Abstract
Class Mulgedio-Aconitetea comprises plant communities that develop primarily in the upper montane to subalpine vegetation belts. It is vegetation where tall herb species (forbs, ferns, grasses) occur dominantly; in the Balkans, it was studied only marginally. In this article, based on floristic composition we assess two questions: (1) what is the diversity of the class Mulgedio-Aconitetea in Bulgaria and (2) what environmental factors are responsible for the vegetation assemblages in the area? We analyzed 387 plots. We created an expert system that classifies this vegetation and tested environmental variables for their influence on these vegetation types. In total, 15 associations and one uncategorized community were classified using the Braun-Blanquet approach. The length of the growing season (number of growing degree days > 0 °C) and climate moisture have a significant effect on the vegetation. Disturbance severity, disturbance frequency and reaction have influence over the species scores. Moreover, all variables significantly differed between the vegetation types.
1. Introduction
The tall-herb vegetation of the class Mulgedio-Aconitetea is distributed in the mountains of Europe (Michl et al. Citation2010; Mucina et al. Citation2016; Preislerová et al. Citation2022) and Asia (Ermakov et al. Citation2000; Heim and Chepinoga Citation2019; Nowak et al. Citation2020). The vegetation usually contains nutrient- and moisture-demanding hemicryptophytes (Illa et al. Citation2006). They disperse seeds mainly by wind, which is crucial in combination with plant height (Thomson et al. Citation2011; Morgan and Venn Citation2017), as their habitats are unevenly distributed throughout the mountains. The tall-herb vegetation usually has distinct dominants accompanied by a low number of ecologically indifferent species. The centre of occurrence of Mulgedio-Aconitetea is in the upper montane to subalpine belts; however, when the conditions are favourable, this vegetation can develop in alpine or lower montane vegetation belts (Karner and Mucina Citation1993; Kočí Citation2007; Michl et al. Citation2010; Świerkosz and Reczyńska Citation2022).
Bulgaria lies on the border of the forest-steppe, temperate deciduous forest and Mediterranean biomes (Loidi et al. Citation2022). Even though tall-herb vegetation is diverse in forest-steppe and deciduous forest biomes, in the Mediterranean biome, it is rather rare (Mucina et al. Citation2016). Bulgaria has several vertically diverse mountain ranges with well-developed alpine belts (Strid et al. Citation2003). During the last glacial maximum, the area of the Balkans was sparsely forested (Velasquez et al. Citation2021) and functioned as a refugium for temperate flora (Stewart et al. Citation2009); however, the alpine floras are not phylogenetically very diverse there (Padullés Cubino et al. Citation2022), which can be caused by long-term processes in relatively low mountain ranges of southeastern Europe and indicates only limited saturation by taxa distributed in other biomes. Nonetheless, local mountains host several endemic species (Petrova and Vladimirov Citation2010; Apostolova et al. Citation2013), some of them also occur in the tall-herb vegetation and can form dominant stands (e.g. Cirsium appendiculatum Griseb.).
In the Balkans, the vegetation of Mulgedio-Aconitetea was studied by several authors using the Braun-Blanquet approach (e.g. Horvat Citation1949; Horvat et al. Citation1974; Čarni and Matevski Citation2010; Ranđelović and Zlatković Citation2010); however, the information provided by them is, in most cases, fragmentary. Four orders of the tall-herb vegetation are distinguished in the Balkans: Adenostyletalia alliariae, Calamagrostietalia villosae, Petasito-Chaerophylletalia and Senecioni rupestris-Rumicetalia alpini (Horvat et al. Citation1974; Mucina et al. Citation2016; Preislerová et al. Citation2022). They differ mainly in dominant species, which can be either tall grasses (e.g. Calamagrostis sp. pl.), tall forbs (e.g. Adenostyles sp. pl., Rumex alpinus L.) or ferns (e.g. Athyrium sp. pl.). In Bulgaria, this vegetation was studied mainly in the Rila Mts. (Horvat et al. Citation1937; Roussakova Citation2000) and Stara Planina Mts. (Michalik Citation1990; Szokala Citation2023). Recently, only vegetation dominated by Petasites sp. pl. was investigated (Nazarov et al. Citation2022). However, there are also studies following the Russian dominance-based approach dealing with this vegetation, as this approach was historically widely used in Bulgaria (Apostolova and Slavova Citation1997).
In this article, we ask: (1) how diverse are the subalpine tall-herb communities in Bulgaria and (2) which environmental factors are responsible for the differentiation of these plant communities in the area?
2. Material and methods
The data were collected during our field sampling (2002–2021) (Westhoff and Van Der Maarel Citation1978), which was done preferentially with the aim to document fully-developed vegetation or obtained from the Balkan Vegetation Database (EU-00-019; Vassilev et al. Citation2020). Our syntaxonomical concept of the class Mulgedio-Aconitetea follows the work of Mucina et al. (Citation2016) and excludes subalpine scrub vegetation. For the list of aggregated species, see Appendix B. The species were aggregated when uncertain determinations could occur or when subspecies were stated only partially (e.g. Juniperus communis L., Sesleria comosa Velen.).
In total, 544 vegetation plots were compiled; selected for further analysis were those that were recorded in 5–100 m2. Before the analyses, vegetation plots with shrubs (cover > 8%) or tree species (cover > 1%) were removed, as well as vegetation plots with dominant Pteridium aquilinum (L.) Kuhn. Vegetation plots occurring below 1000 m a. s. l. were removed as well; left were 387 plots, of which 307 (79%) were our own and 80 (21%) originated from the Balkan Vegetation Database. The data were processed in R 4.3.1 (R Core Team Citation2021) in the RStudio environment (RStudio Team Citation2023). All non-vascular plants were excluded from the analysis.
To describe the studied vegetation types, an expert system (Tichý et al. Citation2019) was created for the classification of vegetation plots, which was developed to reflect an unsupervised classification with the modified Twinspan algorithm (Roleček et al. Citation2009). At first, the dataset was divided according to dominant species or by occurrence of diagnostic species described mainly by Roussakova (Citation2000). For most such divided dataset sub-groups, modified Twinspan algorithm was conducted. The exceptions were groups with a low number of vegetation plots. The clusters created by modified Twinspan were then compared with classification in other sources and either accepted or rejected.
Modified Twinspan analysis was created using nine pseudospecies cut levels of species covers (0, 5, 10, 25, 40, 50, 60, 70, 90). To compare different methods modified Twinspan was created with 23 clusters with the average of Simpson’s dissimilarity index and the numerical agglomerative classification was produced using Ward.D2 algorithm (R Core Team Citation2021) with euclidean distances for 17 clusters. An alluvial diagram was created to compare four different classification methods: developed expert system, modified Twinspan, Ward.D2 algorithm and EUNIS expert system (Chytrý et al. Citation2020). The analysis were done on vegetation plots that were classified by the created expert system (in total 387 vegetation plots); included were vegetation plots classified to multiple vegetation types as in nature vegetation types can be transitional (Krebs Citation2014). DCA (Detrended correspondence analysis) was created using the vegan package (Oksanen et al. Citation2022) and displayed using ggplot2 package (Wickham Citation2016); weighted species scores were calculated using command ‘weights’ (R Core Team Citation2021). Beforehand an 0.6 transformation of species cover (Tichý et al. Citation2019) was done. Climatic factors were acquired through the Chelsa model (Brun et al. Citation2022). Averages of Ellenberg indicator values (EIV) for Europe were obtained from the work of Tichý et al. (Citation2023); if not available, the data were complemented by EIV primarily from Italy (Guarino and La Rosa Citation2019; Tichý et al. Citation2023) and secondarily from Greece (Böhling et al. Citation2002). Disturbance indicator values were taken from Midolo et al. (Citation2023). Elevation of relevés was obtained from the Digital Elevation Model (Mapzen Citation2023) and data were filtered again with the removal of badly georeferenced vegetation plots occurring below 1000 m a. s. l. (). CCA (Canonical correspondence analysis) was done with scaled scores using the vegan package (Oksanen et al. Citation2022) to test the significance of environmental factors for 324 vegetation plots for each environmental variable separately. The significance of environmental factors over site scores was tested with anova.cca test (Oksanen et al. Citation2022) with 9999 permutations. EIV were tested separately for species scores (by anova.cca test) due to biased compositional similarity of mean EIV of sites (Zelený and Schaffers Citation2011). The differences of environmental factors between vegetation types were tested using the second type ANOVA (cmi_max, ngd0) and other factors derived from EIV were tested using type six fourth corner permutation test from ade4 package (Dray and Dufour Citation2007) with 9999 permutations. Maps were created using the QGis program (QGis.org Citation2023). The grid was created with 12 × 11.1 km cells.
The fidelity of species to defined groups was calculated in the Juice program (Tichý Citation2002) with the standardization of all groups to equal sizes and using Fisher’s exact test with p < .01 (Chytrý et al. Citation2002) for correction of randomly occurring species. The fidelity threshold was set up to phi > 0.3, the frequency threshold was set to f > 40% and the cover threshold to 50%. A synoptic table was created for the comparison of constancy of occurrences of species between vegetation types (see Appendix A). New syntaxa are produced according to the International Code of Phytosociological Nomenclature (Theurillat et al. Citation2021). In the Subsection 3.2 Vegetation classification, fidelity is multiplied by 100, highly diagnostic (phi > 0.5) and highly frequent (f > 50%) species are marked with bold font. If the dominant species occurred among diagnostic species (phi > 0.3) or frequent species (f > 40%), there is a note (dom.) after the values.
3. Results
3.1. Vegetation classification
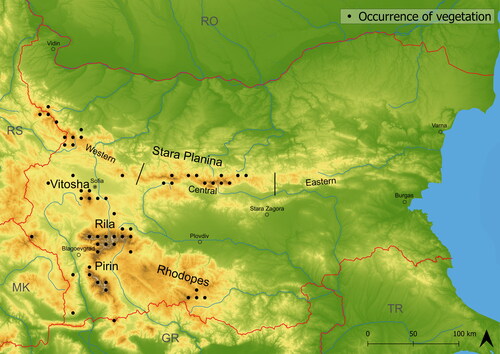
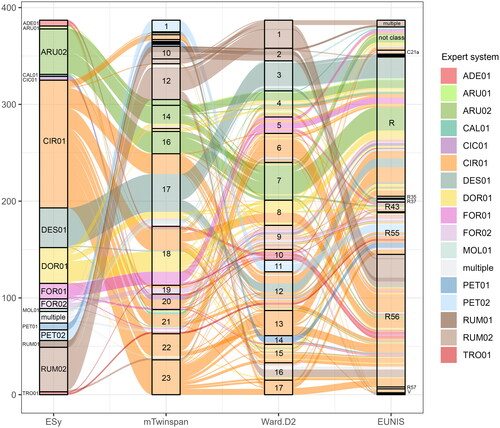
The dataset was classified by two numerical methods (modified Twinspan, Ward.D2), and an expert system was created for final classification (https://doi.org/10.5281/zenodo.8304799). The modified Twinspan classification was divided into 23 clusters due to many single plot clusters (groups 2–9, see ), in Ward’s methods, the vegetation was classified into 17 clusters with relatively even group sizes. The expert system distinguishes 16 vegetation types on the association level. Modified Twinspan method reflects more species composition; conversely, Ward’s algorithm with euclidean distances is more susceptible to dominant species. The modified Twinspan divided well vegetation dominated by Calamagrostis arundinacea (L.) Roth, Rumex alpinus and Petasites sp. pl.; however, other vegetation types were not clearly distinguishable. In comparison to the modified Twinspan, Ward.D2 algorithm well distinguished vegetation dominated by Deschampsia cespitosa (L.) P.Beauv. The vegetation type of Angelico sylvestris-Heracleetum verticillati is closely related to Rumicion alpini, as indicated by the shared species composition of nutrient-demanding plants (see ). The fern vegetation (all. Dryopterido filicis maris-Athyrion distentifolii) is closely related to Adenostylion alliariae. Both vegetation types are usually species-rich and nutrient-demanding; however, they are not disturbed as often as the vegetation of Rumicion alpini and Cirsion appendiculati (see ). In the EUNIS classification, most of the vegetation plots are classified as grasslands and lands dominated by forbs, mosses or lichens (R), montane to subalpine moist or wet tall-herb and fern fringes (R56) and lowland moist or wet tall-herb and fern fringes (R55). Seven vegetation plots were classified as springs and fens (C21a, Q24, Q41, Qb), sixteen as grasslands (R35, R37, R43), two as subalpine to subarctic deciduous scrub (S25) and six as vegetated man-made habitats (V, V15, V39, see ).
Figure 2. Alluvial diagram showing differences in classifications between created expert system (ESy) with 17 distinguished groups, modified twinspan (mTwinspan) for 23 clusters, Ward’s agglomerative algorithm (Ward.D2) for 17 clusters, and EUNIS expert system (EUNIS); ADE01 – Ranunculo platanifolii-Adenostyletum alliariae, ARU01 – Astrantio majoris-Calamagrostietum arundinaceae, ARU02 – Roso pendulinae-Calamagrostietum arundinaceae, CAL01 – Crepido conyzifoliae-Calamagrostietum villosae, CIC01 – Moehringio pendulae-Cicerbitetum alpinae, CIR01 – Angelico sylvestris-Heracleetum verticillati, DES01 – Carici lazarei-Deschampsietum cespitosae, DOR01 – Aquilegio aureae-Doronicetum columnae, FOR01 – Saxifrago rotundifoliae-Athyrietum distentifolii, FOR02 – Cardamino acri-Athyrietum filicis-feminae, MOL01 – Sphagno compacti-Molinietum cearuleae, PET01 – Petasitetum albi, PET02 – Petasitetum hybridi, RUM01 – Senecioni rupestris-Rumicetum alpini, RUM02 – Rumicetum alpini, TRO01 – Trollius europaeus communities; C21a – base-poor spring and spring brook, R – grasslands, R35 – moist to wet mesotrophic to eutrophic hay meadows, R37 – temperate and boreal moist or wet oligotrophic grassland, R43 – temperate acidophilous alpine grassland, R55 – lowland moist or wet tall-herb and fern fringe, R56 – montane to subalpine moist or wet tall-herb and fern fringe, R57 – herbaceous Forest clearing vegetation, V – man-made habitats.
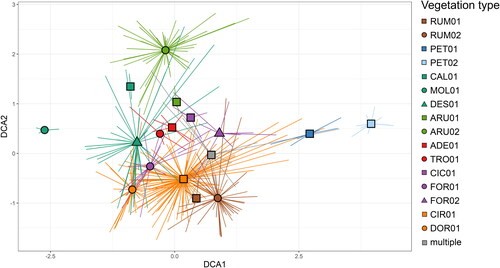
In the ordination diagram () there is a clear gradient of disturbances and nutrient demandingness of vegetation types (along DCA1). Moreover, it also shows a gradient of reaction as Sphagno compacti-Molinietum cearuleae is acidophilous vegetation, whereas Petasitetum albi is neutrophilous. Second ordination axes (DCA2) does not show a clear environmental gradient; it, however, shows a division between grass-dominated vegetation types (Calamagrostietalia villosae) and more ruderal vegetation with species that are comparatively larger (Senecioni rupestris-Rumicetalia alpini); this division can be caused by nutrient or water demandingness, but also intensity and frequency of disturbances.
Figure 3. DCA ordination diagram with ESy groups. Projected are sample scores. RUM01 – Senecioni rupestris-Rumicetum alpini, RUM02 – Rumicetum alpini, PET01 – Petasitetum albi, PET02 – Petasitetum hybridi, CAL01 – Crepido conyzifoliae-Calamagrostietum villosae, MOL01 – Sphagno compacti-Molinietum cearuleae, DES01 – Carici lazarei-Deschampsietum cespitosae, ARU01 – Astrantio majoris-Calamagrostietum arundinaceae, ARU02 – Roso pendulinae-Calamagrostietum arundinaceae, ADE01 – Ranunculo platanifolii-Adenostyletum alliariae, TRO01 – Trollius europaeus communities, CIC01 – Moehringio pendulae-Cicerbitetum alpinae, FOR01 – Saxifrago rotundifoliae-Athyrietum distentifolii, FOR02 – Cardamino acri-Athyrietum filicis-feminae, CIR01 – Angelico sylvestris-Heracleetum verticillati, DOR01 – Aquilegio aureae-Doronicetum columnae.
3.2. Vegetation description
3.2.1. Senecioni rupestris-Rumicetalia alpini
3.2.1.1. Rumicion alpini
RUM01 Senecioni rupestris-Rumicetum alpini ass. nov.Footnote1
Diagnostic species: Trifolium repens L. 74.5, Ochlopoa annua (L.) H.Scholz 72.3, Tripleurospermum caucasicum (Willd.) Hayek 70.7, Arenaria biflora L. 60.9, Viola dacica Borbás 51.4, Spergularia rubra (L.) J.Presl & C.Presl 50.1, Senecio rupestris Waldst. & Kit. 49.2, Scleranthus perennis L. 48.3, Rumex alpinus 48.0 (dom.), Campanula patula agg. 45.3, Veratrum album L. 44.7, Taraxacum sp. 44.2, Verbascum sp. 44.0, Blitum bonus-henricus (L.) Rchb. 43.5, Ranunculus montanus agg. 38.9, Chrysosplenium alternifolium L. 31.1
Constant species: Deschampsia cespitosa 71, Festuca rubra agg. 57, Veronica chamaedrys L. 43, Urtica dioica L. 43, Poa ursina Velen. 43, Poa alpina L. 43, Crocus sp. 43
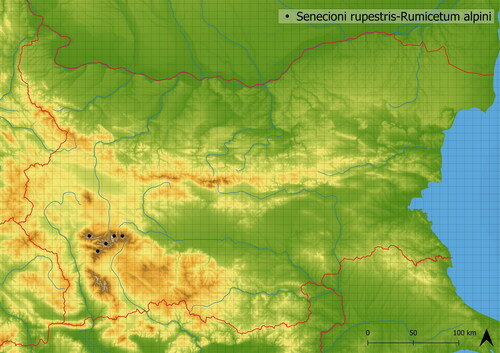
This mediately species-rich vegetation creates enclosed stands with dominant Rumex alpinus accompanied by other nitrophilous and ubiquitous species such as Ochlopoa annua and Trifolium repens. It contains species typical of alpine meadows, such as Campanula patula agg., Viola dacica and Ranunculus montanus agg. Occurring there through spatial mass effect. The vegetation is sparser in comparison to Rumicetum alpini, and as such, can host a greater number of species. It develops in a human-altered environment in disturbed places. It is less nutrient demanding than Rumicetum alpini and generally occurs in higher altitudes (mean altitude of occurrence is in 2104 m a. s. l.). This vegetation occurs very rarely only in the Rila Mts. (see ), where it was documented by Roussakova (Citation2000). In the Balkans it is also distributed in Macedonia (Horvat et al. Citation1974). It is relatively species-poor; in analyzed vegetation, there were approximately 15 species in plots; however, it is species richer than Rumicetum alpini.
RUM02 Rumicetum alpini Beger 1922
Diagnostic species: Rumex alpinus 48.0 (dom.), Urtica dioica 30.8
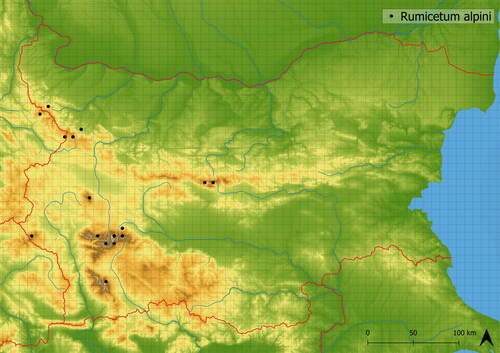
Rumicetum alpini is an anthropogenic vegetation occurring in nutrient-rich places, usually near mountain settlements and huts. It has a lower number of species (∼12 species in analyzed vegetation plots), and the sole dominant Rumex alpinus is the deterministic species of this vegetation. This vegetation is more frequently disturbed (see ), usually by animals, so fewer plants survive than in association Senecioni rupestris-Rumicetum alpini. The accompanying species have low cover values and are ruderal such as Urtica dioica. An average occurrence of this vegetation is in 1917 m a. s. l. It is distributed throughout Europe (Karner and Mucina Citation1993; Kočí Citation2007). In Bulgaria, this vegetation is found in all high-mountain ranges: Osogovska Planina, Pirin, Rila, Stara Planina and Vitosha Mts. (see ). There are two recognizable varieties of this vegetation: the first contains a few species that are typical of fens (e.g. Geum coccineum Sm.), and the other one is more typical, accompanied only by a few ruderal species (e.g. Aegopodium podagraria L., Lamium maculatum (L.) L., Urtica dioica).
3.2.2. Petasito-Chaerophylletalia
3.2.2.1. Arunco-Petasition albi
PET01 Petasitetum albi Dihoru ex Nazarov et al. Citation2022
Diagnostic species: Petasites albus (L.) Gaertn. 87.0 (dom.), Ranunculus repens L. 57.0, Telekia speciosa (Schreb.) Baumg. 52.8, Carex remota L. 50.7, Calystegia sepium (L.) R.Br. 50.2, Stachys sylvatica L. 47.8, Pteridium aquilinum 47.0, Geum urbanum L. 46.2, Silene baccifera (L.) Durande 44.7, Lapsana communis L. 44.0, Agrostis stolonifera L. 39.6, Ranunculus serbicus Vis. 35.0, Chrysosplenium alternifolium L. 31.1
Constant species: Urtica dioica 71, Stellaria nemorum L. 43, Angelica sylvestris L. 43
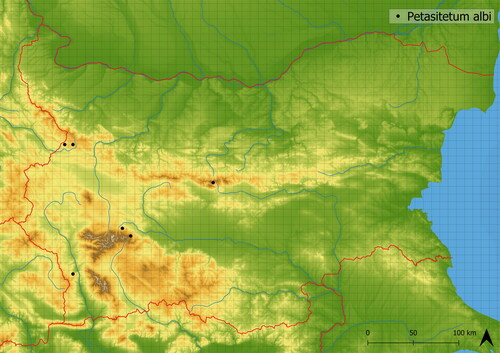
The vegetation types in which Petasites albus dominates have only a small number of accompanying species. In the Petasitetum albi, species typical of southeastern Europe (e.g. Telekia speciosa, Ranunculus serbicus) occur (Nazarov et al. Citation2022). These species are mainly shade- and nutrient-demanding. The vegetation can also host ruderal species such as Urtica dioica and Geum urbanum. It contains 18 species on average in the analyzed plots, and the average altitude of occurrence is in 1456 m a. s. l. It is not frequently disturbed; however, severity of the dsturbances is high (see ). It is documented from Malashevska Planina, Rila and Stara Planina Mts. (see ). In the Balkans, vegetation of Arunco-Petasition albi is reported also from Croatia (Škvorc et al. Citation2017).
3.2.3. Petasition officinalis
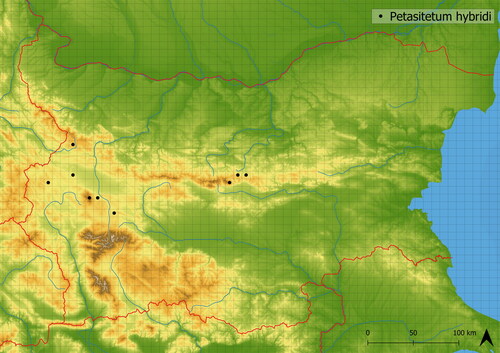
PET02 Petasitetum hybridi Imchenetzky 1926
Diagnostic species: Petasites hybridus (L.) G.Gaertn., B.Mey. & Scherb 100.0 (dom.), Elytrigia repens (L.) Gould 59.1, Rumex conglomeratus Murray 50.2, Galium aparine L. 49.0, Equisetum sylvaticum L. 48.9, Artemisia vulgaris L. 47.0, Conium maculatum L. 41.5, Circaea lutetiana L. 41.5, Lythrum salicaria L. 39.6, Carex hirta L. 37.9, Equisetum arvense L. 37.1, Ranunculus serbicus 33.2
Constant species: Urtica dioica 64
This vegetation develops in wet places within the forest belt. It is species-poor (there were 12 species in analysed vegetation plots on average), created by dominant Petasites hybridus and accompanied by a few other nutrient-demanding species such as Elytrigia repens, Galium aparine and Rumex conglomeratus. It contains species typical of forest-zone such as Circaea lutetiana, and ubiquitous species typical of lower altitudes, such as Carex hirta and Lythrum salicaria. It develops near the lower parts of alpine rivers and springs in relatively low altitudes (mean altitude of occurrence is 1354 m a. s. l.). This vegetation is documented in Bulgaria in Cherni Rid, Stara Planina, Strazhata, Viskyiar and Vitosha Mts. (see ). In the work of Nazarov et al. (Citation2022) Petasites hybridus-dominated communities from Romania and Bulgaria are classified as Telekio-Petasitetum hybridi. However, in our dataset, the vegetation type dominated by Petasites hybridus does not contain any locally distributed species.
3.2.4. Calamagrostietalia villosae
3.2.4.1. Calamagrostion villosae
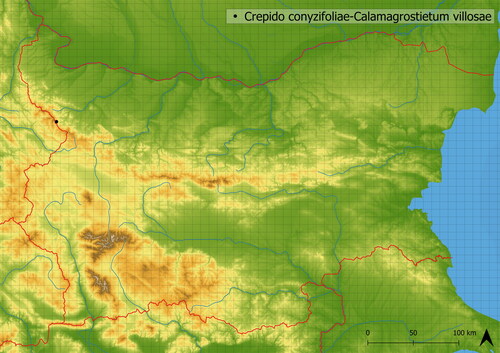
CAL01 Crepido conyzifoliae-Calamagrostietum villosae (Zlatník 1925) Jeník 1961
Diagnostic species: Calamagrostis villosa (Chaix) J.F.Gmel. 75.4 (dom.)
Constant species: Senecio nemorensis agg. 100, Hypericum maculatum Crantz 100, Veratrum lobelianum Bernh. 50, Rumex alpinus 50, Rubus idaeus L. 50, Rubus fruticosus agg. 50, Potentilla erecta (L.) Raeusch. 50, Luzula sylvatica (Huds.) Gaudin 50, Gentiana asclepiadea L. 50, Deschampsia cespitosa 50, Calamagrostis arundinacea 50, Bistorta officinalis Delarbre 50, Adenostyles alliariae (Gouan) Kern. 50
This species-poor vegetation (∼9 species on average in sampled vegetation) is created by dominant Calamagrostis villosa accompanied by ubiquitous species such as Senecio nemorensis agg., Hypericum maculatum and Rubus fruticosus agg. The moss layer is usually poorly developed and suppressed by dead biomass. It was documented in the Western Stara Planina Mts (see ) where it grows at the edge of the forest zone in ∼1725 m a. s. l. This association is known from Czech Republic (Kočí Citation2007), Poland (Świerkosz and Reczyńska Citation2022) and Bulgaria (Szokala Citation2023), however, vegetation dominated by Calamagrostis villosa is known also from other regions (Karner and Mucina Citation1993; Kliment et al. Citation2007).
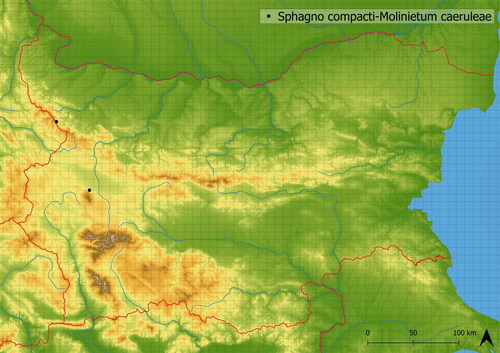
MOL01 Sphagno compacti-Molinietum caeruleae Wagnerová in Berciková 1976
Diagnostic species: Molinia caerulea (L.) Moench 83.9 (dom.), Pinguicula balcanica Casper 79.8, Eriophorum latifolium Hoppe 79.2, Carex pallescens L. 76.0, Carex nigra (L.) Reichard 64.5, Potentilla erecta 60.3, Carex echinata Murray 59.7, Juncus conglomeratus L. 56.5, Epilobium tetragonum L. 56.5
Constant species: Veratrum lobelianum 67, Nardus stricta L. 67, Deschampsia cespitosa 67
This vegetation develops in wet, undisturbed places in the subalpine belt. It is created by dominant Molinia caerulea. The vegetation is species-poor, with only 13 species occurring in the analyzed vegetation plots on average. The moss layer is well developed and contains Sphagnum sp. pl. (S. capillifolium (Ehrh.) Hedw., S. papillosum Lindb., S. russowii Warnst.; Szokala Citation2023). Sphagno compacti-Molinietum caeruleae most likely occurs as a late successional stage of Montio-Cardaminetea or Scheuchzerio palustris-Caricetea nigrae in climatically suitable places where disturbances occur rarely (see ) near the upper timberline (mean altitude of occurrence is in 1772 m a. s. l.). This vegetation contains, beside Molinia caerulea, species of fens and springs. The occurrence of Eriophorum latifolium indicates acidophilous soils (Hájková et al. Citation2008). It is distributed in central Europe (Kočí Citation2007) and Bulgaria (Szokala Citation2023), where it occurs only in Vitosha and Western Stara Planina Mts. (see ).
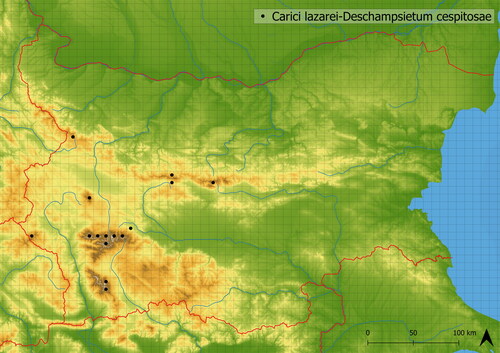
DES01 Carici lazarei-Deschampsietum cespitosae Roussakova Citation2000 nom. corr.Footnote2
Diagnostic species: Luzula campestris agg. 50.0, Geum coccineum 49.4 (dom.), Nardus stricta 39.1, Anthoxanthum odoratum agg. 35.0, Sanguisorba officinalis L. 33.9, Carex lazarei 33.9, Alchemilla sp. 32.3, Plantago gentianoides Sm. 31.0, Deschampsia cespitosa 30.3 (dom.)
Constant species: Festuca rubra agg. 73, Veratrum lobelianum Bernh. 54, Ligusticum mutellina (L.) Crantz 44, Hypericum maculatum Crantz 41
Carici lazarei-Deschampsietum cespitosae is vegetation distinguished by dominant Deschampsia cespitosa. It is mediately species-rich (17 species on average); the species occurring in this vegetation are either ubiquitous herbs (e.g. Anthoxanthum odoratum agg., Festuca rubra agg., Hypericum maculatum, Nardus stricta) or specialists of freshly wet places (e.g. Geum coccineum). The vegetation can have a chionophilous character, indicated by the occurrence of Plantago gentianoides, snow bed specialist (Roussakova Citation2000). It is typical of concave terrain of higher altitudes (average altitude of occurrence is in 2095 m a. s. l.). The vegetation can create relatively large stands and can be grazed. In Bulgaria, it occurs in all main mountain ranges: Osogovska, Pirin, Rila, Stara Planina and Vitosha Mts. (see ).
3.2.5. Calamagrostion arundinaceaeFootnote3
ARU01 Astrantio majoris-Calamagrostietum arundinaceae ass. nov. prov.
Diagnostic species: Crepis viscidula Froel. 65.0, Lilium jankae A.Kern. 64.5, Astrantia major L. 62.3, Athyrium filix-femina (L.) Roth 51.0, Luzula luzuloides (Lam.) Dandy & Wilmott 45.6, Ranunculus platanifolius L. 37.4
Constant species: Veratrum lobelianum 100, Silene vulgaris (Moench) Garcke 100, Senecio nemorensis agg. 100, Rumex arifolius All. 100, Calamagrostis arundinacea 100 (dom.), Rubus idaeus 67, Knautia arvensis agg. 67, Hypericum maculatum 67, Gentiana asclepiadea 67, Dryopteris filix-mas (L.) Schott 67, Dactylis glomerata L. 67, Angelica sylvestris L. 67, Alchemilla sp. 67
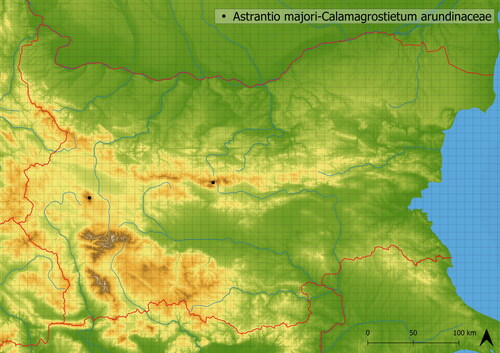
This vegetation contains mostly nutrient-demanding species. It develops in relatively high altitudes (mean altitude of occurrence is in 2029 m a. s. l.) and is mediately species-rich (in studied vegetation, there were 25 species). It is transitional to other vegetation types of the alliance Dryopterido filicis-maris-Athyrion distentifolii through the occurrence of Athyrium filix-femina (L.) Roth and Dryopteris filix-mas; however, it mainly contains ubiquitous species such as Veratrum lobelianum, Silene vulgaris and Knautia arvensis agg. This vegetation occurs only in Central Stara Planina and Vitosha Mts. (see ).
ARU02 Roso pendulinae-Calamagrostietum arundinaceae Szokala Citation2023
Diagnostic species: Genista depressa M.Bieb. 40.2, Avenella flexuosa (L.) Drejer 38.4, Calamagrostis arundinacea 38.0 (dom.), Luzula luzuloides 37.9, Juniperus communis agg. 36.6, Thymus praecox s. l. 34.7, Chamaecytisus austriacus (L.) Link 34.7, Verbascum longifolium Ten. 34.2, Genista sagittalis L. 33.3, Agrostis capillaris L. 32.0, Betonica officinalis L. 31.7, Carduus carduelis (L.) Gren. 31.4, Galium verum L. 30.5
Constant species: Vaccinium myrtillus L. 53, Rubus idaeus 45, Geranium sylvaticum L. 45
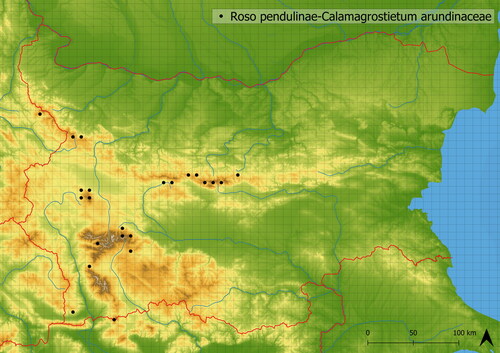
This vegetation is distinguishable by the dominance of Calamagrostis arundinacea. It is mediately species-rich and contains approximately 24 species on average in studied vegetation plots. The accompanying species are either small shrubs (e.g. Chamaecytisus austriacus, Genista depressa, G. sagittalis) or ubiquitous forbs (Avenella flexuosa, Agrostis capillaris, Luzula luzuloides). Roso pendulinae-Calamagrostietum arundinaceae can be slightly thermophilous, which is indicated by the occurrence of Verbascum longifolium and Galium verum. It typically develops in the lower subalpine belt (in 1742 m a. s. l. on average). Two variations of the association are recognizable within the dataset. In the first one, small shrubs (e.g. Juniperus communis agg., Genista depressa, G. sagittalis) are common, whereas the second one is species-poorer and does not contain shrub species. This vegetation develops in all main mountain ranges in Bulgaria: Ograzden, Pirin, Rila, Stara Planina, Slavyanka and Vitosha Mts. (see ).
3.2.6. Adenostyletalia alliariae
3.2.6.1. Adenostylion alliariae
ADE01 Ranunculo platanifolii-Adenostyletum alliariae (Krajina Citation1933) Dúbravcová et Hadač ex Kočí Citation2001
Diagnostic species: Adenostyles alliariae 70.0 (dom.), Primula elatior (L.) Hill 63.8, Soldanella sp. 40.7, Doronicum columnae Ten. 39.9, Saxifraga rotundifolia L. 39.6, Anemonastrum narcissiflorum (L.) Holub 36.4, Rumex arifolius 35.6, Heracleum sphondylium agg., 35.6, Trollius europaeus L. 35.5, Valeriana tripteris L. 34.6, Bistorta officinalis Delarbre 33.4
Constant species: Alchemilla sp. 100, Geranium sylvaticum 83, Hypericum maculatum 67, Veratrum lobelianum 50, Vaccinium myrtillus 50, Silene vulgaris 50, Senecio nemorensis agg. 50, Rubus idaeus 50, Ranunculus platanifolius L. 50, Ligusticum mutellina 50
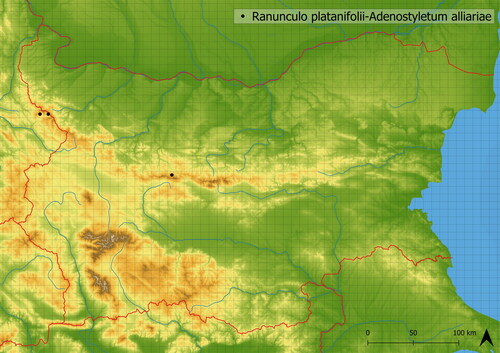
This multilayer vegetation is created by dominant Adenostyles alliariae and accompanied by other tall-herb species such as Heracleum sphondylium agg., Ranunculus platanifolius and Senecio nemorensis agg. In Romania, the vegetation dominated by Adenostyles alliariae is classified within the Adenostylo-Doronicetum austriaci (Sanda et al. Citation2008), which also contains species endemic to southern Carpathians such as Phyteuma vagneri A.Kern. and Pulmonaria filarszkyana Jáv. and species which do not occur in Bulgaria such as Aconitum tauricum Wulfen and Cirsium waldsteinii Rouy. Compared to the vegetation occurring in central Europe, in Bulgaria, Ranunculo platanifolii-Adenostyletum alliariae contains Doronicum columnae and Soldanella sp. pl. (Krajina Citation1933; Kočí Citation2001, Citation2007). However, the differences within vegetation structure are minor and should be considered at the subassociation level. This vegetation is mediately species-rich; in analyzed vegetation, there are 25 species on average. In Bulgaria, the vegetation occurs only in Stara Planina Mts. (C, W; see ), usually on wet screes near rock outcrops. The average altitude of occurrence is in 2004 m a. s. l.
TRO01 Trollius europaeus communities
Diagnostic species: Trollius europaeus 79.4 (dom.), Filipendula ulmaria (L.) Maxim. 70.5, Crepis paludosa (L.) Moench 68.5, Centaurea nervosa Willd. 66.4, Myosotis scorpioides agg. 58.2, Traunsteinera globosa (L.) Rchb. 56.5, Pedicularis hoermanniana K.Malý 56.5, Gymnadenia conopsea (L.) Rich. 56.5
Constant species: Geranium sylvaticum 100, Alchemilla sp. 100, Veratrum lobelianum 67, Rumex arifolius 67, Luzula luzuloides 67, Hypericum maculatum Crantz 67, Geum coccineum 67, Gentiana asclepiadea 67, Festuca rubra agg. 67, Deschampsia cespitosa 67, Cirsium appendiculatum 67, Caltha palustris L. 67, Bistorta officinalis Delarbre 67
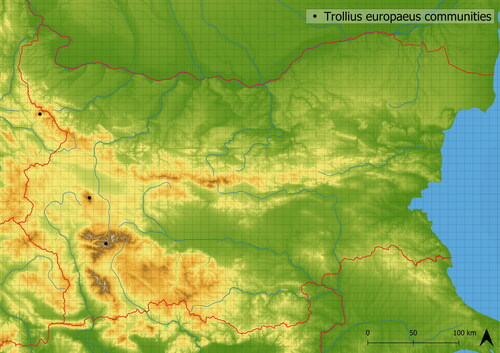
Communities dominated by Trollius europaeus are species-rich (in analysed vegetation plots, there were ∼30 species on average), and in Bulgaria, they occur only rarely. These vegetation types are accompanied by species typical of fens and wet meadows (e.g. Filipendula ulmaria, Crepis paludosa, Myosotis scorpioides agg.), tall-herb vegetation (e.g. Geranium sylvaticum, Veratrum lobelianum, Cirsium appendiculatum) and mesic meadows (e.g. Festuca rubra agg., Hypericum maculatum, Luzula luzuloides, Traunsteinera globosa). They were documented only in a few mountain ranges (Stara Planina, Vitosha, Rila; see ) with rather inhomogeneous species composition. The vegetation occurs in 1978 m a. s. l. on average.
3.2.7. Dryopterido filicis-maris-Athyrion distentifolii
FOR01 Saxifrago rotundifoliae-Athyrietum distentifolii ass. nov.Footnote4
Diagnostic species: Athyrium distentifolium Tausch ex Opiz 85.0 (dom.), Ligusticum mutellina 34.9, Sedum acre L. 34.4, Cystopteris sp. 34.4
Constant species: Veratrum lobelianum 88, Rumex arifolius 69, Festuca rubra agg. 62, Senecio nemorensis agg. 56, Saxifraga rotundifolia L. 50, Doronicum columnae 44
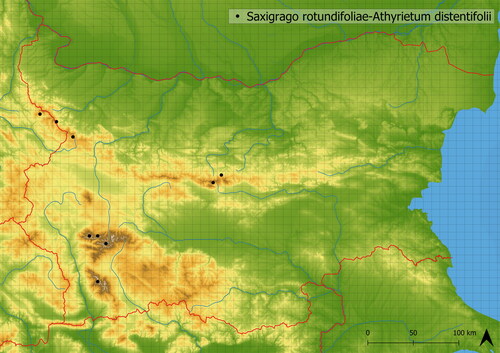
The association of Saxifrago rotundifoliae-Athyrietum distentifolii is a vegetation dominated by Athyrium distentifolium. It is mediately species-rich (in analyzed vegetation plots, there are ∼19 species). The vegetation usually develops in boulder fields and rock screes in almost all main mountain ranges (see ). It contains tall-herb species (e.g. Athyrium distentifolium, Veratrum lobelianum, Senecio nemorensis agg.), species not typically bound to tall herb vegetation such as Ligusticum mutellina, Sedum acre, but also scree specialists such as Doronicum columnae and Valeriana tripteris. This vegetation’s mean altitude of occurrence is 1990 m a. s. l. There are two varieties recognizable within the dataset. The occurrence of Athyrium distentifolium distinguishes the first variety which is usually species poor. Conversely, the second variety is typically species-rich vegetation without dominants and distinguishable by the occurrence of Athyrium filix-femina. This variety contains species not typical to the tall-herb vegetation.
FOR02 Cardamino acris-Athyrietum filicis-feminae ass. nov. prov.
Diagnostic species: Cardamine amara L. 65.9, Caltha palustris L. 49.4, Athyrium filix-femina 44.6 (dom.), Cardamine acris Griseb. 43.6, Luzula luzulina (Vill.) Racib. 40.4, Impatiens noli-tangere L. 33.8, Milium effusum 31.9
Constant species: Senecio nemorensis agg. 60, Calamagrostis arundinacea 60, Vaccinium myrtillus L. 50, Saxifraga rotundifolia L. 50, Rubus idaeus 50, Pulmonaria rubra Schott 50, Oxalis acetosella L. 50, Myosotis scorpioides agg. 50
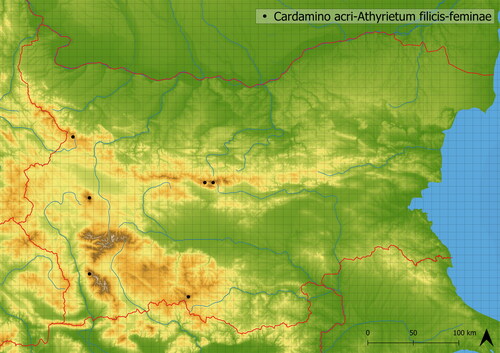
This wet-demanding vegetation dominated by Athyrium filix-femina develops in boulder fields and screes at the edge of subalpine and forest belts (the mean altitude of occurrence is at 1719 m a. s. l.). It is mediately species-rich and contains approximately 20 species on average in studied vegetation plots. It hosts species typical of springs and fens, such as Cardamine amara, Cardamine acris, Caltha palustris, Saxifraga rotundifolia, but also species of broader niche that occur at lower altitudes, such as Impatiens noli-tangere, Milium effusum, Senecio nemorensis agg. and ubiquitous species such as Rubus idaeus and Vaccinium myrtillus. This vegetation is documented in Bulgaria in Pirin, Rhodopi, Stara Planina and Vitosha Mts. (see ).
CIC01 Moehringio pendulae-Cicerbitetum alpinae ass. nov.Footnote5
Diagnostic species: Moehringia pendula (Waldst. & Kit.) Fenzl 89.9, Veronica urticifolia Jacq. 67.9, Sorbus aucuparia L. 66.5, Lilium martagon L. 60.3, Geranium macrorrhizum L. 57.0, Dryopteris filix-mas 55.8 (dom.), Melampyrum sylvaticum agg. 53.2, Pulmonaria rubra Schott 48.1, Oxalis acetosella L. 46.6, Ranunculus platanifolius L. 43.5, Cicerbita alpina (L.) Wallr. 39.1 (dom.), Valeriana tripteris 34.6
Constant species: Senecio nemorensis agg. 100, Silene vulgaris 75, Rubus idaeus 75, Geranium sylvaticum 75, Calamagrostis arundinacea 75, Viola biflora L. 50, Veratrum lobelianum 50, Solidago virgaurea L. 50, Rumex arifolius 50, Poa nemoralis L. 50, Milium effusum 50, Hypericum maculatum 50, Gentiana asclepiadea 50, Epilobium angustifolium L. 50
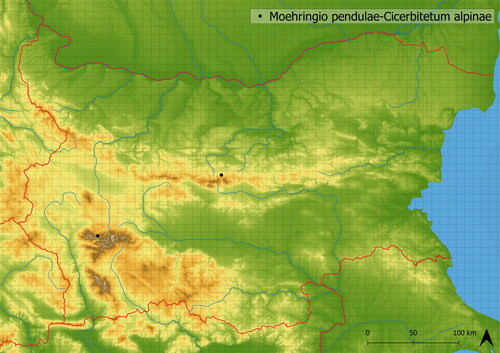
The association Moehringio pendulae-Cicerbitetum alpinae occurs rarely at the edge of the forest and subalpine belts (the mean altitude of occurrence is at 1723 m a. s. l.). Cicerbita alpina dominates in the vegetation and contains species of forest belt (such as Veronica urticifolia, Lilium martagon, Geranium macrorrhizum, Oxalis acetosella) and species with the centre of distribution in higher altitudes (such as Ranunculus platanifolius and Veratrum sp. pl.). The vegetation is mediately species-rich (in analyzed vegetation, there were ∼24 species on average). Similar vegetation dominated by Cicerbita alpina, Chaerophyllum hirsuti-Cicerbitetum alpinae is known from central Europe (Kočí Citation2007). Moehringio pendulae-Cicerbitetum alpinae is distinguishable by the occurrence of higher number of montane and subalpine species (Geranium sylvaticum, Ranunculus platanifolius, Veronica urticifolia) and species typical to southeastern Europe (Geranium macrorrhizum, Moehringia pendula, Pulmonaria rubra). It is documented in the Rila and Central Stara Planina Mts. (see ).
3.2.8. Cirsion appendiculati
CIR01 Angelico sylvestris-Heracleetum verticillati Horvat et al. Citation1937 mut. Szokala Citation2023
Diagnostic species: Cirsium appendiculatum 41.1 (dom.)
Constant species: Alchemilla sp. 69, Veratrum lobelianum 60, Heracleum sphondylium subsp. verticillatum (Pančić) Brummitt 53, Geum coccineum 52, Geranium sylvaticum 50, Festuca rubra agg. 46, Deschampsia cespitosa 46, Saxifraga rotundifolia 45
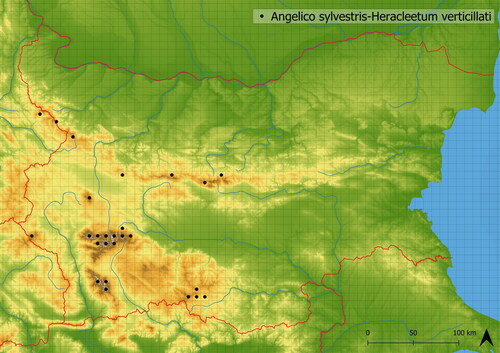
The association of Angelico sylvestris-Heracleetum verticillati is distinguishable by the dominance of either Cirsium appendiculatum or Doronicum austriacum Jacq. Companion species are tall herbs (such as Heracleum sphondylium subsp. verticillatum and Veratrum lobelianum), small species of springs (such as Saxifraga rotundifolia, Geum coccineum, Stellaria nemorum) or other ubiquitous species (such as Festuca rubra agg., Deschampsia cespitosa). It is typically mediately species-rich (in analyzed vegetation, there were ∼19 species on average). It is typical of the upper subalpine belt where it occurs in 2097 m a. s. l. on average. It develops in all main mountain ranges: Osogovska Planina, Pirin, Rhodopi, Rila, Stara Planina and Vitosha Mts. (see ). However, in NW Bulgaria it has untypical species composition (see Szokala Citation2023). This vegetation is also known from Northern Macedonia (Čarni and Matevski Citation2010).
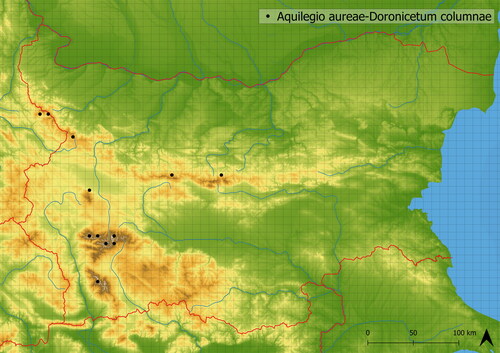
DOR01 Aquilegio aureae-Doronicetum columnae ass. nov.Footnote6
Diagnostic species: Aquilegia aurea Janka 52.8, Rhodiola rosea 43.6, Galium anisophyllon 41.8, Verbascum cf. roripifolium (Halácsy) I.K.Ferguson 39.7, Geum bulgaricum Pančić 39.0, Lavandula angustifolia Mill. 34.7, Arabis alpina 33.2, Botrychium lunaria (L.) Sw. 32.9, Koenigia alpina (All.) T.M.Schust. & Reveal 32.6, Galium rhodopeum Velen. 32.5, Sesleria coerulans s. l. 31.9, Festuca valida (R.Uechtr.) Pénzes 31.7, Carex sempervirens Vill. 31.7, Gentiana punctata 31.0
Constant species: Festuca rubra agg. 73, Veratrum lobelianum 62, Silene vulgaris 62, Geranium sylvaticum 57, Alchemilla sp. 57, Doronicum columnae 51, Saxifraga rotundifolia 49, Luzula alpinopilosa 49, Vaccinium myrtillus 43, Senecio nemorensis agg. 43, Ligusticum mutellina 43, Ranunculus montanus agg. 41, Anthoxanthum odoratum agg. 41
This vegetation develops mainly in alpine and subalpine screes, boulder fields and rock outcrops. It is relatively low vegetation, which typically has no distinct dominants. Scree specialists such as Arabis alpina, Doronicum columnae, Galium anisophyllon, Geum bulgaricum and Rhodiola rosea occur there frequently and are accompanied by graminoids such as Anthoxanthum odoratum agg., Carex sempervirens and Festuca sp. pl. The vegetation, if developing near springs, hosts a greater number of wet demanding species (e.g. Saxifraga rotundifolia and Geum coccineum) or, if occurring on screes or near rock outcrops, more mesic species. Species of the alliance Cirsion appendiculati (e.g. Angelica sylvestris, Cirsium appendiculatum) occur there commonly; however, they have only small cover values. This vegetation is mediately species-rich (in analysed vegetation, there were ∼25 species) and occurs in high altitudes (on average in 2249 a. s. l.). It is distributed in almost all main Bulgarian mountain ranges: Pirin, Rila, Stara Planina and Vitosha Mts. (see ).
3.3. Environmental factors
For the analysis of environmental factors, CCA ordination was done. The significance of axes was tested through anova.cca permutation test (see and ). The number of growing degree days > 0 °C, climate moisture index, reaction, disturbance frequency and disturbance severity were chosen for the ordination analysis. Together, they explained approximately 21% of the total variance within the dataset. The variables regarding temperature (ngd0) and precipitation (cmi) are limiting for the European alpine vegetation (Körner Citation2003), and tall-herb vegetation is affected in the alpine environment by disturbances (Kočí Citation2007), i.e. by its frequency and severity. Because species occurring in this vegetation are usually nutrient-demanding (Ma et al. Citation2021), but tall-herb vegetation can occur on different bedrock types, we decided to test for the influence of reaction. Moreover, the EIV for nutrients and reaction are highly correlated within the dataset. The variables were projected to DCA ordination (see ), and each variable was then displayed within the associations in boxplots (see ).
Figure 20. DCA ordination with vectors; cmi_max – maximum monthly climate moisture index, Dis_severity – disturbance severity, Dis_frequency – disturbance frequency, ngd0 – number of growing degree days > 0 °C, reaction – reaction; projected are sample scores (grey) and species scores (dark grey) with weights > 200; Alchemilla – Alchemilla sp., Calaru – Calamagrostis arundinacea, Cirapp – Cirsium appendiculatum, Desces – Deschampsia cespitosa, Doraus – Doronicum austriacum, Fesval – Festuca valida, Fesrub – Festuca rubra agg., Gersyl – Geranium sylvaticum, Geucoc – Geum coccineum, Herver – Heracleum sphondylium subsp. verticillatum, Hypmac – Hypericum maculatum, Luzalp – Luzula alpinopilosa, Rubida – Rubus idaeus, Rumalp – Rumex alpinus, Rumari – Rumex arifolius, Saxrot – Saxifraga rotundifolia, Sennem – Senecio nemorensis agg., Stelnem – Stellaria nemorum, Verlob – Veratrum lobelianum.
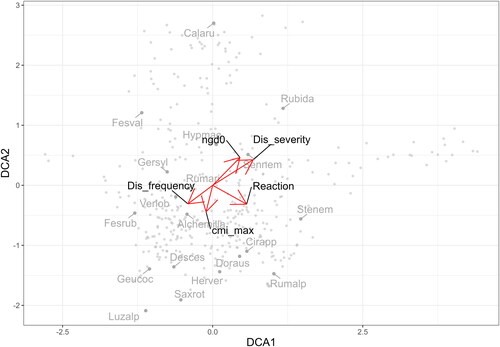
Figure 21. Differences in environmental factors between vegetation types. The ngd0 and cmi_max were tested with the ANOVA test, variables derived from EIV with the fourth corner permutation test (significance indicated at the upper right corner; *** < .001). The line in the boxplots represents the median, and the whiskers show maximum and minimum values at a maximum of 1.5 times the interquartile range. Boxes represent first and third quartiles, and dots represent outliers. The red dashed line represents the overall mean. ADE01 – Ranunculo platanifolii-Adenostyletum alliariae, ARU01 – Astrantio majoris-Calamagrostietum arundinaceae, ARU02 – Roso pendulinae-Calamagrostietum arundinaceae, CAL01 – crepido conyzifoliae-Calamagrostietum villosae, CIC01 – Moehringio pendulae-Cicerbitetum alpinae, CIR01 – Angelico sylvestris-Heracleetum verticillati, DES01 – Carici lazarei-Deschampsietum cespitosae, DOR01 – Aquilegio aureae-Doronicetum columnae, FOR01 – Saxifrago rotundifoliae-Athyrietum distentifolii, FOR02 – Cardamino acri-Athyrietum filicis-feminae, MOL01 – Sphagno compacti-Molinietum cearuleae, PET01 – Petasitetum albi, PET02 – Petasitetum hybridi, RUM01 – Senecioni rupestris-Rumicetum alpini, RUM02 – Rumicetum alpini, TRO01 – Trollius europaeus communities.
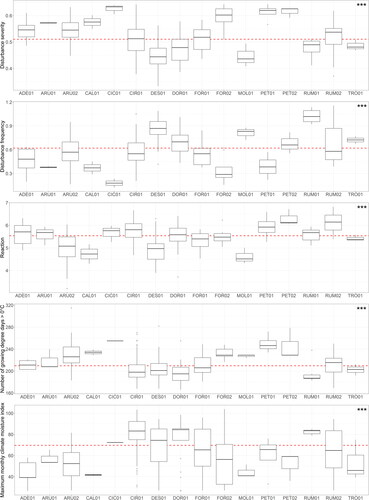
Table 1. Variables of CCA analysis tested by anova.cca permutation test on sample scores, significance * < .05, ** < .01.
The number of growing degree days > 0 °C shows the temperature demands of vegetation types. The relatively most warm-demanding communities are Moehringio pendulae-Cicerbitetum alpinae and Petasitetum albi, which develop in the forest zone. Conversely, the most cold-demanding vegetation types are those that require specific topographic conditions provided by high mountains, such as Aquilegio aureae-Doronicetum columnae or where high-altitude environment suppress the dominant species such as in Senecioni rupestris-Rumicetum alpini. As the vegetation of tall-herb species does not occur zonally and grows only if there are met specific conditions (regarding water availability), the maximum monthly climate moisture index is a rather complex geographical variable, where seasonality and other factors may also play an important role. The highest values are within the Angelico sylvestris-Heracleetum verticillati, Carici lazarei-Deschmpsietum cespitosae and Aquilegio aureae-Doronicetum columnae. The vegetation of Angelico sylvestris-Heracleetum verticillati and Aquilegio aureae-Doronicetum columnae most frequently occur in relatively high altitudes, which causes higher rainfall and mist occurrence. However, the vegetation of Carici lazarei-Deschampsietum cespitosae also benefits from frequent disturbance (grazing; see ), which keeps this vegetation from becoming more productive; the combination of higher altitude (where pasture occurs) and moisture availability plays here the most crucial role. Most severe disturbances occur in vegetation developing in lower altitudes (e.g. Moehringio pendulae-Cicerbitetum alpinae, Petasitetum hybridi, Petasitetum albi) or at the edge of the forest and subalpine zones (e.g. Astrantio majoris-Calamagrostietum arundinaceae, Cardamino acris-Athyrietum filicis-feminae).
Reaction, disturbance severity and disturbance frequency had significant effect on species score distribution (see ). Disturbance frequency and severity were correlated in the dataset. The disturbance variables are linked to the management practices, and it is more frequent in Carici lazarei-Deschampsietum cespitosae, Rumicetum alpini and Senecioni rupestris-Rumicetum alpini. Disturbance is most severe in Moehringio pendulum-Cicerbitetum alpinae, Cardamino acris-Athyrietum filicis-feminae and in vegetation dominated by Petasites sp. pl. The reaction demandingness is highest in vegetation types typically developing at sites highly influenced by human activity (Senecioni rupestris-Rumicetum alpini) or by the transfer of sediments (Petasitetum hybridi, Petasitetum albi) and lowest in vegetation types developing without frequent disturbances (Crepido conyzifoliae-Calamagrostietum villosae, Sphagno compacti-Molinietum caeruleae).
Table 2. Variables of CCA analysis tested by anova.cca permutation test on species scores, significance *** < .001.
4. Discussion
4.1. Vegetation differentiation and diversity
The analyzed vegetation plots were classified into 15 vegetation types on the level of association and one community. They were classified into seven alliances which belong to four orders. The most common vegetation type is the alliance of Cirsion appendiculati, distributed in most of Bulgaria. The rarest are the vegetation types of the alliances Adenostylion alliariae and Dryopterido filicis maris-Athyrion distentifolii. The vegetation of the alliances Petasition officinalis and Arunco-Petasition albi have a relatively low number of relevés included in the dataset; hence, their distribution is most likely not covered representatively.
For the final classification of vegetation, the expert system was created, which classifies the vegetation into recognizable vegetation types. Created expert system is mostly dominance-based. This way of classification (based on dominant species) was chosen because most of the vegetation types have a distinct dominant species (e.g. Petasitetum hybridi, Rumicetum alpini, Ranunculo platanifolii-Adenostyletum alliariae) with only a few same species co-occurring in most of the vegetation types (Karner and Mucina Citation1993; Roussakova Citation2000; Kočí Citation2007). The accompanying species are mostly ubiquitous (e.g. Anthoxanthum odoratum agg., Festuca rubra agg., Deschampsia cespitosa) and with a strong affinity for disturbed places (e.g. Filipendula ulmaria, Rubus idaeus, Telekia speciosa). Compared to numerical classification methods, the vegetation dominated by Calamagrostis arundinacea, Petasites sp. pl. and Rumex alpinus are the most robust groups. The smaller the distinguished group by the expert system, the less robust it is in other classification methods (see ). The vegetation with the largest number of samples (Cirsion appendiculati) and related vegetation types were distinguished by the numerical methods very poorly. This can be due to the predominance of these vegetation types in the dataset and thus by captured variability of the gradients towards other vegetation types. The most related vegetation type to the Cirsion appendiculati is Carici lazarei-Deschampsietum cespitosae (all. Calamagrostion villosae) which is classified by several authors (e.g. Roussakova Citation2000; Tzonev et al. Citation2009) in the alliance Cirsion appendiculati.
Few interpretations can be made regarding the classification methods. The vegetation of Adenostylion alliariae is related to the vegetation of Cirsion appendiculati, which replaces it in most of the areas of the Balkan peninsula (Horvat et al. Citation1937, Citation1974; which confirms their classification into the same order of Adenostyletalia alliariae; see ; Mucina et al. Citation2016). The alliance of Cirsion appendiculati is also connected to the vegetation of Rumicion alpini due to the occurrence of nutrient-demanding and ruderal species (see and ). The vegetation of tall grasses (order Calamagrostietalia villosae) forms a well-distinguished and specific group within the dataset. The association of Carici lazarei-Deschampsietum cespitosae is mainly related to Angelico sylvestris-Heracleetum verticillati because of similar species-pool (of relatively wet-demanding species) and area of occurrence (the accompanying species have southeast-European distribution); it was classified into Cirsion appendiculati by Roussakova (Citation2000). Conversely, Calamagrostion arundinaceae prefer more mesic conditions in which species with broader niches (e.g. Astrantia major, Geranium sylvaticum, Athyrium sp. pl.) occur and thus share them with Adenostyletalia alliariae. The association of Sphagno compacti-Molinietum cearuleae is more related to the alpine spring and fen vegetation and shares a minimal species pool with other vegetation of the class Mulgedio-Aconitetea. Very similar in this regard is the vegetation dominated by Petasites sp. pl., which contains many species occurring in the forest belt.
Roussakova (Citation2000) distinguished in her work three associations of the class Mulgedio-Aconitetea: Angelico sylvestris-Heracleetum verticillati, Carici lazarei-Deschampsietum cespitosae and Senecioni rupestris-Rumicetum alpini, same vegetation types are accepted in national compilation of Tzonev et al. (Citation2009). Nazarov et al. (Citation2022) distinguished one plot of Petasitetum kablikiani in Bulgaria, which we are missing in the analyzed dataset. European distribution of higher syntaxa provided by Preislerová et al. (Citation2022) distinguish in Bulgaria only two alliances: Cirsion appendiculati and Rumicion alpini, however, they predict in the area numerous other alliances. In the work of Szokala (Citation2023) and the compilation prepared by Apostolova (Citation2023) other alliances were added for Bulgaria that were not distinguished in previous studies. The presented article contains the largest amount of vegetation plots and captures the diversity on a national scale.
4.2. Environmental factors
For the subalpine vegetation of class Mulgedio-Aconitetea, season length (ngd0; correlated strongly to altitude), moisture (cmi_max) and disturbance severity play a significant role in their occurrences (see ). The temperature is a crucial environmental factor in alpine environments (Körner Citation2003). However, tall-herb vegetation does not occur zonally (Karner and Mucina Citation1993; Kočí Citation2007) and thus should be more adaptive to local small-scale deviations (e.g. soil depth, nutrient availability) rather than to climatic factors (Walter and Breckle Citation1985; Breckle Citation2002). This study shows that the tall-herb vegetation is also susceptible to average temperature and has limited zonality. This affinity for certain climatic variables in tall-herb vegetation was also shown in Siberia by Heim and Chepinoga (Citation2019), and Ermakov et al. (Citation2000), and a similar pattern was shown in South African wetland vegetation (considered previously azonal) by Sieben (Citation2018). Although the tall-herb vegetation is not truly zonal (in the sense that it does not develop on prevalent soils; Breckle Citation2002), it is also not truly azonal but more likely intrazonal (Alexander Citation2009; Sieben Citation2018). The subalpine tall-herb vegetation occurs in multiple biomes (temperate, boreal, arctic) and the species composition usually shares at least the same genera in dominant taxa; however, it is also enriched with species occurring only in specific biomes, in part also due to mass effect.
The climate moisture index (cmi) is defined as the difference between precipitation and potential evapotranspiration (Hogg Citation1997; Brun et al. Citation2022) and thus informs about moisture availability in the landscape (Brun et al. Citation2022) which is essential for the occurrence of appropriate sites for the tall-herb vegetation. We can expect that in areas with high values of cmi the vegetation will be more widespread and have a higher diversity. Conversely, in less wet areas, the vegetation will be more limited to specific microhabitats. This can be seen when comparing two distinct mountain ranges of Western Stara Planina (with low cmi) and Rila Mts. (with high cmi). In the Western Stara Planina occur vegetation types (Sphagno compacti-Molinietum cearuleae, Ranunculo platanifolii-Adenostyletum alliariae) that are rare in the country and are bound to subalpine springs or wet sandstone screes; the vegetation is generally not very floristically diverse but unique. Conversely, in Rila Mts., where almost all vegetation types of tall-herb vegetation occurring in Bulgaria were distinguished, some of them behave zonally (such as the case with Rumicetum alpini and Senecioni rupestris-Rumicetum alpini). However, the climate moisture index is also affected by local specifications (e.g. homogeneity of landscape, height, insularity and geographical position of the mountain ranges).
The disturbance severity and frequency are linked to management practices and regimes, as well as the origin of the vegetation. The tall-herb vegetation naturally occurs in nutrient-rich places, usually at the bases of slopes, in boulder fields, and in terrain depressions. They are typically affected there by the movement of different materials (either stones or snow). In higher altitudes, the plants need to adapt to continuous disturbance and movement of small rock materials. The plants can adapt either by forming large underground mass that fixates the screes (e.g. Aquilegia aurea, Geum sp. pl. and Rhodiola rosea), by creeping stolons that permit some movement of weathered rock (e.g. Arabis alpina and Galium anisophyllon) or form a dense tussock that fixates the immediate surroundings (e.g. Festuca valida) (Reisigl and Keller Citation1987). The vegetation type specifically bound to these conditions is the ass. Aquilegio aureae-Doronicetum columnae. In the lower altitudes, at the bases of slopes, the movement of stones is not that frequent, and they accumulate together with organic matter. The areas can have a reduced upper forest line, meaning the sites have a longer-lasting season (see ), and the temperature is not that extreme. Plants produce larger amounts of biomass and have a longer lifespan there. In these habitats, the vegetation of tall forbs (e.g. all. Dryopterido filicis-maris-Athyrion distentifolii) occurs. Other vegetation types (especially ord. Calamagrostietalia villosae and Senecioni rupestris-Rumicetalia alpini) are more linked to human-induced disturbances (such as mowing, grazing or fire management).
The soil reaction affects the mobility of nutrients and soil processes (White Citation2012; Neina Citation2019); however, vegetation can also influence the soil reaction (Faget et al. Citation2013). Generally, the analyzed vegetation types are neutrophilous (see ). They usually develop on soils where organic matter accumulates, which affects the soil properties (Neina Citation2019). Tall-herb communities with the highest values are most affected by human activity. They are affected not only by livestock keeping but also possibly by soil additives (they usually develop near mountain settlements). These specific conditions most probably overwrite the effect of the geological composition. In Bulgarian mountains, siliceous rocks dominate (Strid et al. Citation2003); hence, it is possible that within the country, there are missing calciphilous vegetation types of the class Mulgedio-Aconitetea. The highest reaction values have vegetation types dominated by Rumex alpinus and Petasites sp. pl. These plants are also nutrient-demanding and usually establish after severe environmental alterations.
5. Conclusion
This article presents the first national classification in the Balkans and the first classification of the class Mulgedio-Aconitetea in Bulgaria. Revision of existing information regarding this vegetation was made in a wide context of the Balkans and neighboring areas. This vegetation class is very diverse in the area; we distinguished 15 association and one uncategorised community. Six associations are newly described, two of them are described as provisional. The vegetation is affected by growing season length and climate moisture. This shows that even though Mulgedio-Aconitetea is not the dominant vegetation type in the mountains, it still preserves limited zonal patterns. Moreover, vegetation types are strongly affected by disturbances that are either human-induced or naturally occurring and by reaction, which is strongly correlated with nutrient availability in the dataset.
Authors’ contributions
DS conceived the idea, analysed the data and interpreted them, wrote the expert system, text and created maps. All authors sampled the vegetation. All authors revised the text and approved of its final form.
Acknowledgements
We would like to thank Jakub Tešitel for suggestions regarding the analysis of data, Milan Chytrý and Zdeňka Lososová for revising the text, Irena Axmanová and Salza Palpurina for useful comments and help with species nomenclature, Michal Hájek and Petra Hájková for useful comments on early version of the manuscript.
Disclosure statement
No potential conflict of interest was declared by the authors.
Additional information
Funding
Notes
1 Nomenclature type: Roussakova Citation2000, p. 116, tab. 42, relevé no. 1 (typus).
2 Synonym: Carici bulgaricae-Deschampsietum cespitosae Roussakova Citation2000; Carex lazarei Jac.Koopman, Niketic, Wieclaw & Govaerts [nom. illeg. Carex bulgarica (Domin) Lazare].
3 In the Balkans, this alliance is reported from Kosovo (Rexhepi Citation1994), North Macedonia (Horvat Citation1960), and Bulgaria (Szokala Citation2023). There is historically only one association in the Balkans named Knautio-Calamagrostietum arundinaceae. It occurs in several literature sources (Horvat Citation1960, Horvat et al. Citation1974, Rexhepi Citation1994); however, they most likely refer to the hand-written manuscript of Horvat from 1949 (see Lakušić et al. Citation2015), and thus it is not an effectively published name (Theurillat et al. Citation2021 Art. 1).
4 Typus Saxifrago rotundifoliae-Athyrietum distentifolii. Author: M. Kočí. Date: 12.07.2003. GPS 23°29’38″ N, 42°07’14″ E. Locality: Rila, Ribni jezera ca 0.5 km NNE of the Ribni jezera cottage. Habitat: Heel of a rock, near a scree cone. Relevé area: 10 m2. Altitude: 2172 m a. s. l. Slope: 20°. Cover: Etotal 60%, E1 60%, E0 5%. Species – E1: Athyrium distentifolium 3, Doronicum austriacum 2a, Festuca rubra L. 2a, Rumex arifolius 2a, Dryopteris filix-mas 1, Gentiana punctata L. 1, Geranium sylvaticum 1, Geum montanum L. 1, Jasione bulgarica Stoj. & Stef. 1, Milium effusum L. 1, Saxifraga rotundifolia 1, Vaccinium myrtillus 1, Veratrum lobelianum 1, Anthoxanthum odoratum agg. +, Avenella flexuosa +, Cirsium appendiculatum +, Doronicum columnae +, Homogyne alpina (L.) Cass. +, Ligusticum mutellina +, Poa ursina +, Potentilla crantzii (Crantz) Beck ex Fritsch +, Ranunculus montanus Willd. +, Sedum alpestre Vill. +, Senecio nemorensis L. +, Vaccinium uliginosum L. +, Crocus veluchensis Herb. R, Gymnocarpium dryopteris (L.) Newman r. E0: not analysed.
5 Typus Moehringio pendulae-Cicerbitetum alpinae. Author: M. Kočí. Date: 13.07.2003. GPS 23°24’05″ N, 42°29’27″ E. Locality: Rila river, valley near Rila monastir, ca 2 km E of Kirilova poljana. Habitat: Slight depression in opening of spruce-fir dominated forest. Relevé area: 16 m2. Altitude: 1768 m a. s. l. Slope: 10°. Cover: Etotal 100%, E1 100%, E0 40%. Species – E1: Cicerbita alpina 4, Dryopteris filix-mas 2b, Calamagrostis arundinacea 2a, Geranium sylvaticum 2a, Prenanthes purpurea L. 2a, Senecio nemorensis 2a, Solidago virgaurea +, Campanula trichocalycina Ten. 1, Geum coccineum 1, Epilobium angustifolium 1, Hypericum maculatum 1, Lilium martagon 1, Luzula sylvatica 1, Ranunculus platanifolius 1, Rumex arifolius 1, Vaccinium myrtillus 1, Veratrum lobelianum 1, Veronica urticifolia 1, Cruciata laevipes Opiz +, Melampyrum sylvaticum L. +, Moehringia pendula +, Melica nutans L. +, Myosotis sylvatica Ehrh. ex Hoffm. +, Silene vulgaris +, Sorbus aucuparia +, Oxalis acetosella +, Viola biflora +. E0: not analysed.
6 Typus Aquilegio aureae-Doronicetum columnae. Author: M. Kočí. Date: 08.07.2003. GPS 23°38’25″ N, 42°10’01″ E. Locality: Kazana glacial cirque, base of rock outcrops, middle part of eastern slope. Habitat: base of rock otcrops. Relevé area: 15 m2. Altitude: 2350 m a. s. l. Slope: 40°. Cover: Etotal 90%, E1 90%, E0 0.5%. Species – E1: Geranium sylvaticum 3, Aquilegia aurea 2b, Saxifraga rotundifolia 2b, Festuca rubra 2a, Geum coccineum 2a, Senecio nemorensis 2a, Soldanella pindicola Hausskn. 2a, Veratrum lobelianum 2a, Aconitum lycoctonum L. 1, Angelica sylvestris 1, Cirsium appendiculatum 1, Doronicum columnae 1, Festuca valida 1, Hypericum maculatum 1, Knautia arvensis agg. 1, Luzula alpinopilosa (Chaix) Breistr. 1, Rhodiola rosea L. 1, Rumex arifolius 1, Koenigia alpina +, Alchemilla glabra Neygenf. +, Alchemilla vulgaris agg. +, Arabis alpina L. +, Carex sempervirens Vill. +, Centaurea nervosa +, Crepis conyzifolia (Gouan) A.Kern. +, Epilobium angustifolium +, Galium anisophyllon Vill. +, Homogyne alpina +, Ligusticum mutellina +, Myosotis alpestris F.W.Schmidt +, Phleum alpinum +, Poa nemoralis +, Polystichum lonchitis +, Ranunculus montanus +, Saxifraga paniculata +, Sedum alpestre +, Sesleria coerulans agg. +, Vaccinium myrtillus +, Viola tricolor L.+. E0: Pleuropteropyrum undulatum (Murr.) A.Löve & D.Löve +.
References
- Alexander EB. 2009. A comment on the zonal, intrazonal, and azonal concepts and serpentine soils. Madroño. 56(1):57–57.
- Apostolova I. 2023. Progress of vegetation studies in Bulgaria: an updated phytosociological checklist of the high-rank syntaxa. PhB. 29(2):225–258. doi: 10.7546/PhB.29.2.2023.9.
- Apostolova I, Pedashenko H, Sopotlieva D, Velev N, Vassilev K, Meshinev T. 2013. Arctic-Alpine plants in Bulgarian mountains. LAZA. 34(1):55–63. doi: 10.5209/rev_LAZA.2013.v34.n1.43180.
- Apostolova I, Slavova L. 1997. Compendium of Bulgarian plant communities. Sofia: Bulgarian Academy of Sciences.
- Böhling N, Greuter W, Raus T. 2002. Zeigerwerte der Gefäßpflanzen der Südägäis (Griechenland). Braun-Blanquetia. 32:1–108.
- Breckle S-W. 2002. Walter’s vegetation of the earth. Berlin: Springer.
- Brun P, Zimmermann NE, Hari C, Pellissier L, Karger DN. 2022. Global climate-related predictors at kilometer resolution for the past and future. Earth Syst Sci Data. 14(12):5573–5603. doi: 10.5194/essd-14-5573-2022.
- Čarni A, Matevski V. 2010. Vegetation along mountain streams in the southern part of the Republic of Macedonia. Braun-Blanquetia. 46:157–170.
- Chytrý M, Tichý L, Hennekens SM, Knollová I, Janssen JAM, Rodwell JS, Peterka T, Marcenò C, Landucci F, Danihelka J, et al. 2020. EUNIS habitat classification: expert system, characterisic species combinations and distribution maps of European habitats. Appl Veg Sci. 23(4):648–675. doi: 10.1111/avsc.12519.
- Chytrý M, Tichý L, Holt J, Botta-Dukát Z. 2002. Determination of diagnostic species with statistical fidelity measures. J Veg Sci. 13(1):79–90. doi: 10.1658/1100-9233(2002)013[0079:DODSWS]2.0.CO;2.
- Dray S, Dufour A. 2007. The ade4 Package: implamanting the duality diagram for ecologists. J Stat Soft. 22(4):1–20. doi: 10.18637/jss.v022.i04.
- Ermakov N, Shaulo D, Maltseva T. 2000. The class Mulgedio-Aconitetea in Siberia. phyto. 30(2):145–192. doi: 10.1127/phyto/30/2000/145.
- Faget M, Blossfeld S, von Gillhaussen P, Schurr U, Temperton VM. 2013. Disentangling who is who during rhizosphere acidification in root interactions: combining fluorescence with optode techniques. Front Plant Sci. 4:392. doi: 10.3389/fpls.2013.00392.
- Guarino R, La Rosa M. 2019. Flora d’Italia digitale. In: Pignatti S, Guarino R, La Rosa M, editors. Flora d’Italia. 2nd ed. Bologna: Edagricole.
- Hájková P, Hájek M, Apostolova I, Zelený D, Dítě D. 2008. Shifts in the ecological behaviour of plant species between two distant regions: evidence from the base richness gradient in mires. J Biogeogr. 35(2):282–294. doi: 10.1111/j.1365-2699.2007.01793.x.
- Heim RJ, Chepinoga VV. 2019. Subalpine tall-herb vegetation patterns: a case study from the Khamar-Daban Range (southern Baikal region, Eastern Siberia). Bot Pac. 8(1):39–49. doi: 10.17581/bp.2019.08111.
- Hogg EH. 1997. Temporal scaling of moisture and the forest–grassland boundary in western Canada. Agric For Meteorol. 84:115–122.
- Horvat I. 1949. Nauka o biljnim zajednicama. Zagreb: Nakladni Zavod Hrvatske.
- Horvat I. 1960. Planinska vegetacija Makedonije u svijetlu suvremenih Istraživanja. Izdanja na Prirodnonačniot Muzej. 6:164–204.
- Horvat I, Glavač V, Ellenberg H. 1974. Vegetation Südosteuropas. Jena: Gustav Fisher.
- Horvat I, Pawłowski B, Walas J. 1937. Studia fitosocjologiczne nad wysokogórską roślinnością gór Riła w Bulgarii – Phytosoziologishe Studien über die Hochgebirgsvegetation der Rila Planina in Bulgaria. Bulletin de L’Acad. Polonaise Des Sciences Sér B. (1):159–189.
- Illa E, Carrillo E, Ninot JM. 2006. Patterns of plant traits in Pyrenean alpine vegetation. Flora. 201(7):528–546. doi: 10.1016/j.flora.2005.10.007.
- Karner P, Mucina L. 1993. Mulgedio-Aconitetea. In: Grabherr G, Mucina L, editors. Die Pflantengesellschaften Österreichs Teil II. Jena, Stuttgart & New York (NY): Gustav Fischer; p. 468–505.
- Kliment J, Jarolímek I, Šibík J. 2007. Mulgedio-Aconitetea. In: Kliment J, Valachovič M, editors. Rastlinné společenstvá Slovenska, 4. Vysokohorská vegetácia. Bratislava: VEDA; p. 21–129.
- Kočí M. 2001. Subalpine tall-herb vegetation (Mulgedio-Aconitetea) in the Czech Republic. Preslia. 73:289–331.
- Kočí M. 2007. Subalpínská vysokobylinná a křovinná vegetace (Mulgedio-Aconitetea) [Subalpine tall-forb and deciduous-shrub vegetation]. In: Chytrý M, editor. Vegetace České republiky 1. Praha: Academia; p. 92–131.
- Körner C. 2003. Alpine plant life, functional plant ecology of high mountain ecosystems. Berlin: Springer.
- Krajina V. 1933. Die Pflanzengesellschaften des Mlynica-Tales in den Vysoké Tatry (Hohe Tatra). Beihefte Zum Botanischen Centralblatt. 50:774–957.
- Krebs CJ. 2014. Ecology: the experimental analysis of distribution and abundances. Harlow: Pearson.
- Lakušić D, Ranđelović V, Di Pietro R. 2015. Nomenclature adjustments to neglected syntaxa of the tall-herb hygrophilous communities of the SE-Europe. Period Biol. 117(3):383–397. doi: 10.18054/pb.2015.117.3.3141.
- Loidi J, Navarro-Sánchez G, Vynokurov D. 2022. Climatic definitions of the world’s terrestrial biomes. VCS. 3:231–271. doi: 10.3897/VCS.86102.
- Ma M, Zhu Y, Wei Y, Zhao N. 2021. Soil nutrient and vegetation diversity patterns of Alpine wetlands on the Qinghai-Tibetian Plateau. Sustainability. 13(11):6221. doi: 10.3390/su13116221.
- Mapzen. 2023. Mapzen Terrain Tile. Mapzen. https://github.com/tilezen/joerd/tree/master/docs.
- Michalik S. 1990. Plant communities in the Boatin biosphere reserve on the northern slopes of the Stara Planina Mts. (Central Bulgaria). Zakład Ochrony Przyrody i Zasobów Naturalnych Polskiej Akademii Nauk. 47:9–36.
- Michl T, Dengler J, Huck S. 2010. Montane-subalpine tall-herb vegetation (Mulgedio-Aconitetea) in central Europe: large-scale synthesis and comparison with northern Europe. Phytocoenologia. 40(2–3):117–154. doi: 10.1127/0340-269X/2010/0040-0377.
- Midolo G, Herben T, Axmanová I, Marcenò C, Pätsch R, Bruelheide H, Karger DN, Aćić S, Bergamini A, Bergmeier E, et al. 2023. Disturbance indicator values for European plants. Global Ecol Biogeogr. 32(1):24–34. doi: 10.1111/geb.13603.
- Morgan JW, Venn SE. 2017. Alpine plant species have limited capacity for long-distance seed dispersal. Plant Ecol. 218(7):813–819. doi: 10.1007/s11258-017-0731-0.
- Mucina L, Bültmann H, Dierßen K, Theurillat J, Raus T, Čarni A, Šumberová K, Willner W, Dengler J, García RG, et al. 2016. Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci. 19(S1):3–264. doi: 10.1111/avsc.12257.
- Nazarov M, Velev N, Mardari C, Grigorov B, Georgiev S, Genova B, Vassilev K. 2022. Syntaxonomy and ecology of Petasites albus (L.) Gaertn., P. hybridus and P. kablikianus phytocoenoses in Bulgaria and Romania. C R Acad Bulg Sci. 75(1):43–55. doi: 10.7546/CRABS.2022.01.06.
- Neina D. 2019. The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci. 2019:1–9. doi: 10.1155/2019/5794869.
- Nowak A, Świerszcz S, Nowak S, Nobis M. 2020. Classification of tall-forb vegetation in the Pamir-Alai and western Tian Shan Mountains (Tajikistan and Kyrgyzstan, Middle Asia). –VCS. 1:191–217. doi: 10.3897/VCS/2020/60848.
- Oksanen J, Simpson G, Blanchet F, Kindt R, Legedre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, et al. 2022. vegan: community Ecology Package [R package version 2.6-4]. https://CRAN.R-project.org/package=vegan.
- Padullés Cubino J, Chytrý M, Divíšek J, Jiménez-Alfaro B. 2022. Climatic filtering and temporal instability shape the phylogenetic diversity of European alpine floras. Ecography. 2022(11):e06316. doi: 10.1111/ecog.06316.
- Petrova A, Vladimirov V. 2010. Balkan endemics in the Bulgarian flora. Phytol Balcan. 16:293–311.
- Preislerová Z, Jiménez-Alfaro B, Mucina L, Berg C, Bonari G, Kuzemko A, Landucci F, Marcenò C, Monteiro-Henriques T, Novák P, et al. 2022. Distribution maps of vegetation alliances in Europe. Appl Veg Sci. 25(1):e12642. doi: 10.1111/avsc.12642.
- QGIS.org. 2023. QGIS Geographic Information System. QGIS Association. http://www.qgis.org.
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/.
- Ranđelović VN, Zlatković BK. 2010. Flora i vegetacija Vlasinske visoravni. Niš: Prirodno-Matematički Fakultet.
- Reisigl H, Keller R. 1987. Alpenpflanzen im Lebensraum. Stuttgart & New York (NY): Gustav Fischer.
- Rexhepi F. 1994. Vegetacioni i Kosovës. Pristinë: Universiteti i Prishtinës.
- Roleček J, Tichý L, Zelený D, Chytrý M. 2009. Modified TWINSPAN classification in which the hierarchy respects cluster heterogeneity. J Veg Sci. 20(4):596–602. doi: 10.1111/j.1654-1103.2009.01062.x.
- Roussakova V. 2000. Végétation alpine et sous alpine supérieure de la montagne de Rila (Bulgarie). Braun Blanquetia. 25:3–132.
- RStudio Team. 2023. RStudio: integrated development for R. Boston: RStudio, PBC. http://www.rstudio.com/.
- Sanda V, Vicol I, Ştefănuţ S. 2008. Biodiversitatea ceno-structurală a învelişului vegetal din România. – Bucuresti: Ars Docendi.
- Sieben EJJ. 2018. Zonal and azonal vegetation revisited: how is wetland vegetation distributed across different zonobioms. Austral Ecol. 44(3):449–460. doi: 10.1111/aec.12679.
- Škvorc Ž, Jasprica N, Alegro A, Kovačić S, Franjić J, Krstonošić D, Vraneša A, Čarni A. 2017. Vegetation of Croatia: phytosociological classification of the high-rank syntaxa. Acta Bot Croat. 76(2):200–224. doi: 10.1515/botcro-2017-0014.
- Stewart JR, Lister AM, Barnes I, Dalén L. 2009. Refugia revisited: individualistic responses of species in space and time. Proc Biol Sci. 277(1682):661–671. doi: 10.1098/rspb.2009.1272.
- Strid A, Andonoski A, Andonoski V. 2003. The high mountain vegetation of the Balkan Peninsula. In: Nagy L, Grabherr G, Körner Ch, Thompson DBA. Alpine biodiversity in Europe. Berlin: Springer; p. 113–121.
- Świerkosz K, Reczyńska K. 2022. Diversity of Mulgedio-Aconitetea communities in the Sudetes Mts. (SW Poland) in the Central European context. VCS. 3:67–86. doi: 10.3897/VCS.70200.
- Szokala D. 2023. Alpine and subalpine acidophilous vegetation on the eastern side of the Chiprovska Planina Mts. Tuexenia. 43:109–158.
- Theurillat J, Willner W, Fernández-González F, Bültmann H, Čarni A, Gigante D, Mucina L, Weber H. 2021. International code of phytosociological nomenclature. 4th edition. Appl Veg Sci. 24(1):e12491. doi: 10.1111/avsc.12491.
- Thomson FJ, Moles AT, Auld TD, Kingsford RT. 2011. Seed dispersal distance is more strongly correlated with plant height than seed mass. J Ecol. 99(6):1299–1307. doi: 10.1111/j.1365-2745.2011.01867.x.
- Tichý L. 2002. JUICE, software for vegetation classification. J Veg Sci. 13(3):451–453. doi: 10.1111/j.1654-1103.2002.tb02069.x.
- Tichý L, Axmanová I, Dengler J, Guarino R, Jansen F, Midolo G, Nobis MP, Van Meerbeek K, Aćić S, Attorre F, et al. 2023. Ellenberg-type indicator values for European vascular plant species. J Veg Sci. 34(1):e13168. doi: 10.1111/jvs.13168.
- Tichý L, Chytrý M, Landucci F. 2019. GRIMP: a machine-learning method for improving groups of discriminating species in expert systems for vegetation classification. J Vegetation Science. 30(1):5–17. doi: 10.1111/jvs.12696.
- Tichý L, Hennekens SM, Novák P, Rodwell JS, Schaminée JHJ, Chytrý M. 2019. Optimal transformation of species cover for vegetation classification. Appl Veg Sci. 23(4):710–717. doi: 10.1111/avsc.12510.
- Tzonev RT, Dimitrov MA, Roussakova VH. 2009. Syntaxa according to the Braun-Blanquet approach in Bulgaria. Phytol Balcan. 15:209–233.
- Vassilev K, Pedashenko H, Alexandrova A, Tashev A, Ganeva A, Gavrilova A, Macanović A, Assenov A, Vitkova A, Genova B, et al. 2020. Balkan vegetation database (BVD) – updated information and current status. VCS. 1:151–153. doi: 10.3897/VCS/2020/61348.
- Velasquez P, Kaplan JO, Messmer M, Ludwig P, Raible CC. 2021. The role of land cover in the climate of glacial Europe. Clim Past. 17(3):1161–1180. doi: 10.5194/cp-17-1161-2021.
- Walter H, Breckle S-W. 1985. Ecological zonation of the geobiosphere. In: Walter H, Breckle S-W, editors. Ecological systems of the geobiosphere. Berlin: Springer; p. 15–40.
- Westhoff V, Van Der Maarel E. 1978. The Braun-Blanquet approach. In: Whittaker RH, editor. Classification of plant communities. The Hague, Boston & London: Dr W. Junk bv Publishers; p. 287–399.
- White PJ. 2012. Ion uptake mechanisms of individual cells and roots: short-distance transport. In: Marschner P, editor. Marschner’s mineral nutrition of higher plants. Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney & Tokyo: Elsevier; p. 7–47.
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York (NY): Springer Verlag.
- Zelený D, Schaffers AP. 2011. Too good to be true: pitfall of using mean Ellenberg indicator values in vegetation analysis. J Veg Sci. 23(3):419–431. doi: 10.1111/j.1654-1103.2011.01366.x.
Appendix A
Synoptic table
Synoptic table of diagnostic species with percantage frequencies in the upper index. The coloured cells indicate solely dominant species on which the vegetation definition is based on.
Combined synoptic table of fidelity and frequency.
Appendix B
Table B1. Aggregation of species.