?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The efficacy of low salinity waterflooding (LSWF) as an enhanced oil recovery (EOR) method in carbonate formations has been well established; however, its geochemical modelling in such reservoirs remains insufficiently explored. This study aims to bridge this gap by conducting a comparative analysis of three well-recognised geochemical mechanisms including fines migration, rock dissolution/precipitation, and multi-ion exchange (MIE). Our research encompasses two parts: experimental and modelling. First, we conducted four coreflooding experiments to study the effect of LSWF on oil recovery in core scale as well as on rock wettability by measuring the contact angle of the crude oil-brine-rock system. In the next step, we created a one-dimensional compositional model in CMG-GEMTM for LSWF, validated it using the obtained experimental results, and compared the accuracy of the three geochemical mechanisms one-by-one. The results of the experimental section, confirm the positive effect of LSWF on oil recovery: 34.3% with seawater (40,000 ppm), 42.5% with LoSal-01 (10,000 ppm), 49.7% with LoSal-02 (5,000 ppm), and 60.9% with LoSal-03 (2,000 ppm). The experiments also show the wettability alteration through the reduction in the contact angle. Furthermore, the modelling results reveal that choosing MIE as the governing mechanism and sulphate concentration ([]) as the relevant interpolation factor (IF) leads to the most accurate LSWF model. After identifying the most accurate mechanism, we detected the wettability alteration in the model by showing the movement of oil and water relative permeability curves intersection point on the Kr-Sw diagram toward the right side.
Introduction
LSWF is a promising method for enhancing oil extraction from carbonate reservoirs, known for its cost-effectiveness and low carbon dioxide emissions. This technique has consistently demonstrated its effectiveness in EOR at various stages of production, both in laboratory settings and field applications globally. Originating from Bernard’s pioneering work in 1967 by incorporating NaCl ranging from 0 to 1 % in distilled water, LSWF has sparked extensive research and practical trials worldwide (Amiri & Gandomkar, Citation2019; Arain et al., Citation2024; Gandomkar et al., Citation2022).
Numerous studies have confirmed the positive impact of LSWF on oil recovery from carbonate rocks (Gandomkar & Rahimpour, Citation2015, Citation2017). For instance, research by Strand et al. (Citation2006) highlighted how specific ions in seawater can modify the oil recovery process by altering the surface properties of rocks, particularly under elevated temperatures (Strand et al., Citation2006). Similarly, Zhang et al. (Citation2006) observed enhanced oil recovery attributed to these alterations, supported by experimental evidence (Zhang et al., Citation2006). Further investigations by Yousef et al. (Citation2010) revealed that reducing water salinity enhances oil recovery from oil-contaminated carbonate rocks (Yousef et al., Citation2010). Conversely, Fathi et al. (Citation2011) noted that high sodium chloride levels could impede oil recovery (Fathi et al., Citation2011). Additionally, various researchers have conducted extensive experimental tests to highlight the positive influence of water composition on oil recovery factors in carbonate rocks(Alshakhs & Kovscek, Citation2015; Ashraf et al., Citation2010; Austad et al., Citation2015; Chandrasekhar et al., Citation2018; Fan et al., Citation2023; Fathi et al., Citation2011; Hussain et al., Citation2013; Jalilian et al., Citation2017; Kumar Saw & Mandal, Citation2023; Mazinani et al., Citation2023; Puntervold et al., Citation2015; Romero et al., Citation2013; Sanaei et al., Citation2019; Yousef et al., Citation2010; Zhang et al., Citation2006).
Considering LSWF’s proven success, researchers have delved into understanding the underlying mechanisms, particularly focusing on how it alters the wettability of carbonate rocks (Austad et al., Citation2015; Fan et al., Citation2023; Katende & Sagala, Citation2019; Lyu et al., Citation2022; Shalabi et al., Citation2014). Multiple mechanisms have been suggested regarding the mysteries behind the LSWF in carbonate reservoirs, and so far, a unanimous agreement on the fundamental mechanisms has not been reached. Generally, the mechanisms behind the LSWF performance in carbonate rocks, can be categorized into rock-fluid interactions and fluid-fluid interactions. Rock-fluid interactions focus on wettability alteration, transitioning the reservoir rock from oil-wet to water-wet states. Mechanisms such as electrostatic interactions, mineral dissolution, fine particle migration, and pH influence are involved in this alteration (Bidhendi et al., Citation2018; Fredriksen et al., Citation2018; Khaksar Manshad et al., Citation2016; Liu et al., Citation2019; Sandengen et al., Citation2016). Fluid-fluid interactions involve the interactions between the injected low-salinity water and reservoir brine, leading to enhanced oil recovery. These interactions result in reduced interfacial tension, improved capillary flow, and decreased oil entrapment. Mechanisms encompassed within fluid-fluid interactions include chemical osmosis, reduction in viscoelasticity, and formation of microdispersions (Bartels et al., Citation2019; Mahani, Keya, et al., Citation2015; Mahani, Menezes, et al., Citation2017). Understanding these mechanisms is crucial for optimizing LSWF and achieving effective EOR. Experimental studies have provided insights into the mechanisms involved, but further research is necessary for a comprehensive understanding and practical application in reservoir scale.
The exploration of LSWF within carbonate reservoirs has been limited due to their complex pore-scale heterogeneity, the presence of fractures, and the predominance of oil-wet rocks (Sagbana et al., Citation2023). Despite these challenges, significant advancements have been made in modelling LSWF’s effects on oil recovery, thanks to pioneering efforts like those of Jerauld et al. (Citation2008), who developed salinity-dependent oil/water relative permeability functions (Jerauld et al., Citation2008). This work, along with subsequent studies, has enabled predictions of LSWF’s impact at pilot field scales by considering factors such as salt’s influence on the aqueous phase, density, viscosity, relative permeability, and capillary pressure.
Further progress was made by researchers like Tripathi and Mohanty (Citation2008), who explored the tertiary mode instability of LSWF and the process of WA through mathematical models (Tripathi & Mohanty, Citation2008; Wu, Citation2002). These models highlighted the importance of adjusting key parameters like relative permeability and residual oil saturation in response to the injected brine salinity. Subsequent advancements integrated comprehensive approaches, including ion exchange mechanisms and mineral dissolution reactions, into multiphase flow and transport equations. Notably, Al-Shalabi et al. (Citation2015a) enhanced the accuracy of geochemical simulators by incorporating models for activity coefficients (Al-Shalabi, Sepehrnoori, & Delshad, Citation2015a; Al-Shalabi, Sepehrnoori, & Pope, Citation2015a, Citation2015b), while Kazemi Nia Korrani et al. (Citation2015) and Al-Shalabi and Sepehrnoori (Citation2016) furthered the understanding of LSWF’s mechanisms through innovative modelling approaches (Al-Shalabi & Sepehrnoori, Citation2016; Kazemi Nia Korrani et al., Citation2015; Kazemi et al., Citation2015). Recent studies by Sanaei et al. (Citation2019), Korrani and Jerauld (Citation2019), and Magzymov et al. (Citation2021) have focused on surface complexation reactions and the impact of reaction kinetics on WA in low salinity circumstances (Korrani & Jerauld, Citation2019; Magzymov et al., Citation2021; Sanaei et al., Citation2019). Additionally, Elakneswaran et al. (Citation2020) and Kalantariasl et al. (Citation2022) have employed geochemical modelling to examine the effects of rock lithology and the injected brine’s composition on oil recovery (Iptc-20250-Ms, Citationn.d.; Kalantariasl et al., Citation2022).
In recent affords, LSWF in carbonate reservoirs was simulated by employing a Bond-Product-Sum (BPS) approach and directly accounted the effect of water composition, pH, oil composition (acid and base numbers), and surface reaction site densities on the wetting characteristics and consequently the incremental oil recovery by engineered waterflooding (Almasiyan & Mahani, Citation2023). Other recent research investigated the combination of LSWF with other methods such as carbonated water and nanoparticle addition to the injected water composition (Alvarez et al., Citation2024). Through a comprehensive review of the existing literature, it has become evident that a significant gap in knowledge exists regarding the accurate modelling of LSWF especially in terms of selecting the mechanism as the main governing mechanism. Therefore, the principal objective of this study was to undertake the modelling of LSWF in carbonate rocks and conducting a comparative analysis of three distinct geochemical mechanisms (fines migration, rock dissolution/precipitation, MIE). The comparison will lead to the identification of the mechanism by which the most accurate modelling will be acquired. The modelling procedure for LSWF, particularly in carbonate rocks, is typically time-consuming and complex due to the heterogeneity of the rock structure. The methodology and outcomes of this research will benefit a broad spectrum of scholars focused on modelling LSWF in carbonate reservoirs by providing guidance on the judicious selection of interpolants. This work’s contributions are expected to enhance the efficiency and accuracy of LSWF modelling in heterogeneous carbonate formations, potentially leading to improved oil recovery strategies in such challenging reservoirs.
Materials and methods
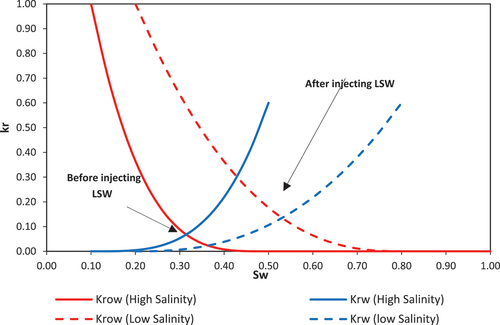
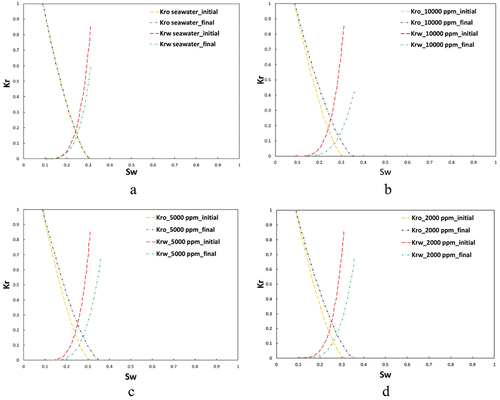
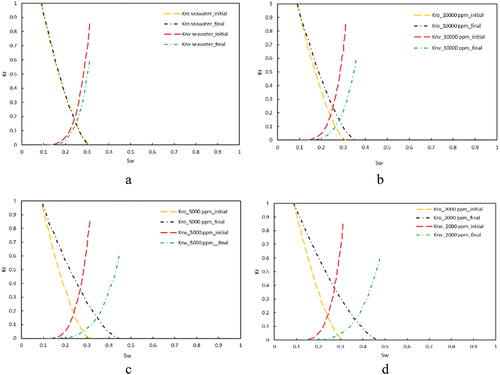
In carbonate formations, the WA is the key effect of LSWF and is typically marked by the shifting of the crossover point on the oil-water relative permeability (kr-Sw) graph towards higher water saturation levels, as depicted in .
To estimate the altered Krl for both of oil and water phases, equation (1) is employed in the modelling:
The terms Krl, KrlHS, and KrlLS refer to the interpolated, initial, and final relative permeability of the liquid phase (either oil or water), respectively. The Ө parameter, which is called interpolation factor (IF), may correspond to different parameters such salt concentration (Jerauld et al., Citation2008), PDIs concentration (Kazemi Nia Korrani et al., Citation2015), contact angle, molar Gibbs free energy (Adegbite et al., Citation2017; Al-Shalabi, Sepehrnoori, & Delshad, Citation2015b; Al-Shalabi, Sepehrnoori, Pope, et al., Citation2014; Al-Shalabi, Sepehrnoori, Delshad, et al., Citation2014), and fraction of Mg2+ (Egbe et al., Citation2021). In this study, the IF value is calculated according to the chemical reactions stoichiometric happening during LSWF. Then, the obtained IF is used to estimate the final Krl and the movement of the crossover point of the oil and water relative permeability curves.
Experimental section
The experimental section entails the preparation and collection of all necessary materials, including rock, crude oil, and brine samples, followed by running the coreflooding experiments. The results of these experiments will be detailed in the subsequent sections.
Materials (rock and fluid)
The materials comprise the crude oil, brines including formation water (FW), seawater (SW), and low salinity (LoSal) waters and the cylindrical cores.
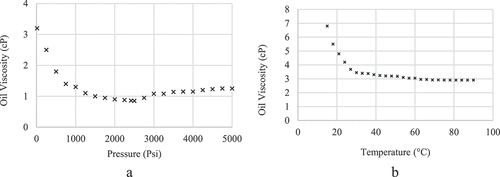
Crude oil. The oil sample used in this study was taken from Asmari oil reservoir, which is a carbonate reservoir located in the southwest of Iran. Its viscosity was 3.18 cp at 60 °C and 15 psi. The oil’s acid number was found to be 3.62 mg KOH/g of oil, and 2.12 wt % asphaltene content after saturates- aromatics- resin-asphaltene (SARA) test analysis as shown in .
Table 1. SARA components before and after distillation of oil.
The asphaltene fraction was separated from the oil after distilling the oil at a temperature of 260 °C. Compared to asphaltenes, resins are heavier and stayed in the oil even at this temperature. Asphaltenes in crude oil might include polar substances important for the positive effects of LSWF on ORF, but since asphaltenes make up a small portion of the crude oil, removing them does not significantly reduce the oil’s polar substance content. After distillation, the acid number was measured at 3.59 mg KOH/g, which did not show a remarkable reduction from the crude oil’s pre-distillation acid number of 3.62 mg KOH/g. depict the relationship between oil viscosity and temperature and pressure, respectively, based on studies done at 60 °C.
Brines. The brines were synthesized artificially in the lab by dissolving salts with 99.99 % purity into the freshwater. To ensure the salt dissolved evenly, a rotating centrifuge was used at room temperature and normal atmospheric pressure. The SW salinity was adjusted to 40,000 ppm to mimic the Persian Gulf’s water, while the FW salinity was set at 192,350 ppm based on the Asmari reservoir’s brine. The chemical composition of the used brines is listed in . The brines injected were diluted to 4, 8, and 20 times less than the SW’s salinity level.
Table 2. FW, Asmari water, and SW chemical composition (ppm).
Cores. The cores were taken from a bigger core sample collected from drilling the Asmari carbonate formation using a fully automated device designed for extracting plugs. The XRD analysis of the cores is presented in .
Table 3. mineralogy of the rock samples using XRD.
The core plugs were cleaned using a two-stage process with a Soxhlet extractor, where hot solvents, specifically toluene and ethanol, were circulated through them. This process continued until the column of the Soxhlet extractor was clear, ensuring that all fluids and impurities were removed from the rock. The cleaning phase lasted between 72 to 96 hours for each sample. Afterward, the cores were dried in an oven and positioned on a horizontal core holder to measure their porosity (φ) and permeability (k). The petrophysical properties of the core samples are summarized in .
Table 4. Core plugs’ petrophysical properties.
Coreflood apparatus
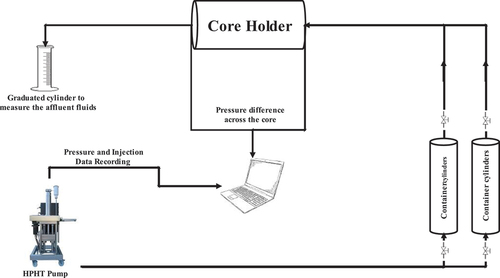
In the next step, the core samples were positioned in a core holder, and oil was injected into them until they reached a level of water saturation that could not be reduced further (Swirr), as detailed in . These oil-saturated cores were then submerged in a container filled with oil for a period of 56 days, with the aim of achieving the original thermodynamic and wettability state of the reservoir. We set up a coreflood apparatus suitable for conducting experiments at high temperatures and pressures, as well as under ambient conditions. A simplified schematic diagram of this setup is presented in . The setup consisted of a coreflood cylinder capable of withstanding pressures up to 5000 psi, a DBR pump for adjusting the confined pressure and fluids injection rates, a computer for displaying and recording relevant data including pressure, temperature, and injection rate. Additionally, two fluid vessels were utilized to store the injected fluids such as FW, SW, LoSal, and oil, along with calibrated gauges for visually monitoring and controlling the pressure.
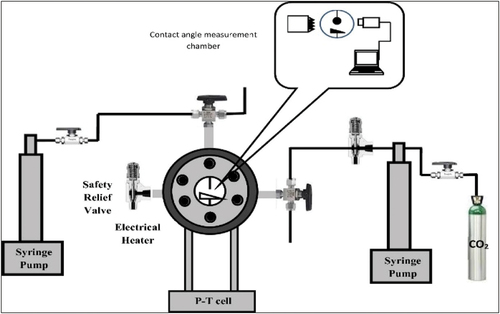
Also, in order to check the WA occurrence during the coreflooding, the contact angle between the rock and the brine drops before (Өi) and after (Өf) the coreflooding tests. In this regard, we used a pendant drop with tilted plate contact angle method as its schematic diagram of this device is shown in .
Modelling section
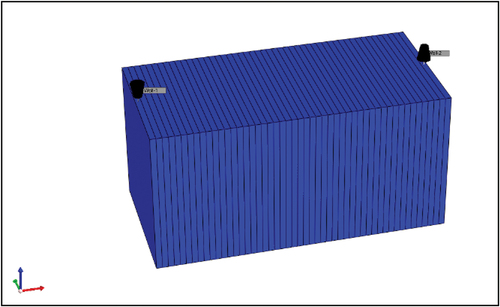
The model was developed with the CMG-GEMTM software and run through the Low-Salinity Waterflooding Wizard. GEM™ is a prominent compositional reservoir simulation tool for geochemical modelling of LSWF, WA, MIE, and zeta potential influences during this water based EOR method in different rock types (Compositional, Unconventional & Advanced Processes Simulator, Citationn.d.). The pre-processing tool from the CMG, known as Builder, was used to create a one-dimensional, horizontally uniform cubic Cartesian grid model system (50 × 1 × 1) with defined porosity and permeability, as shown in .
In order to replicate the waterflooding procedure, an injection well was positioned at the coordinates (1 1 1) within the grid, while a production well was placed at the end coordinates (50 1 1). Furthermore, the injection rate was established at 0.2 cc/min for a duration exceeding five PVI.
Three key LSWF mechanisms including fines migration, rock dissolution/precipitation, MIE in carbonate reservoirs were examined to identify the most accurate model.
Fines migration focuses on the migration of calcite fines as the primary mechanism during LSWF, where calcite particles are dislodged from the carbonate surface and move into the flowing fluid. This migration alters the hydrodynamic properties of the rock, like porosity and permeability, reduces the residual oil by blocking small pore spaces, which creates resistance to fluid flow, increases pressure drop, and redirects the fluid flow within the pores, leading to higher oil recovery. Because of the significant impact of Ca2+, this cation’s concentration has been selected as the IF, and its calculation formula is as follows in equation (2):
The next modelled mechanism, i.e., the rock dissolution/precipitation leads to an augmentation in the porosity and permeability of the rock. It should be noted that dissolution solely occurs as a result of the activation of dissolution reactions and is not associated with the migration of fines (Abbasi & Khamehchi, Citation2021). The main chemical reactions happening during this mechanism are shown in , and many more can be found in IPHREEQC database.
Table 5. rock solution/precipitation reactions.
The total ionic strength (TIS) of the injected brine is considered the most important parameter in rock dissolution/precipitation. This parameter is referred to as the IF in this mechanism, and its definition is given by equation (3):
In equation (3), TIS max denotes the highest TIS level where no WA is observed. TIS (x,t) indicates the TIS in each grid section at a certain time during the simulation, and TIS min is the lowest total ionic strength at which the greatest WA is noted. This approach considers the geochemical interactions, including precipitation and dissolution, between the water’s ionic components and the minerals on the carbonate surface. WA happens due to the alterations in the mineral and chemical surface charge of the rock, which are a result of the organic compounds being released and dissolved. The increased attraction of divalent PDIs, like , to the rock surface changes the charge of the rock’s surface from positive to negative. The carboxylic group’s presence causes repulsion, creating a water-wet condition, lowering the IFT, and enhancing the ORF.
The third mechanism was MIE, which involves swapping PDIs (e.g., Ca2+, Mg2+, ) with the carboxylic groups on the carbonate surface through the injected brine, changing the rock’s ability to absorb water. The IF for this mechanism is calculated based on the concentration of the sulphate cation, as outlined in equation (4).
In the given equation, []max represents the highest concentration of the sulphate cation in the injected LSW at the point of injection. On the other hand, [
] min refers to the lowest concentration of the sulphate cation in the FW. Lastly, [
] (x, t) denotes the concentration of the sulphate cation in each grid block at a specific time. The summary of the three geochemical models have been outlined in .
Table 6. Characteristics of modelling approaches.
The initial relative permeability (KrlHS) curves were generated using the Brooks-Corey relative permeability functions, described in equations (5) to (7), and input them into the model:
In this context, krw and kro represent the end-point relative permeabilities for water and oil, respectively. Swn denotes the normalized water saturation, while Swirr signifies the irreducible water saturation and Sor the residual oil saturation. Additionally, no and nw are the power law parameters applicable to oil and water, correspondingly, and are referred to as the Corey exponents. Given that each test was performed using a new core sample that exhibited nearly identical characteristics in terms of rock type, porosity, and permeability, the exponents for relative permeability correlations were determined by regression analysis and maintained at a constant value for all the tests. The trials yielded measurements of the connate water saturation (Swc) and the residual oil saturation (Sor). The oil relative permeability at the critical water saturation (kro (Swirr)) and the water relative permeability at the residual oil saturation ((krw)Sorw) were determined using the SW flooding data.
To build the fluid model and its PVT behavior, the CMG-WinProp was used by putting the chemical composition and characteristics of the Asmari oil sample inside the simulator. Then, we used regression analysis to check that the oil physical and chemical traits matched the data from the lab. For all our simulations, we used the Peng-Robinson equation of state to predict the oil’s density and viscosity.
Results and discussion
In this section the results of the experimental coreflooding and the modelling outputs will be elucidated separately.
Experimental results
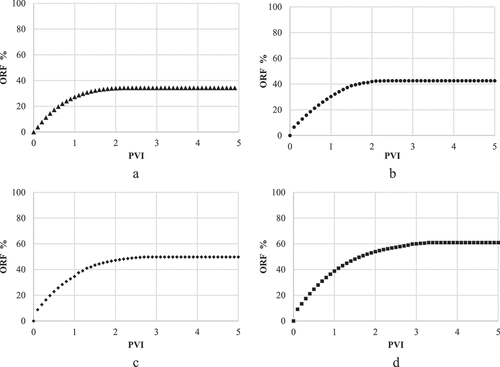
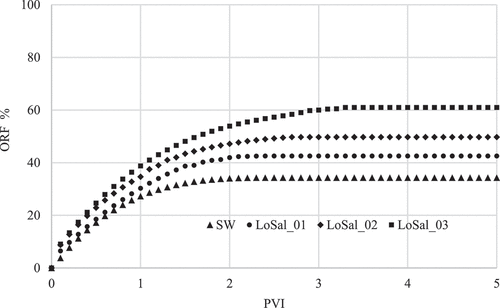
During the coreflooding tests, four brine samples were injected into the core including the SW, LoSal-01, LoSal-02, and LoSal-03 with salinity degree of 40,000, 10000, 5,000, and 2,000 ppm respectively. The coreflooding were implemented in secondary recovery mode, and the injection was continued until no further oil was observed in the effluent streams. The results of the experimental coreflooding tests and the relevant oil recovery factors are shown in .
Furthermore, illustrates the comparison of all experimental findings, elucidating the direct relation between the salinity reduction and increasing the recovery factor.
Decreasing salinity from 40,000 to 2,000 ppm resulted in a 26.7% enhancement in ORF, showing a remarkable correlation between the reduced salinity and increased ORF. Through a meticulous analysis of the concentration of potential determining ions (PDIs) including , Mg2+ and Ca2+ well as the contact angle measurements prior to and after the flooding process, it was ascertained that fines migration and WA happened during the LSWF.
describes the concentration of PDIs and contact angles before and after LSWF across various experiments.
Table 7. PDIs’ concentration (ppm) and contact angle (degree) before and after LSWF.
Upon interaction of LSWF with PDIs, the process of sulphate ions adsorption onto the surface of the rock is observed. This occurrence is attributed to the presence of an additional positive charge on the carbonate rock, a result of electrical induction facilitated by the aforementioned anions. Subsequently, Ca2+ facilitates the separation of adsorbed polar oil components from the rock surface, as shown in equation (8):
Likewise, the mechanism by which Mg2+ facilitates the detachment of oil in the presence of SO42- is demonstrated by equation (9). In both instances, SO42- acts as a catalyst for the reactions.
Modelling results
Each time we selected one of the mechanisms of fines migration, rock dissolution/precipitation, and MIE. Then, based on the accuracy of the obtained history match between the model and the experiment, we recognized each mechanism efficiency in providing an accurate match. Depending on the simulation accuracy of a particular mechanism, we made the decision for running further analysis for the effect of the LSWF on the oil/water relative permeabilities. This helped us to examine the effect of LSWF on the wettability of the rock through visual checking of the movement of the intersection point on the Kr-Sw curve.
Fines migration
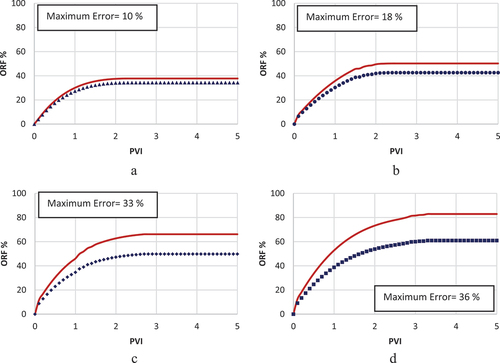
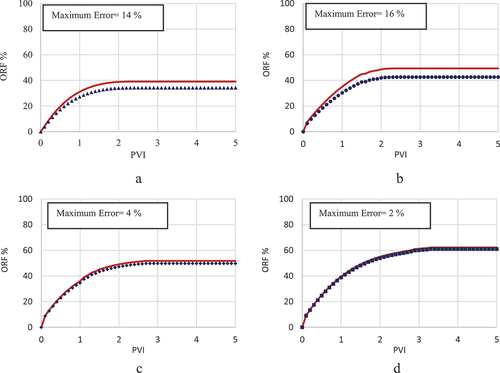
Choosing fines migration as the governing mechanism was not efficient enough to achieve an accurate history match, as shown by . The model’s limited precision, with error rates peaking at approximately 36% in the history match illustrated in , underscores its inadequacy in accurately replicating the experimental results for LSWF in carbonate rocks. This study, therefore, deems this mechanism as an ineffective strategy for modelling. The investigation did not extend to evaluating the influence of LSWF under this geochemical process on the relative permeabilities of oil and water. Nevertheless, it is suggested that the fines migration model may hold greater applicability in scenarios waterflooding processes with a salinity level of 10,000 ppm, where the difference between the model and experimental outcomes is reduced to under 18%, as shown in .
Rock dissolution/precipitation
The results of the LSWF simulation by considering the rock dissolution/precipitation as the governing mechanism are shown in . In this mechanism the ionic strength of the injected water is selected as the interpolation factor and its value is determined utilizing the (3), assigning it a range between 0.2 and 0.9. The TIS, conceptualized as a reflection of the overall salinity level and treated as an independent variable, is adopted as the IF for the purpose of interpolating relative permeability. The outcomes derived from this modelling approach are depicted in , showcasing the effectiveness of the TIS in influencing the simulation’s accuracy.
Figure 9. Modelling results of LSWF using rock dissolution/precipitation (a) SW (b) LoSal-01 (c) LoSal-02 (d) LoSal-03.
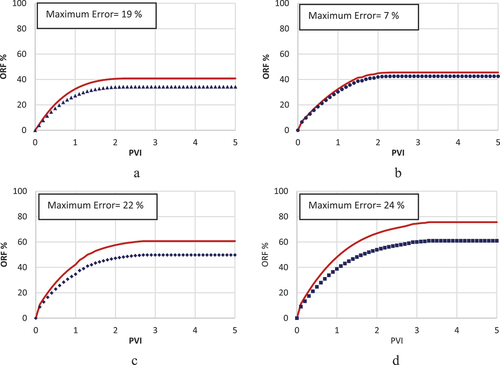
While the rock dissolution/precipitation model demonstrates a markedly improved accuracy over the fines migration model in history matching, it still falls short of being deemed the optimal model for such analysis. This model exhibits efficiency in simulating scenarios with intermediate salinity levels around 10,000 ppm, achieving a relatively low error margin of 7% between the experimental results and the model predictions (). However, its performance diminishes in high-salinity conditions, where the error rate escalates to 19% (), and in scenarios involving low-salinity injected water, with the error increasing to 24% (). The analysis further explores the adjustment of oil and water relative permeability curves, specifically their shift towards higher water saturations on the graph, as an indicator of WA. In this context, depict this shift from an oil-wet state towards a more hydrophilic condition, serving as evidence of WA. This shift is instrumental in understanding the dynamics of fluid flow within the reservoir and the efficiency of the waterflooding process.
Multi-ion exchange results
In this scenario, the MIE and [] are selected as the governing mechanism and IF, respectively. This framework facilitated the most accurate correlation between the model and experimental data, as depicted in . The error percentage in results between the model and experimental observations was approximately 15% at 40 000 and 10 000 ppm salinity levels (), and less than 4% at lower salinities (). The precision of the MIE model substantiates its selection as the dominant geochemical mechanism and [
] as the pertinent IF, establishing it as the optimal modelling approach for LSWF in carbonate reservoirs.
During the MIE analysis, [] was utilized as the IF for deriving relative permeability curves, with the outcomes depicted in . Notably, the crossover point of the oil and water relative permeability curves exhibited a shift towards the right, albeit to a lesser extent than observed in the low-salinity injection studies illustrated in . The most significant modifications were observed in salinity level of 2,000 ppm, thereby affirming the beneficial impact of salinity reduction on enhancing hydrophilicity and WA. A comparative analysis of SW with the brines LoSal_01 to LoSal_03 elucidates a distinctly positive shift in the curve intersection, attributable to the salinity reduction from 40,000 ppm in to 2,000 ppm in .
Conclusions
In this study, we conducted an experimental analysis of LSWF in carbonate reservoirs, investigating its impact on the oil recovery factor and water wetness. Subsequently, we developed a one-dimensional compositional model to accurately simulate this process, considering three distinct geochemical mechanisms namely fines migration, rock dissolution and precipitation, and multi-ion exchange. For each simulation, we selected a specific governing geochemical mechanism and an interpolation factor. Our findings revealed that fines migration alone was insufficient in achieving a precise history match. While rock dissolution/precipitation exhibited improved accuracy by incorporating the ionic strength in its model, it also fell short of meeting the criteria for a recommended LSWF modelling approach. Based on our results, the most effective strategy for enhancing model accuracy in carbonate reservoirs during LSWF is to consider the MIE as the principal mechanism, with sulphate concentration ([SO42-]) as the IF. Additionally, our research confirmed the WA in both experimental section through contact angle measurement, as well as in the modelling section by showing the shift of the relative permeability curves intersection points toward the right side of the Kr-Sw.
The results of this study are highly beneficial for facilitating faster and more accurate simulations of Low Salinity Water Flooding (LSWF) in heterogeneous carbonate reservoirs, thereby enhancing the research capabilities in this field. For future investigations, we recommend the exploration of additional mechanisms and a comprehensive comparison of multiple mechanisms using commercial simulation software. Furthermore, it is strongly advised that each mechanism be evaluated at the core scale utilizing advanced analytical methods such as Computed Tomography (CT) scanning and Nuclear Magnetic Resonance (NMR) techniques. These approaches would provide more detailed insights into the underlying processes and validate the simulation results with experimental data.
Financial interests
The authors declare they have no financial interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasi, S., & Khamehchi, E. (2021). Investigation of permeability decline due to coupled precipitation/dissolution mechanism in carbonate rocks during low salinity co-water injection. Energy Reports, 7, 125–135. https://doi.org/10.1016/j.egyr.2020.11.169
- Adegbite, J. O., Al-Shalabi, E. W., & Ghosh, B. (2017). Modeling the effect of engineered water injection on oil recovery from carbonate cores. http://onepetro.org/SPEOCC/proceedings-pdf/17OCC/2-17OCC/D021S008R008/1310057/spe-184505-ms.pdf/1
- Almasiyan, P., & Mahani, H. (2023). A bond-product-sum (BPS) approach for modeling wettability alteration and enhanced oil recovery by engineered salinity waterflooding in carbonate rocks. Energy & Fuels, 38(6), 5007–5021. https://doi.org/10.1021/acs.energyfuels.3c05055
- Alshakhs, M. J., & Kovscek, A. R. (2015). SPE-175147-MS an experimental study of the impact of injection water composition on oil recovery from carbonate rocks. http://onepetro.org/SPEATCE/proceedings-pdf/15ATCE/3-15ATCE/D031S037R008/1383328/spe-175147-ms.pdf/1
- Al-Shalabi, E. W., & Sepehrnoori, K. (2016). A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. Journal of Petroleum Science & Engineering, 139, 137–161. https://doi.org/10.1016/j.petrol.2015.11.027
- Al-Shalabi, E. W., Sepehrnoori, K., & Delshad, M. (2015a). Numerical simulation of the LSWI effect on hydrocarbon recovery from carbonate rocks. Petroleum Science and Technology, 33(5), 595–603. https://doi.org/10.1080/10916466.2014.1003940
- Al-Shalabi, E. W., Sepehrnoori, K., & Delshad, M. (2015b). Simulation of wettability alteration by low-salinity water injection in waterflooded carbonate cores. Petroleum Science and Technology, 33(5), 604–613. https://doi.org/10.1080/10916466.2014.919005
- Al-Shalabi, E. W., Sepehrnoori, K., Delshad, M., & Pope, G. (2014). SPE-169674-MS a novel method to model low salinity water injection in carbonate oil reservoirs. http://onepetro.org/SPEOGWA/proceedings-pdf/14OGWA/All-14OGWA/SPE-169674-MS/1543424/spe-169674-ms.pdf/1
- Al-Shalabi, E. W., Sepehrnoori, K., & Pope, G. (2014). Mysteries behind the low salinity water injection technique. Journal of Petroleum Engineering, 2014, 1–11. https://doi.org/10.1155/2014/304312
- Al-Shalabi, E. W., Sepehrnoori, K., & Pope, G. (2015a). Geochemical interpretation of low-salinity-water injection in carbonate oil reservoirs. SPE Journal, 20(6), 1212–1226. https://doi.org/10.2118/169101-PA
- Al-Shalabi, E. W., Sepehrnoori, K., & Pope, G. (2015b). New mobility ratio definition for estimating volumetric sweep efficiency of low salinity water injection. Fuel, 158, 664–671. https://doi.org/10.1016/j.fuel.2015.06.018
- Alvarez, A. C., Bruining, J., & Marchesin, D. (2024). Modeling low saline carbonated water flooding including surface complexes. Computational Geosciences, 28(3), 373–393. https://doi.org/10.1007/s10596-024-10274-1
- Amiri, S., & Gandomkar, A. (2019). Influence of electrical surface charges on thermodynamics of wettability during low salinity water flooding on limestone reservoirs. Journal of Molecular Liquids, 277, 132–141. https://doi.org/10.1016/j.molliq.2018.12.069
- Arain, A. H., Negash, B. M., Yekeen, N., Farooqi, A. S., & Alshareef, R. S. (2024). Synergising nanoparticles and low salinity waterflooding for enhanced oil recovery: A state-of-the-art review. Journal of Molecular Liquids, 400, 124495. https://doi.org/10.1016/j.molliq.2024.124495
- Ashraf, A., Hadia, N. J., & Torsaeter, O. (2010). SPE 129012 laboratory investigation of low salinity waterflooding as secondary recovery process: Effect of Wettability. http://onepetro.org/SPEOGIC/proceedings-pdf/10OGIC/All-10OGIC/SPE-129012-MS/1767328/spe-129012-ms.pdf/1
- Austad, T., Shariatpanahi, S. F., Strand, S., Aksulu, H., & Puntervold, T. (2015). Low salinity EOR effects in Limestone Reservoir cores containing anhydrite: A discussion of the chemical mechanism. Energy & Fuels, 29(11), 6903–6911. https://doi.org/10.1021/acs.energyfuels.5b01099
- Bartels, W. B., Mahani, H., Berg, S., & Hassanizadeh, S. M. (2019). Literature review of low salinity waterflooding from a length and time scale perspective. Fuel, 236, 338–353. Elsevier Ltd. https://doi.org/10.1016/j.fuel.2018.09.018
- Bidhendi, M. M., Garcia-Olvera, G., Morin, B., Oakey, J. S., & Alvarado, V. (2018). Interfacial viscoelasticity of crude oil/brine: An alternative enhanced-oil-recovery mechanism in smart waterflooding. SPE Journal, 23(3), 803–818. https://doi.org/10.2118/169127-pa
- Chandrasekhar, S., Sharma, H., & Mohanty, K. K. (2018). Dependence of wettability on brine composition in high temperature carbonate rocks. Fuel, 225, 573–587. https://doi.org/10.1016/j.fuel.2018.03.176
- Compositional, Unconventional & Advanced Processes Simulator. (n.d.). https://www.Cmgl.ca/Solutions/Software/Gem/
- Egbe, D. I. O., Jahanbani Ghahfarokhi, A., Nait Amar, M., & Torsæter, O. (2021). Application of low-salinity waterflooding in carbonate cores: A geochemical modeling study. Natural Resources Research, 30(1), 519–542. https://doi.org/10.1007/s11053-020-09712-5
- Elakneswaran, Y., Takeya, M., Ubaidah, A., Shimokawara, M., Okano, H., & Nawa, T. (2020). Integrated geochemical modelling of low salinity waterflooding for enhanced oil recovery in carbonate reservoir. Paper presented at the International Petroleum Technology Conference, Dhahran, Kingdom of Saudi Arabia, January 2020. https://doi.org/10.2523/IPTC-20250-MS
- Fan, P., Liu, Y., He, Y., Hu, Y., Chao, L., Wang, Y., Liu, L., & Li, J. (2023). Experimental study on the mechanism and law of low-salinity water flooding for enhanced oil recovery in tight sandstone reservoirs. American Chemical Society Omega. https://doi.org/10.1021/acsomega.3c07960
- Fathi, S. J., Austad, T., & Strand, S. (2011). Water-based enhanced oil recovery (EOR) by “smart water”: Optimal ionic composition for EOR in carbonates. Energy & Fuels, 25(11), 5173–5179. https://doi.org/10.1021/ef201019k
- Fredriksen, S. B., Rognmo, A. U., & Fernø, M. A. (2018). Pore-scale mechanisms during low salinity waterflooding: Oil mobilization by diffusion and osmosis. Journal of Petroleum Science & Engineering, 163, 650–660. https://doi.org/10.1016/j.petrol.2017.10.022
- Gandomkar, A., Ghorbani Sheykhneshin, M., Nasriani, H. R., Yazdkhasti, P., & Safavi, M. S. (2022). Enhanced oil recovery through synergy of the interfacial mechanisms by low salinity water alternating carbon dioxide injection. Chemical Engineering Research & Design, 188, 462–472. https://doi.org/10.1016/j.cherd.2022.09.053
- Gandomkar, A., & Rahimpour, M. R. (2015). Investigation of low-salinity waterflooding in secondary and tertiary enhanced oil recovery in limestone reservoirs. Energy & Fuels, 29(12), 7781–7792. https://doi.org/10.1021/acs.energyfuels.5b01236
- Gandomkar, A., & Rahimpour, M. R. (2017). The impact of monovalent and divalent ions on wettability alteration in oil/low salinity brine/limestone systems. Journal of Molecular Liquids, 248, 1003–1013. https://doi.org/10.1016/j.molliq.2017.10.095
- Hussain, F., Zeinijahromi, A., Bedrikovetsky, P., Badalyan, A., Carageorgos, T., & Cinar, Y. (2013). An experimental study of improved oil recovery through fines-assisted waterflooding. Journal of Petroleum Science & Engineering, 109, 187–197. https://doi.org/10.1016/j.petrol.2013.08.031
- iptc-20250-ms. (n.d.).
- Jalilian, M., Pourafshary, P., Sola, B. S., & Kamari, M. (2017). Optimization of smart water chemical composition for carbonate rocks through comparison of active cations performance. Journal of Energy Resources Technology, Transactions of the ASME, 139(6). https://doi.org/10.1115/1.4037215
- Jerauld, G. R., Lin, C. Y., Webb, K. J., & Seccombe, J. C. (2008). Modeling Low-Salinity Waterflooding. http://onepetro.org/REE/article-pdf/11/06/1000/2550514/spe-102239-pa.pdf/1
- Kalantariasl, A., Tale, F., Parsaei, R., Keshavarz, A., Jahanbakhsh, A., Mercedes Maroto-Valer, M., & Mosallanezhad, A. (2022). Optimum salinity/composition for low salinity water injection in carbonate rocks: A geochemical modelling approach. Journal of Molecular Liquids, 362, 119754. https://doi.org/10.1016/j.molliq.2022.119754
- Katende, A., & Sagala, F. (2019). A critical review of low salinity water flooding: Mechanism, laboratory and field application. Journal of Molecular Liquids, 278, 627–649. Elsevier B.V. https://doi.org/10.1016/j.molliq.2019.01.037
- Kazemi, A., Korrani, N., Fu, W., Sanaei, A., & Sepehrnoori, K. (2015). SPE-175102-MS mechanistic modeling of modified salinity waterflooding in carbonate reservoirs. http://onepetro.org/SPEATCE/proceedings-pdf/15ATCE/2-15ATCE/D021S026R001/1382657/spe-175102-ms.pdf/1
- Kazemi Nia Korrani, A., Sepehrnoori, K., & Delshad, M. (2015). Coupling IPhreeqc with UTCHEM to model reactive flow and transport. Computers & Geosciences, 82, 152–169. https://doi.org/10.1016/j.cageo.2015.06.004
- Khaksar Manshad, A., Olad, M., Taghipour, S. A., Nowrouzi, I., & Mohammadi, A. H. (2016). Effects of water soluble ions on interfacial tension (IFT) between oil and brine in smart and carbonated smart water injection process in oil reservoirs. Journal of Molecular Liquids, 223, 987–993. https://doi.org/10.1016/j.molliq.2016.08.089
- Korrani, A. K. N., & Jerauld, G. R. (2019). Modeling wettability change in sandstones and carbonates using a surface-complexation-based method. Journal of Petroleum Science & Engineering, 174, 1093–1112. https://doi.org/10.1016/j.petrol.2018.12.016
- Kumar Saw, R., & Mandal, A. (2023). Experimental investigation on fluid/fluid and rock/fluid interactions in enhanced oil recovery by low salinity water flooding for carbonate reservoirs. Fuel, 352, 352. https://doi.org/10.1016/j.fuel.2023.129156
- Liu, Y., Kaszuba, J., & Oakey, J. (2019). Microfluidic investigations of crude oil-brine interface elasticity modifications via brine chemistry to enhance oil recovery. Fuel, 239, 338–346. https://doi.org/10.1016/j.fuel.2018.11.040
- Lyu, C., Zhong, L., Ning, Z., Chen, M., & Cole, D. R. (2022). Review on underlying mechanisms of low salinity waterflooding: Comparisons between Sandstone and carbonate. Energy & Fuels, 36(5), 2407–2423. American Chemical Society. https://doi.org/10.1021/acs.energyfuels.1c04248
- Magzymov, D., Purswani, P., Karpyn, Z. T., & Johns, R. T. (2021). Modeling the effect of reaction kinetics and dispersion during Low-Salinity Waterflooding. SPE Journal, 26(5), 3075–3093. https://doi.org/10.2118/193909-PA
- Mahani, H., Keya, A. L., Berg, S., Bartels, W. B., Nasralla, R., & Rossen, W. R. (2015). Insights into the mechanism of wettability alteration by low-salinity flooding (LSF) in carbonates. Energy & Fuels, 29(3), 1352–1367. https://doi.org/10.1021/ef5023847
- Mahani, H., Menezes, R., Berg, S., Fadili, A., Nasralla, R., Voskov, D., & Joekar-Niasar, V. (2017). Insights into the impact of temperature on the wettability alteration by low salinity in carbonate rocks. Energy & Fuels, 31(8), 7839–7853. https://doi.org/10.1021/acs.energyfuels.7b00776
- Mazinani, S., Farhadi, H., & Fatemi, M. (2023). Experimental and theoretical investigation of the impact of crude-oil on the wettability behavior of calcite and silicate due to low salinity effect. Fuel, 349. https://doi.org/10.1016/j.fuel.2023.128608
- Puntervold, T., Strand, S., Ellouz, R., & Austad, T. (2015). Modified seawater as a smart EOR fluid in chalk. Journal of Petroleum Science & Engineering, 133, 440–443. https://doi.org/10.1016/j.petrol.2015.06.034
- Romero, M. I., Gamage, P., Jiang, H., Chopping, C., & Thyne, G. (2013). Study of low-salinity waterflooding for single- and two-phase experiments in Berea sandstone cores. Journal of Petroleum Science & Engineering, 110, 149–154. https://doi.org/10.1016/j.petrol.2013.08.050
- Sagbana, P. I., Sarkodie, K., & Nkrumah, W. A. (2023). A critical review of carbonate reservoir wettability modification during low salinity waterflooding. Petroleum, 9(3), 317–330. KeAi Communications Co. https://doi.org/10.1016/j.petlm.2022.01.006
- Sanaei, A., Tavassoli, S., & Sepehrnoori, K. (2019). Investigation of modified water chemistry for improved oil recovery: Application of DLVO theory and surface complexation model. Colloids and Surfaces: A, Physicochemical and Engineering Aspects, 574, 131–145. https://doi.org/10.1016/j.colsurfa.2019.04.075
- Sandengen, K., Kristoffersen, A., Melhuus, K., & Jøsang, L. O. (2016). Osmosis as mechanism for low-salinity enhanced oil recovery. SPE Journal, 21(4), 1227–1235. https://doi.org/10.2118/179741-pa
- Shalabi, E. W. A., Sepehrnoori, K., & Delshad, M. (2014). Mechanisms behind low salinity water injection in carbonate reservoirs. Fuel, 121, 11–19. https://doi.org/10.1016/j.fuel.2013.12.045
- Strand, S., Standnes, D. C., & Austad, T. (2006). New wettability test for chalk based on chromatographic separation of SCN– and SO42–. Journal of Petroleum Science & Engineering, 52(1–4), 187–197. https://doi.org/10.1016/j.petrol.2006.03.021
- Tripathi, I., & Mohanty, K. K. (2008). Instability due to wettability alteration in displacements through porous media. Chemical Engineering Science, 63(21), 5366–5374. https://doi.org/10.1016/j.ces.2008.07.022
- Wu, Y.-S. (2002). SPE 118830 efficient simulation for low-salinity waterflooding in porous and fractured reservoirs. http://onepetro.org/spersc/proceedings-pdf/09RSS/All-09RSS/SPE-118830-MS/2721247/spe-118830-ms.pdf/1
- Yousef, A. A., Al-Saleh, S., Al-Kaabi, A., Al-Jawfi, M., & Aramco, S. (2010). CSUG/SPE 137634 laboratory investigation of novel oil recovery method for carbonate reservoirs. http://onepetro.org/SPEURCC/proceedings-pdf/10CURIPC/All-10CURIPC/SPE-137634-MS/1753384/spe-137634-ms.pdf/1
- Zhang, P., Tweheyo, M. T., & Austad, T. (2006). Wettability alteration and improved oil recovery in chalk: The effect of calcium in the presence of sulfate. Energy & Fuels, 20(5), 2056–2062. https://doi.org/10.1021/ef0600816