine (Pinus sylvestris) stand on a podzol . Plant Soil , 294 : 41 – 54 .
- van Schöll , L , Keltjens , W G , Hoffland , E and van Breemen , N . 2004 . Aluminium concentration versus base cation to aluminium ratio as predictors for aluminium toxicity in Pinus sylvestris and Picea abies seedlings . For Ecol Manag , 195 : 301 – 309 .
- van Schöll , L , Keltjens , W G , Hoffland , E and van Breemen , N . 2005 . Effects of ectomycorrhizal colonization on the uptake of Ca and Mg and Al by Pinus sylvestris under aluminum toxicity . For Ecol Manag , 215 : 352 – 360 .
- Vogt , A , Publicover , D A , Bloomfield , J , Perez , J M , Vogt , D J and Silver , W L . 1993 . Belowground responses as indicators of environmental changes . Environ Exp Bot , 133 : 189 – 205 .
- Wargo , P M , Vogt , K , Vogt , D , Holifield , Q , Tilley , J , Lawrence , G and David , M . 2003 . Vitality and chemistry of roots of red spruce in forest floors of stands with a gradient of soil Al/Ca ratios in the northeastern United States . Can J Forest Res , 33 : 635 – 652 .
- Watanabe , T , Jansen , S and Osaki , M . 2005 . The beneficial effect of aluminum and the role of citrate in aluminum accumulation in Melastoma malabathricum . New Phytol , 165 : 773 – 780 .
- Wissemeier , A H , Hahn , G and Marschner , H . 1998 . Callose in roots of Norway spruce (Picea abies (L.) Karst.) is a sensitive parameter for aluminum supply at a forest site (Höglwald) . Plant Soil , 199 : 53 – 57 .
- Zysset , M , Brunner , I , Frey , B and Blaser , P . 1996 . Response of European chestnut to varying calcium/aluminum ratios . J Environ Qual , 25 : 702 – 708 .