Abstract
The history of Actinodontum lomaense has been marked by taxonomic revisions since its initial discovery in samples from Sierra Leone during 1984. The reassignment from the genus Docidium to the genus Actinodontum primarily resulted from pronounced morphological differences, including variations in cell size and shape, ornamentations on the cell wall, and the presence of conical teeth-like structures at the isthmus. This article reports on the discovery of A. lomaense in two locations in southern Africa, marking a significant expansion of the geographical distribution range of the genus, from the tropical regions of Sierra Leone to sub-tropical environments in South Africa and the Kingdom of Lesotho. Additionally, the preference of Actinodontum species for relatively high altitudes, observed in this study, confirms a biogeographical pattern characterising this genus. Cell dimensions recorded in the southern African samples slightly exceeded dimensions previously reported in the literature. As a result, an extention of the dimension ranges for this species is proposed. Findings presented in this article, along with physico-chemical variables measured in their habitat, contribute to our understanding of the ecological preferences and distribution patterns of A. lomaense. It also provides valuable insights into the biogeography of desmid species on the African continent.
Introduction
In 1984, a novel desmid species was discovered on the north-eastern slopes of Peak Bintimani, located in the Loma Mountains of Sierra Leone. Two years later, this species was formally described as Docidium lomaensis by Alfinito and Mazzoni (Citation1986). However, in their initial description, Alfinito and Mazzoni (Citation1986) noted the species’ distinctiveness from all other known species within the genus Docidium Brébisson ex Ralfs. This distinction primarily centred on two key features, namely the presence of a granulated cell wall and the significantly shorter cell length compared to other species within the genus.
Subsequently, Alfinito and Coesel (Citation2013) conducted a re-examination of the type material of the Sierra Leone specimens and described it as an Actinotaenium-like desmid. However, cells in the genus Actinotaenium (Nägeli) Teiling are characterised by elongated, omniradiate (circular in cross-section) forms, with a shallow sinus, and smooth, unornamented, but porous cell walls (Teiling Citation1954). In contrast, the cells discovered in Sierra Leone exhibited distinctive characteristics, including an ornamented cell wall and the presence of conical teeth-like structures at the base of the semicells. These differentiating characteristics distinguish them from Actinotaenium. Furthermore, the cells also diverged markedly from the genus Docidium, particularly in terms of isthmial cell wall ornamentation and general semicell outline. In Docidium, the semicells exhibit an elongate-cylindric form (Ralfs Citation1848), whereas the cells from Sierra Leone displayed a pyramidal-elliptical shape (Alfinito and Coesel Citation2013). In the light of these distinguishing features, Alfinito and Coesel (Citation2013) concluded that these characteristics justified the establishment of a new genus, Actinodontum Alfinito & Coesel, within the Family Desmidiaceae. Consequently, the cells previously identified as Docidium lomaensis by Alfinito and Mazzoni (Citation1986) were reclassified in the genus Actinodontum and were henceforth referred to as Actinodontum lomaense (Alfinito & Mazzoni) Alfinito & Coesel. The latter was also assigned as the type species of the new genus. Two additional species were similarly re-assigned to the new genus Actinodontum, namely Cosmarium basituberculoides Bourelly & Couté (now Actinodontum basituberculoides (Bourelly & Couté) Alfinito & Coesel) and Cosmarium elgonense Kusel-Fetzmann (now Actinodontum elgonense (Kusel-Fetzmann) Alfinito & Coesel).
Since its initial discovery in Sierra Leone in 1984, A. lomaense has remained undetected with no subsequent encounters or reports anywhere else in the world. In this article, we present an update on the species’ distribution by documenting its rediscovery in two locations in southern Africa. Both light (LM) and scanning electron microscope (SEM) images of A. lomaense are presented, and the species distribution is discussed with reference to its ecology. The finding of such rare species emphasises the importance of continuous monitoring and research efforts to enhance our understanding of unique algal species.
Study area, material and methods
During the study Actinodontum lomaense cells were found in high altitude samples from the Drakensberg area, spanning both the South African and Lesotho sides.
South African side of Drakensberg escarpment
In June 2008 samples were collected from the eastern slopes of the Drakensberg escarpment, situated in the KwaZulu-Natal Province of South Africa. Three samples were obtained from a waterfall (coordinates 29°41′04.2″S, 29°21′10.5″E; elevation: 1 149 m a.s.l.) located within the Cobham Nature Reserve, part of the uKhahlamba-Drakensberg Park (designated as a UNESCO World Heritage site in 2000). This park, covering 240,000 hectares, features a diverse landscape comprising rivers, wetlands, indigenous forests, grasslands, valleys, and cliffs. The first sample was taken by squeezing the roots of plants hanging in the waterfall (), the second from the splash zone of the waterfall, and the third from dripping water from the roof of the waterfall. These samples were deposited in the South African National Diatom Collection (SANDC), North-West University, Potchefstroom, South Africa (accession numbers 08-511, 08-512, and 08-513, respectively).
Figure 1. Field photos of the sampling sites. A: Sampling site on the South African (eastern) side of the Drakensberg escarpment, showing the cascading roots of the plants from which the sample was taken; B: Sampling site on the Lesotho (western) side of the Drakensberg escarpment, indicating algal growth in the stream from which scrapes were made.

Lesotho side of Drakensberg escarpment
During June 2008, a single sample was collected from the western slopes of the Drakensberg escarpment in the Leribe Administrative District, Kingdom of Lesotho. The sample was collected from the shallower, slower flowing section of a fast-flowing stream by scraping material from the rock face (). The stream was situated alongside the A25 road to Katse Dam, between Pitseng and Lejone (coordinates: 29°03′36.6″S, 28°21′21.9″E; elevation: 2 250 m a.s.l.). The sample was also deposited in the same collection (accession number 08-524). Water quality was measured with a YSI 556 Multi-Probe System (MPS) multimeter.
All collected samples were preserved in 10% ethanol and transported to the phycology laboratory at the North-West University, Potchefstroom, South Africa. Here, the samples were screened for algae using both a Carl Zeiss light microscope, equipped with DIC optics, and a Leica DM 2500 LED light microscope with phase contrast. During the light microscopic examinations, cells of A. lomaense were identified and photographed using digital cameras mounted on the microscopes (a JVC TK-1270 camera on the Zeiss microscope, and a Leica Flexacam C3 camera on the Leica microscope). Cell dimensions were ascertained using standard linear stage micrometers.
The sample was also prepared for scanning electron microscope (SEM) investigation. A drop of sample was pipetted on two circular glass cover slips with diameters of 18 mm and left to air dry. After the sample dried out, the cover slips were transferred to a desiccator and left for 24 h. The cover slips were mounted on aluminium SEM stubs with carbon tape, and sputter-coated with gold palladium for 90s using a SPI Module Sputter Coater (SPI Supplies: Westchester, PA, USA). The samples were investigated using a Phenom Pro-Desktop SEM (Phenom-World BV, Eindhoven, Netherlands). A total of 20 cells were measured during the SEM investigation.
Additionally, an exhaustive review of scientific literature, phycological inventories, technical reports, and internet databases was undertaken to compile existing geographical records of the species.
Results
Several cells (moderate density) of A. lomaense were detected in the samples from South Africa and Lesotho ( and ). The cells were elliptical, up to three times longer than wide () and characterised by very shallow U-shaped to widely opened V-shaped median constrictions, when present. Median constrictions were observed in all cells viewed with light microscopy (), and some with scanning electron microscopy (). However, most cells observed with scanning electron microscopy () had no visible median constrictions, probably due to cell deformation during sample preparation. The semicells had a pyramidal to cylindrical shape, attenuating towards truncately rounded apices. At the base of each semicell 15 conical teeth (Figure 21–L) encircled the isthmus and the teeth of one semicell alternated and interlocked with those of the other semicell ( and –E). No cells were observed in apical views. The cell walls were decorated with pits (scrobiculi), each having a small pore in the centre. Light microscopy showed a central, axile chloroplast in each semicell, containing a single pyrenoid ().
Figure 2. Light microscope images of Actinodontum lomaense cells from the Drakensberg escarpment. Scale bars = 20 µm.
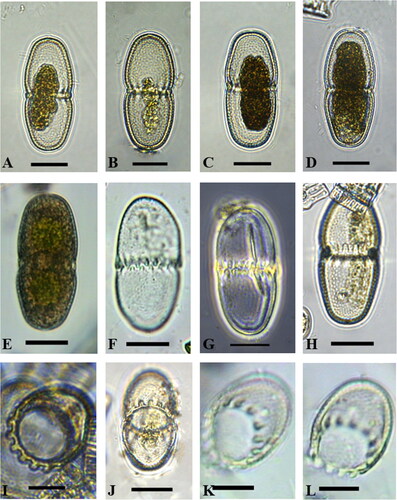
Figure 3. Scanning electron microscope images of Actinodontum lomaense cells from the Drakensberg escarpment. Scale bars: 20 µm.
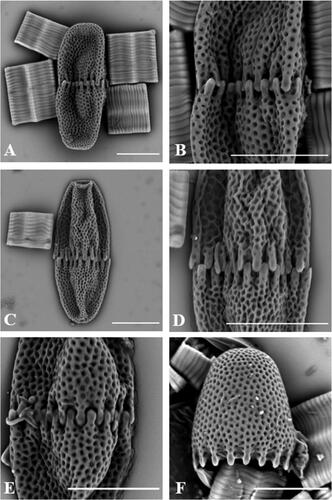
Table 1. Cell dimensions (in µm) found in the samples from the Drakensberg.
The dimensions of cells are illustrated in and can be summarised as follows: Cell length ranged between 47.7 and 64.7 µm (average 58.2 µm) and cell width between 21.2 and 31.0 µm (average 24.9 µm). The average diameter of the isthmus measured with the LM was 20.1 µm.
Physico-chemical environmental variables were measured on the Lesotho side of the Drakensberg escarpment at the sampling site. The stream had a water temperature of 10.7 °C, conductivity of 0.054 mS/cm, percentage dissolved oxygen of 87.8%, and pH of 7.71. Co-occurring species included long zigzag chains of Tabellaria flocculosa (Roth) Kützing (dominant species in the sample). Representatives of other less abundant species found in the samples included: Actinotaenium globosum (Bulnheim) Kurt Förster ex Compère, Cosmarium botrytis Meneghini ex Ralfs and Staurastrum punctulatum Brébisson (desmids), Brachysira sp., Navicula sp., Nitzschia sp. and Pinnularia sp. (diatoms), Mougeotia st. sterile (filamentous green alga) and Trachelomonas volvocina (Ehrenberg) Ehrenberg (euglenoid).
Discussion
Currently there are three taxonomically accepted species in the genus Actinodontum (Guiry and Guiry Citation2023), namely A. basituberculoides (Bourrelly & Couté) Alfinito & Coesel, A. elgonense (Kusel-Fetzmann) Alfinito & Coesel and A. lomaense (Alfinito & Mazzoni) Alfinito & Coesel. The semicells of A. elgonense are characterised by an elliptical shape, distinguishing them from the pyramidal-shaped semicells found in A. basituberculoides and A. lomaense. A. basituberculoides consists of larger cells with more pyramidal semicells containing stelloid chloroplasts, whereas A. lomaense features smaller cells with axial chloroplasts, as described by Alfinito and Coesel (Citation2013). The morphology of cells in our samples corresponded to the morphology of A. lomaense cells from Sierra Leone described by Alfinito and Mazzoni (Citation1986) and Alfinito and Coesel (Citation2013). During their initial LM observations Alfinito and Mazzoni (Citation1986) described the cell wall as being granulated. However, in the later publication by Alfinito and Coesel (Citation2013) the cell wall morphology of the newly established genus was described to be ‘scrobiculate’. SEM investigations of cells () showed that the ornamentations on the surface are in fact pits or scrobiculi (as described by Alfinito and Coesel Citation2013), rather than granules. Each of the pits has a small pore in the centre. The teeth structures observed in specimens collected from the Drakensberg region exhibited different characteristics when compared to those found in Sierra Leone. In the Drakensberg specimens, the teeth displayed a smooth surface (–F). In contrast, the teeth in the Sierra Leone samples were adorned with scrobiculi and pores, mirroring the features present on the surface of the cell wall (Alfinito and Coesel Citation2013). Shallow U-shaped to widely opened V-shaped median constrictions were observed in cells viewed with light microscopy (), but cells observed with scanning electron microscopy () had no visible median constrictions, probably as a result of deformation during sample preparation.
Dimensions of cells in Sierra Leone were reported as (44)–50 µm long and 18–20(23) µm wide by Alfinito and Mazzoni (Citation1986), and 48–59 µm long and 19–23 µm wide by Alfinito and Coesel (Citation2013). In contrast, cells from the Drakensberg samples were generally larger, with an average length of 58 µm (ranging between 47.7 and 64.7 µm) and an average width of 25 µm (ranging between 21.2 and 31 µm), as shown in . Alfinito and Mazzoni (Citation1986) characterised the cells as small, 2.2–2.6 (3) times longer than wide, while Alfinito and Coesel (Citation2013) noted a length-to-width ratio of 1.9–3.4. In the present study, the observed length-to-width ratio ranged between 1.8 and 2.8. As a result, the known dimension ranges for this species have been expanded by the current study to 44.0–64.7 × 18.0–31.0 µm. It’s worth noting that these dimensions are still significantly smaller than those of the genus Docidium (130–430 µm in length), to which the species was previously classified (Alfinito and Mazzoni Citation1986). In the original description of the genus Docidium, Ralfs (Citation1848) noted that cells can occasionally be as much as twenty times longer than wide, further highlighting the substantial difference in size between Actinodontum and Docidium.
All previous records of the genus Actinodontum were from tropical regions in Africa; A. basituberculoides is known from Madagascar (Bourrelly and Couté Citation1991), A. elgonense from Kenya (Kusel-Fetzmann Citation1968), and A. lomaense from Sierra Leone (Alfinito and Mazzoni Citation1986; Alfinito and Coesel Citation2013). The rediscovery of A. lomaense in the Drakensberg region significantly contributes to the previously known geographical distribution of the species by expanding its geographical ranges to also include the sub-tropical regions of the world. It indicates the adaptability of this desmid species and the fact that it can thrive in a wider range of environmental conditions than previously known. Given the significant passage of time since the species was initially found in Sierra Leone and then rediscovered in the Drakensberg region 40 years later, it can be considered endemic to the tropical and sub-tropical regions of the African continent until further research yields additional insights.
Alfinito and Coesel (Citation2013) made an interesting observation that the genus Actinodontum seems to prefer relatively high altitudes. The Sierra Leone samples were collected from the highest peak in the country, namely Peak Bintimani located in the Loma Mountains at 1 650 m a.s.l. (Alfinito and Mazzoni Citation1986; Alfinito and Coesel Citation2013), A. basituberculoides was reported from Madagascar (Ankazobé, north of Tananarive) at an altitude of 1 500 m a.s.l. (Bourrelly and Couté Citation1991) and A. elgonense was collected from a high-altitude pool on Mount Elgon, Kenya (Kusel-Fetzmann Citation1968). Further support for this observation can be found in the current study where A. lomaense cells were also found at high altitudes water bodies, located at 1 149 and 2 250 m a.s.l in South Africa and Lesotho, respectively. The genus Actinodontum is therefore possibly confined to sub-tropical and tropical regions, representing a separate biogeographical unit within the African continent as stated by Coesel (Citation1996).
The absence of prior information regarding the ecological preferences and water quality variables associated with A. lomaense makes this rediscovery particularly interesting. The lack of data regarding the species’ habitat and environmental requirements highlights the need for further research to better understand the ecological niche it occupies. In this study, water quality assessments were conducted at the sampling site in Lesotho. The measured physico-chemical variables, including water temperature, conductivity, dissolved oxygen, and pH, can provide valuable insights into the environmental conditions of the habitats where A. lomaense was found. The pennate diatom Tabellaria flocculosa was the most abundant species in the Drakensberg samples, where it occurred in the form of long zigzag chains. A review of Tabellaria species from freshwater environments in Europe revealed that the genus generally prefers unpolluted, circumneutral to slightly acidic and poorly mineralised waters (Heudre et al. Citation2021). It is, however, important to note that these measurements alone do not fully elucidate the species’ ecological preferences. To comprehensively determine the ecological requirements of A. lomaense future research should consider a broader set of water quality parameters such as nutrient levels (e.g. nitrogen and phosphorus concentrations), light availability, and specific chemical properties.
Acknowledgements
Thank you to Prof. Jonathan Taylor (North-West University, South Africa) who assisted with sampling and diatom identification. We also acknowledge Willie Landman for his help with SEM preparation and investigations.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alfinito S, Coesel PFM. 2013. Actinodontum, a new genus of Desmidiaceae (Streptophyta, Viridiplantae) from tropical Africa. Plant Biosyst. 147(3):672–677. doi: 10.1080/11263504.2012.727488.
- Alfinito S, Mazzoni A. 1986. Some desmids (Chlorophyta) from Sierra Leone. Quaderno Accad Nazion Lincei. 260:97–104.
- Bourrelly P, Couté A. 1991. Desmidiées de Madagascar (Chlorophyta, Zygophyceae). Biblioth Phycol 86. J. Cramer, Berlin and Stuttgart. p. 347.
- Coesel PFM. 1996. Biogeography of desmids. Hydrobiologia. 336(1–3):41–53. doi: 10.1007/BF00010818.
- Guiry MD, Guiry GM. 2023. AlgaeBase. World-Wide Electronic Publication. National University of Ireland, Galway. https://www.algaebase.org. [Accessed 14 Oct 2023].
- Heudre D, Wetzel CE, Lange-Bertalot H, Van de Vijver B, Moreau L, Ector L. 2021. A review of Tabellaria species from freshwater environments in Europe. Fottea. 21(2):180–205. doi: 10.5507/fot.2021.005.
- Kusel-Fetzmann E. 1968. Beiträge zur Kenntnis der Algenflora Ostafrikanischer Hochgebirgsseen. Biologische Beiträge zur Kenntnis des Mount Kenya. Hochgebirgsforschung. 1:69–100.
- Ralfs J. 1848. The British Desmidieae The drawings by Edward Jenner, A.L.S. pp. [i]-xxii, [i], [1]-226, 35 pls. London: Reeve, Benham & Reeve, King William Street, Strand.
- Teiling E. 1954. Actinotaenium, genus Desmidiacearum resuscitatum. Botaniska Notiser. 4:376–426.
