Abstract
Lactarius and Lactifluus are milkcaps that are characterized by the secretion of latex. These two genera are part of a globally distributed cosmopolitan group of ectomycorrhizal fungi that is an important food resource in various ecosystems. Recently, the taxonomy of Lactarius and Lactifluus has been revised based on molecular phylogenetics. Despite the importance of these taxa, Korean species of both genera are poorly understood. In an effort to describe milkcap species that are indigenous to Korea, a long-term study has been initiated. During a recent survey, eight species of milkcaps that were previously unrecorded in Korea were detected based on morphological observation and sequence analysis of the internal transcribed spacer region: five Lactarius species (Lactarius atromarginatus, L. austrotorminosus, L. kesiyae, L. tabidus, and L. vietus) and three Lactifluus species (Lactifluus acicularis, Lf. pilosus, and Lf. pinguis). Detailed morphological descriptions and phylogenetic relationships of these species are provided in this article.
1. Introduction
The term “milkcap” describes a genus of mushroom-forming fungi called Lactarius Pers. because of its latex production, and over 600 milkcap species have been reported worldwide [Citation1]. Lactarius was one of the two major groups treated in the family Russulaceae (together with a larger genus called Russula Pers.) [Citation2,Citation3]. This genus is easily differentiated from its sister genus by the presence of latex and lactifer networks in subhymenium [Citation4,Citation5]. In lamellar trama, most Lactarius species have less complex sphaerocytes than those in Russula species [Citation5]. Lactarius plays a critical ecological role in terrestrial ecosystems through ectomycorrhizal symbiotic relationships with various plants [Citation6,Citation7]. The basidiomata of many milkcap species as an important nutrient source for insects [Citation8] and other animals including humans [Citation9,Citation10]. Some species of Lactarius have been studied in order to investigate the mechanisms for natural rubber synthesis [Citation11].
In the last decade, the generic landscape of the family Russulaceae has changed. Four phylogenetic lineages have been found in this family and milkcap species appear to have evolved in three lineages [Citation12]. Consequently, two additional genera have been defined: Multifurca Buyck & V. Hofst. and Lactifluus (Pers.) Roussel. Two species previously placed in the Lactarius were actually shown to be representatives of the Multifurca: M. furcata [Citation12] and M. stenophylla [Citation13]. Species in another clade within the genus Lactarius (approximately 20% of species) were transferred to the genus Lactifluus [Citation14–16]. The majority of species (approximately 80% of species) remain in the larger clade, Lactarius [Citation17]. Although Lactarius can clearly be distinguished from Multifurca and Lactifluus using molecular sequence data, there are no absolute morphological characters to distinguish species in these genera. Some morphological characters seem common in all or at least two of the three genera. For example, the pileal surface of Lactarius can be viscid or lubricous when moist, often with distinctive zonation, and bearded at the pileus margins. Scrobiculate caps and stipes only occur in Lactarius. Many Lactifluus species generally have pileipellis and stipellis with thick-walled elements, as well as lamprocystidia and only few Lactarius species have these characteristics. A hymenophoral trama consists of sphaerocytes (as in the genus Russula) is general in the genus Lactifluus but is rarely found in Lactarius [Citation18].
Although milkcap species are easy to recognize at the genus level because of their latex exuding fruiting bodies, identification at the species level is difficult due to extensive morphological variations and highly similar morphological features among closely related species. Recently, DNA sequence analysis has improved the precision of species identification in milkcaps. The internal transcribed spacer (ITS) region [Citation19] has been widely used in the identification of new species [Citation20,Citation21] and to delimitate among closely related species in Korea [Citation21,Citation22]. Recently, The National Institute of Biological Resources has organized a project that investigates diversity of Korean indigenous Lactarius species (NIBR, http://www.nibr.go.kr). In this study, we were able to identify eight unrecorded milkcap species based on ITS sequence analysis. Here, we provide detailed morphological descriptions and phylogenetic support for each species.
2. Materials and methods
2.1. Sampling and morphological study
A total of 20 specimens were used in this study (). These samples have obtained from three herbaria in South Korea: the Seoul National University Fungus Collection (SFC) herbarium, Kangwon National University (TPML), and the National Institute of Agricultural Sciences (HCCN). Fruiting bodies in these herbaria were collected from various regions across the Korean peninsula and annexed islands from 1993 to 2016. Specimen information, such as collection date, site, composition of forest, and photographs were obtained from each herbarium. Specimen were putatively identified using field guides [Citation3,Citation5,Citation23] and the Russulales News website (http://www.mtsn.tn.it/russulales-news/). For terminology of macro-morphology, we followed Verbeken and Walleyn [Citation24]. The Methuen Handbook of Color [Citation25]was used as the color standard for description of specimens.
Table 1. Sequenced specimens used in this study, with GenBank accession numbers for the ITS.
In order to observe microscopic features, specimens were rehydrated in 5% (w/v) KOH and stained with 1% (w/v) congo red. Basidiospores were observed and measured in Melzer’s reagent. All measurements of basidiospores and hymenial elements (e.g., Basidia, cystidia, and marginal cells) were performed using a Nikon Eclipse 80i optical microscope (Nikon, Japan) at 40X magnification. Forty basidiospores were measured for each collection and 95% limits were calculated. The height of basidiospore ornamentation was measured separately. The ratio of basidiospore (length/width) was calculated which inferred Q-values. Both basidiospore sizes and Q-value were calculated with 0.1 precision. Basidiospore ornamentation of all collections was observed using the same microscope at 100X magnification. For scanning electron microscope (SEM) analysis, an EM ACE200 platinum coater (Leica, Austria) was used to preserve a piece of lamellae on the surface of an SEM sample stub. All SEM photos were produced using a SUPRA 55VP scanning electron microscope (Carl Zeiss, Germany) at 5000× or 10,000× magnification.
2.2. DNA extraction, PCR amplification, and sequencing
Genomic DNA was extracted from sections of fresh or dried fruiting bodies by using a modified version of the CTAB DNA extraction method described by Rogers and Bendich [Citation26]. ITS regions were amplified using several combinations of primer sets: forward primers ITS1F or ITS5 [Citation27] and reverse primers ITS4B or Russ3R [Citation23,28]. PCR amplification of the ITS was performed as previously described by Park and Lee [Citation23]. PCR products were purified with the Expin PCR Purification Kit (GeneAll Biotechnology, Seoul, Korea) according to the user’s manual. Finally, DNA sequencing was performed by Macrogen (Seoul, South Korea), using the same set of primers for each locus, on an ABI3730 automated DNA Sequencer.
2.3. Sequence alignment and phylogenetic analyses
The ITS sequences were assembled and edited with MEGA version 6 (Pennsylvania State University, State College, PA) [Citation29]. For phylogenetic analyses of Lactarius and Lactifluus, the generated ITS sequences were aligned with those of 17 taxa of Lactarius and Lactifluus from published data. Two representatives of Multifurca were used as an out-group. All ITS sequences generated in this study were deposited in GenBank (). Sequence alignment was performed separately using the online MAFFT version 7 program (CBRC, Tokyo, Japan) [Citation30] with the default settings and edited manually using MEGA version 6 (Pennsylvania State University, State College, PA). Maximum likelihood (ML) analyses were performed with RAxML version 7.03(Bioinformatics Institute, Singapore) [Citation31] using the GTR + G model and 1000 bootstrap replicates. jModelTest version 2.1.7 (National Academy of Sciences, Washington, DC) [Citation32] was used to decide the model of character evolution. Bayesian inference (BI) analyses were executed with MrBayes on XSEDE version 3.2.6 [Citation33] using the HKY + I+G model. The analysis was performed with four independent runs, each with four chains. Each run had 20 million generations and the sampling frequency was set to 100. All phylogenetic analyses were performed on the CIPRES Science Gateway [Citation34].
3. Results
3.1. Phylogenetic analyses
The dataset used in this study contained 58 Russulaceae collections, of which 32 and 24 belonged to the genus Lactarius and Lactifluus, respectively. The ITS dataset consisted of a 770 bp alignment. Tree topology obtained from ML and BI analyses were similar, with slight variation in nodal support (support: ML > 70, BI > 0.95) (). The genera Lactarius (99/1) and Lactifluus (91/0.98) were well supported. Twenty specimens were conspecific to five and three species of Lactarius and Lactifluus, respectively. ITS sequences of each species had more than 98.2% sequence similarity with reference sequences on Genbank. Each species was well supported by both ML bootstrap values and BI posterior probabilities.
Figure 1. Phylogram generated from maximum likelihood analysis based on ITS sequence data of milkcaps. Branch support values are given as maximum likelihood bootstrap values >70 and Bayesian Inference posterior probabilities >0.95 are shown. The scale bar indicates the number of nucleotide substation per site. Superscript T indicates the holotype specimen of each species.
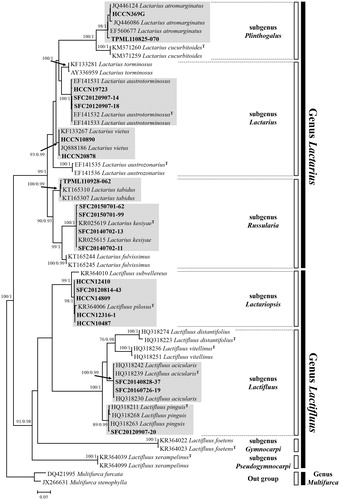
For Lactarius, two specimens (HCCN369G and TPML110825-070) grouped into in a well-supported clade (98/1) with Chinese, Japanese, and Thai samples that were denoted as Lactarius atromarginatus in L. subg. Plinthogalus. Three samples (HCCN19723, SFC20120907-14, and SFC20120907-18) and two collections (HCCN10890 and HCCN20878) matched with L. austrotorminosus (100/1.0) and European L. vietus (93/0.99), respectively. Five specimens grouped with L. fulvissimus, L. kesiyae, and L. tabidus within L. subg. Russularia. Four of these (SFC20140702-11, SFC20140702-13, SFC20150701-62, and SFC20150701-99) formed a monophyletic group with L. kesiyae from Vietnam and Thailand (100/1.0). TPML110928-062 was conspecific with Swedish and Canadian specimens identified as L. tabidus (100/0.99).
For Lactifluus, eight samples were conspecific with three Thai Lactifluus species, Lf. acicularis, Lf. Pilosus, and Lf. pinguis. Five specimens (HCCN10487, HCCN12316-1, HCCN12410, HCCN14809, and SFC20140702-13) matched to the type specimen of Lf. pilosus (LTH205) (98/1) which belongs to Lf. subg. Lactariopsis. Two specimens (SFC20140828-37 and SFC20160726-19) grouped with the holotype of Lf. acicularis (KVP08-029) (100/0.99). One specimen (SFC20120907-20) was conspecific with Lf. pinguis (89/0.99). These two species are members of Lf. subg. Lactifluus.
3.2. Taxonomy
Lactarius atromarginatus Verbeken & E. Horak, Aust. Syst. Bot. 13(5): 688 (2000) (, and )
Figure 2. Fruiting bodies of eight milkcap species. (A) Lactarius atromarginatus, (B) L. austrominosus. (C) L. kesiyae, (D) L. tabidus, (E) L. vietus, (F) Lactifluus acicularis, (G) Lf. pilosus, and (H) Lf. pinguis. Scale bar =1 cm.

Figure 3. SEM photos of basidiospores. (A) Lactarius atromarginatus, (B) L. austrotorminosus, (C) L. kesiyae, (D) L. tabidus, (E) L. vietus, (F) Lactifluus acicularis, (G) Lf. pilosus, and (H) Lf pinguis. Scale bar =5 μm.
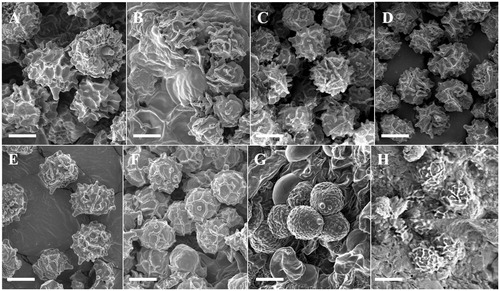
Figure 4. Microscopic features of eight milkcap species. (A) Lactarius atromarginatus, (B) L. austrotorminosus, (C) L. kesiyae, (D) L. tabidus, (E) L. vietus, (F) Lactifluus acicularis, (G) Lf. pilosus, and (H) Lf pinguis. Abbreviation in figure: ba: basidia; cla: cheilolamprocystidia; cle:cheiloleptocystidia; cmc: cheilomacrocystidia; mc: marginal cells; pc: pseudocystidia; plc: pleurolamprocystidia; pmc: pleuromacrocystidia.
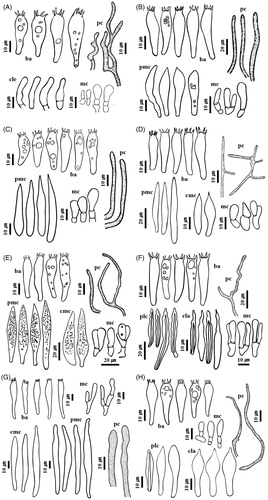
Pileus 25–50 mm, planoconvex when young, later applanate and depressed in the center to infundibuliform with central umbo; surface dry, rugose, not viscid when moist, dark brown to yellowish brown, and paler toward margin. Lamellae adnate to subdecurrent, rather close, white to pale yellow, with abundant lamellulae, and staining purplish grey when bruised; with abundant lamellulae. Stipe 30–60 × 5–8 mm, cylindrical, yellowish brown to pale brown, and paler toward base. Latex watery white, abundant, and turning pale lilac. Basidiospores 7.2–10.6 × 7.2–10.0 μm, Q = 1.0–1.1, and globose to subglobose; ornamentation mostly up to 1.2 μm high, consisting of amyloid ridges which form a complete reticulum. Basidia 46.8–65.0 × 11.0–14.8 μm and subclavate. Pleuromacrocystidia absent. Cheiloleptocystidia and pseudocystidia abundant.
Specimens examined: Korea, Chungcheongbuk-do, Boeun-gun, Mt. Songni. 36°29′28″ N, 127°48′16″ E, August 25 2011, Nam Kyu Kim, TPML110825-070; on the ground of Quercus forest. Korea, Gyeonggi-do, Yangpyeong-gun, Mt. Yongmun. 37°33′15″ N, 127°34′10″ E, July 27 1993, Soon Ja Seok, HCCN369G; on the ground of mixed forest.
Remarks: L. atromarginatus seems to be a widely distributed species in Asia. It was first described from Papua New Guinea [Citation35] and later in China, Japan, and Thailand [Citation16,Citation36]. According to the original description of the species [Citation35], the umber brown pileus with wrinkled surface, pinkish, and lilac discoloration of context and latex and subreticulate basidiospores allow for characterizing of this species. This is the only species with lilac discoloration of latex belonging to the subgenus Plinthogalus in Korea.
Lactarius austrotorminosus H.T. Le & Verbeken, Fungal Diversity 24: 198 (2007) (, and )
Pileus 25–70 mm, convex when young, later applanate and depressed in the center to almost infundibuliform; margin incurved with numerous hairs; surface scaly, zonate, with rings of hairs, greyish orange to pink, greyish brown. Lamellae cream to pale yellow, subdecurrent, crowded, with abundant with abundant lamellulae. Stipe 25–65 × 5–15 mm, cylindrical, white, pale orange to brown toward the base, discoloring orange to brown if bruised, becoming hollow in age, and sometimes scrobiculate. Latex white and unchanging. Basidiospores 6.3–8.0 × 4.5–7.1 μm, Q = 1.05–1.25, subglobose to broadly ellipsoid; ornamentation amyloid, mostly up to 1.2 μm high, consisting of ridges which form incomplete reticulum. Basidia 36.1–62.3 × 7.8–9.6 μm and cylindrical to slightly clavate. Pleuromacrocysti-dia 34.9–71.4 × 6.9–11.2 μm, fusiform, apically constricted, and abundant. Cheilomacrocystidia absent. Pseudocystidia abundant.
Specimens examined: Korea, Jeju-do, Seogwipo-si, Donnaeko campsite. 33°18′01″ N, 126°35′03″ E, September 7 2012, Hyun Lee, SFC20120907-14; on the ground of evergreen Quercus and Castanopsis forest. Korea, Jeju-do, Seogwipo-si, Donnaeko campsite. 33°18′03″ N, 126°35′04″ E, September 7 2012, Hyun Lee, SFC20120907-18; on the ground of evergreen Quercus and Castanopsis forest. Korea, Jeju-do, Seogwipo-si, Namwon-eup. 33°20′09″ N, 126°36′40″ E, July 14 2010, Soon Ja Seok, HCCN19723; On the ground of broad-leaved forest.
Remarks: L. austrotorminosus was originally described in broad-leaf forests in Thailand that were dominated by Fagaceae trees with few Dipterocarpus and Pinus trees [Citation37]. To date, this species was only found on Jeju Island. This species is easily recognized in the field because of it has pinkish orange colors, strikingly hairy, and zonate pileus, and involute margin. Under the microscope, incompletely reticulate basidiospores and the absence of cheilocystidia are useful characteristics to recognize this species.
Lactarius kesiyae Verbeken & K.D. Hyde, Phytotaxa. 207(3): 215–241 (2015) (, and )
Pileus 25–50 mm diameter, convex when young, later applanate and depressed in the center to infundibuliform; margin pectinated with age; surface smooth, slightly viscid when moist, and dark blond to pale orange or pale greyish brown. Lamellae subdecurrent, ivory to cream, apricot when bruised, crowded, and with abundant lamellulae. Stipe 35–60 × 5–10 mm, cylindrical, cream to pale apricot, and hollow. Latex watery white, abundant, and unchanging on exposure. Basidiospores 6.0–7.8 × 5.4–7.0 μm, Q = 1.0–1.2, globose to broadly ellipsoid; ornamentation amyloid, mostly up to 1.2 μm high, consisting of isolated warts and ridges which form an incomplete reticulum. Basidia 30.7–45.0 × 6.7–10.7 μm, clavate. Pleuromacrocystidia 42.1–73.8 × 4.1–7.9 μm and subulate. Lamellar edge fertile. Cheilomacrocystidia 40.9–50.4 × 5.7–7.5 μm and fusiform to subulate. Pseudocystidia abundant.
Specimens examined: Korea, Jeju-do, Seogwipo-si, Andeok valley. 33°15′25″ N, 126°21′08″ E, July 2 2014, Hyun Lee, SFC20140702-11; on the ground of evergreen trees and Pinus densiflora forest. Korea, Jeju-do, Seogwipo-si, Andeok valley. 33°15′25″ N, 126°21′12″ E, July 2 2014, Hyun Lee, SFC20140702-13; on the ground of Pinus densiflora forest. Korea, Jeju-do, Dongbaekdongsan. 33°30′51″ N, 126°32′08″ E, July 1 2015, Hyun Lee, SFC20150701-62; on the ground of evergreen trees and Pinus densiflora forest. Korea, Jeju-do, Dongbaekdongsan. 33°30′57″ N, 126°42′50″ E, July 1 2015, Hyun Lee, SFC20150701-99; On the ground of evergreen trees and Pinus densiflora forest.
Remarks: Lactarius kesiyae is distinguished by its sticky and glossy appearance in moist conditions, its pale brownish grey to pale brownish orange color and the pectinate pileus margin. The latex is watery white and turns yellow on white tissue. Microscopically, pleuromacrocystidia are very long (up to 100 μm long in the original description), protruding from the hymenium. This species was originally described growing with Pinus kesiya in Thailand and Vietnam [Citation38]. However, specimens collected in Korea were found in mixed forests where evergreen broad-leaved trees and Pinus densiflora grow together. This species was found only on Jeju Island in Korea.
Lactarius tabidus Fr., Epicr. syst. mycol. (Upsaliae): 346 (1838) [1836–1838] (, and )
Pileus 15–50 mm diameter, convex when young, later applanate and depressed in the center, sometimes with central umbo; surface even when young, slightly venose toward the center in age, sometimes with wrinkled margin, yellowish brown to greyish orange brown, and sometimes hygrophanous. Lamellae adnexed, rather crowded, cream to pale pink, sometimes forked, and with abundant lamellulae. Stipe 20–70 × 5–10 mm, cylindrical, sometimes clavate, and clay pink to greyish orange brown. Latex white to cream and slightly turning pale yellow. Basidiospores 6.0–7.8 × 5.4–7.0 μm, Q = 1.0–1.2, globose to broadly ellipsoid; ornamentation mostly up to 1.3 μm high, consisting of isolated amyloid warts and ridges which form an incomplete reticulum. Basidia 30.7–45.0 × 6.7–10.7 and clavate. Pleuromacrocystidia 34.3–84.2 × 5.6–10.4 μm, fusiform, and rare. Cheilomacrocystidia 27.3–52.0 × 4.7–8.7 μm, fusiform to subulate, and abundant.
Specimens examined: Korea, Gangwon-do, Taebaek-si, Mt. Hambaek, 37°09′42″ N, 128°55′03″ E, September 29 2011, Nam Kyu Kim, TPML110928-062; on the ground of Betula and Sorbus forest.
Remarks: The most distinctive character of Lactarius tabidus is a hygrophanous cap margin when moist. Basidiospores with acute warts were observed in European specimens [Citation5]; however, basidiospores of Korean samples have more interconnection in ornamentation (). The fruiting body of L. tabidus, with its dull brownish colors, is easily confused with L. subdulcis. The unchanging latex and the presence with Fagus trees can help to discriminate this species from L. tabidus.
Lactarius vietus (Fr.) Fr., Epicr. syst. mycol. (Upsaliae): 344 (1838) [1836–1838] (, and )
Pileus 20–75 mm diameter, planoconvex when young, later applanate and depressed in the center to infundibuliform; azonate to indistinctly greyish zonate near cap margin, surface finely rugulose, and slightly lubricous when moist. Lamellae adnate to subdecurrent, white to cream, rather crowded, sometimes forked, and with abundant lamellulae. Stipe 22–65 × 7–18 mm, cylindrical; surface even, often a narrow pale zone under the gills, and pale cream to olivaceous buff; context hollow in age. Latex white and drying greenish grey. Basidiospores 6.4–9.2 × 5.4–7.6 μm, Q = 1.0–1.3, globose to broadly ellipsoid; ornamentation mostly up to 1.5 μm high, consisting of some isolated amyloid warts and ridges which forms a nearly complete reticulum. Basidia 38.8–50.3 × 8.6–11.4 μm, and clavate. Pleuromacrocystidia 58.8–108.3 × 8.9–13.4 μm, fusiform, and abundant. Cheilomacrocystidia 41.8–55.3 × 9.1–14.2 μm, fusiform, and abundant.
Specimens examined: Korea, Gangwon-do, Inje-gun, Baekdam-sa temple, 38°09′53″ N, 128°22′26″ E, October 14 2010, Soon Ja Seok, HCCN20878; on the ground of Betula and Quercus forest. Korea, Gangwon-do, Wonju-si, Mt. Chiak, 37°23′41″ N, 128°03′07″ E, October 10 2002, Soon Ja Seok, HCCN10890; on the ground of Betula, Pinus and Quercus forest.
Remarks: The pale greyish brown cap, the milk, which turns greenish grey, pinkish buff gills, pale zone at the top of stipe and subreticulate basidiospores are useful in delimitation of this species. L. glyciosmus also has a greyish brown cap and pinkish buff gills but this species has unchanging latex, smaller basidiospores than L. vietus and non-gelatinous cap surface.
Lactifluus acicularis (Van de Putte & Verbeken) Van de Putte, Mycotaxon 120: 444 (2012) (, and )
Pileus 40–70 mm, convex when young, later applanate and depressed in the center to infundibuliform; surface dry, rugose, pruinose, yellowish brown to reddish brown, paler toward the margin. Lamellae subdecurrent to decurrent, rather crowded, and staining brown to greyish brown when bruised; lamellulae of different lengths abundant. Stipe 46–75 × 5–12 mm, cylindrical to subclavate; surface dry, pruinose, longitudinally rugulose, and concolorous with pileus. Latex white, sticky, and slowly changing brownish within few minutes to half an hour. Basidiospores 7.0–9.0 × 6.5–8.0 μm, Q = 1.0–1.2, globose to subglobose; ornamentation amyloid, mostly up to 1.3 μm high, consisting of some isolated warts and ridges which form a nearly complete reticulum. Basidia 41.8–62.0 × 9.2–11.8 μm, clavate. Pleurolamprocystidia 43.7–91.4 × 5.8–10.8 μm, fusiform, and abundant. Lamella edge mixed. Cheilolamprocystidia 25.1–63.6 × 4.2–7.8 μm, fusiform, and abundant.
Specimens examined: Korea, Chungcheongnam-do, Cheonan-si, Mt. Gwangdeok, 36°40′06″ N, 127°01′34″ E, August 28 2014, Young Woon Lim, SFC20140828-37; on the ground of Acer, Pinus densiflora and Quercus forest. Korea, Incheon-si, Ongjin-gun, Jangbong island, 37°32′21″ N, 126°20′12″ E, July 26 2016, Jae Young Park, SFC20160726-19; on the ground of Pinus rigida and Quercus forest.
Remarks: Lactifluus acicularis and Lf. volemus shared some characteristics, such as the very long pileipellis hairs, the brown to reddish-brown pileus color and basidiospores with an almost complete reticulum [Citation39]. Korean collections were often identified as Lactifluus volemus. However, they can be distinguished from Lf. volemus by the average size of basidiospores. Basidiospores of Lactifluus volemus (9.0–10.0 × 8.4–9.3 μm) are much larger than that of Lf. acicularis [Citation40].
Lactifluus pilosus (Verbeken, H.T. Le & Lumyong) Verbeken, Mycotaxon 102: 287 (2007) (, and )
Pileus 80–170 mm, convex when young, later infundibuliform and deeply depressed in the center, sometimes with wavy margin; surface dry, velvety, often rugulose near the margin, whitish to pale yellow, and with orange to brownish spots in age. Lamellae decurrent, distant, cream to greyish cream, turning orange brown when bruised, and with abundant lamellulae of different lengths. Stipe 10–45 × 10–25 mm, cylindrical to slightly tapering downwards, central, sometimes eccentric; surface dry, velvety, and concolorous with pileus. Latex white which changes to pale yellow when dried. Basidiospores 7.0–8.2 × 5.6–7.2 μm, Q = 1.0–1.3, globose to ellipsoidal; ornamentation amyloid, mostly up to 0.7 μm high, and consisting of ridges which form an incomplete reticulum; sometimes isolated ridges present; isolated warts present. Basidia 55.3–71.4 × 8.0–11.3 μm and subclavate. Pleuromacrocystidia 91.1–105.2 × 7.2–9.8 μm, subfusiform to clavate, and abundant. Lamella edge sterile. Cheilomacrocystidia 63.7–74.9 × 5.5–7.8 μm, narrowly clavate, and abundant.
Specimens examined: Korea, Gyeongsangbuk-do, Ulleung-gun, Ulleung island, Mt. Seonginbong, 37°30′35″ N, 130°51′36″ E, August 14 2012, Young Woon Lim, SFC20120814-43; On the ground of Pinus densiflora and Acer okamotoanum forest. Korea, Korea, Gangwon-do, Inje-gun, Baekdam-sa temple, 38°12′03″ N, 128°19′21″ E, August 16 2002, Soon Ja Seok, HCCN10487; on the ground of Pinus densiflora and Quercus forest. Korea, Gangwon-do, Pyeong Chang-gun, Woljeong-sa temple, 37°43′37″ N, 128°35′47″ E, August 30 2004, Soon Ja Seok, HCCN12316-1; on the ground of Abies holophylla, Pinus densiflora and Acer pseudosieboldianum forest. Korea, Gangwon-do, Pyeong Chang-`gun, Sangwon-sa temple, 37°47′06″ N, 128°34′24″ E, August 31 2004, Soon Ja Seok, HCCN12410; on the ground of Abies holophylla and Pinus densifolia forest. Korea, Gangwon-do, Pyeong Chang-gun, Woljeong-sa temple, 37°43′24″ N, 128°35'49'' E, September 20 2006, Soon Ja Seok, HCCN14809; on the ground of mixed forest.
Remarks: These Korean specimens were initially misidentified as Lactifluus vellereus, however, Lf. vellereus differs from Lf. pilosus by having unchanging latex, medium crowded gills, and larger basidiospores with very low ridges (0.2 µm) forming a complete reticulum [Citation41]. The other European species, Lf. bertillonii is also similar but this species can be distinguished by the unchanging latex, medium crowded lamellae, and larger basidiospores with incomplete reticulum [Citation5].
Lactifluus pinguis (Van de Putte & Verbeken) Van de Putte, Mycotaxon 120: 444 (2012) (, and )
Pileus 40–80 mm, convex when young, later applanate and deeply depressed in the center, sometimes with margin wavy in age; surface dry, rugulose when young, pale yellow to wheat straw, and partly pale pink or pale orange when young. Lamellae decurrent, crowded, white to cream, staining pale brown when bruised, and with abundant lamellulae of different lengths. Stipe 45–90 × 8–12 mm, cylindrical to slightly tapered toward pileus, sometimes eccentric; surface dry, and concolorous with pileus. Latex white, sticky, and slowly changing pale brownish. Basidiospores 8.0–9.7 × 7.5–9.2 μm, Q = 1.0–1.15, globose to subglobose; ornamentation amyloid, mostly up to 2.0 μm high, consisting of ridges which form a complete reticulum; sometimes isolated ridges present; isolated warts rare. Basidia 42.2–63.0 × 11.0–13.6 μm and subclavate. Pleurola-mprocystidia 55.2–75.3 × 8.1–12.9 μm, fusiform to clavate, and abundant. Lamella edge mixed. Cheilolamprocystidia 35.2–61.0 × 9.5–12.1 μm, clavate to subclavte, and abundant.
Specimens examined: Korea, Jeju-do, Seogwipo-si, Donnaeko campsite, 33°18′01″ N, 126°35'03'' E, September 7 2012, Hyun Lee, SFC20120907-20; On the ground of evergreen broad-leaved forest.
Remarks: Like Lf. acicularis, this species was previously misidentified as Lactifluus volemus in Korea. However, they can be distinguished from Lf. volemus by the height of basidiospore ornamentation. The height of ornamentation in Lf. volemus (up to 1.5 μm) is lower than that of Lf. pinguis [Citation40]. Furthermore, pleurolamprocystida cell wall thickness of Lf. pinguis (3–7 μm) is much thicker than that of Lf. volemus (1.5–4.5 μm) [Citation39,Citation40].
4. Discussion
To date, 66 milkcap species have been reported in South Korea [Citation42]. These specimens were identified based solely on morphology and many European and North American names were used [Citation23,Citation43,Citation44]. For this reason, it is probable that the number of Korean milkcap species is considerably different from what is currently reported, and DNA sequence analysis can be used for more accurate species identification [Citation21,Citation45]. In this study, eight species in the family Russulaceae were identified as new records to South Korea. All presented species were identified using molecular data from ITS sequences and morphological characteristics. Five species were shown to belong to three subgenera of Lactarius and three species belonged to two subgenera of Lactifluus.
Recent molecular studies showed that the majority of Asian milkcap species were not conspecific to mycoflora found in other continents, such as Europe and North America [Citation16,Citation20,Citation46]. Intercontinental conspecificity of milkcaps can occur infrequently in boreal and alpine forests, as some species were found in both North America and Europe [Citation47,Citation48], and some in both in Europe and Asia [Citation49,Citation50]. In this study, we found that some Korean specimens were conspecific with two European species, Lactarius tabidus and L. vietus. These species have also been reported in North America and Europe [Citation4,Citation5,Citation47]. The wide distribution of L. tabidus has recently been confirmed using ITS sequence data [Citation50]. The remaining six species were originally described in Southeast Asia. Korean collections of Lactifluus acicularis and Lf. pinguis were previously misidentified as European Lf. volemus because of their similar morphological characteristics. Previous studies revealed that there were many species hidden in the Lactifluus volemus species complex and indeed Lf. volemus has not been found in Asia [Citation39,Citation40]. Furthermore, Korean collections of Lactifluus pilosus have been misidentified as Lf. Vellereus, which was first described from Europe. Thus, a thorough study of the Korean collections designated as Lf. vellereus is needed. L. austrotorminosus, L. kesiyae and Lf. pinguis were first described in Thailand [Citation37–39]. Interestingly, these species were found only on Jeju Island. This observation suggests that climate change has affected fungal distribution in Korea. Beginning in the last decade, tropical and subtropical marine organisms [Citation51,Citation52] and plants [Citation53,Citation54] have frequently been reported on Jeju Island, the southernmost part of Korea. Thus, Jeju Island may represent a climate change hot spot. In addition, a recent study showed that the distribution pattern of ectomycorrhizal fungi was affected by climate change, inducing both host migration and host modification [Citation55]. Three other species, Lactarius atromarginatus, Lf. acicularis and Lf. pinguis, were also reported from tropical and subtropical Asia. Lactarius atromarginatus was reported initially in Papua New Guinea [Citation35], and then reported in Thailand, Japan, and southern China. These species were also collected in central Korea. Further studies are needed to establish any relationship between the fruiting of these species in Korea and climate change.
Various tree species serve as hosts for milkcaps through ectomycorrhizal symbiosis. Some species are specific to host trees [Citation56]. For example, Lactarius tabidus and L. vietus both use Betula as their ectomycorrhizal symbiont [Citation5]. The distribution of Betula is seems to be restricted to some regions in Korea, as these species were only collected from high mountain forests mixed with Betula and Quercus trees. Lactarius kesiyae is an ectomycorrhizal symbiont of Pinus kesiya [Citation38]; however, P. kesiya is not found in Korea, and in this study, specimens of L. Kesiyae were collected near P. densiflora. Therefore, the host of L. kesiyae likely includes two Pinus species in Pinus sect. Pinus subsec. Pinus [Citation57]. However, further molecular data obtained from ectomycorrhizal rootlets and fruiting bodies is needed to establish the symbiont host of Korean species.
Acknowledgments
We would like to express our gratitude to Dr. Seok Soon Ja and National Institute of Agricultural Sciences for providing the specimens for this study. We also greatly appreciate two anonymous reviewers who improved our manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kirk PM, Cannon PF, Minter DW, et al. Dictionary of the fungi. Wallingford: CAB International; 2008.
- Singer R. The Agaricales in modern taxonomy. 4th ed. Koenigstein: Sven Koeltz; 1986.
- Kränzlin F. Fungi of Switzerland. Vol. 6. Lucerne: Verlag Mykologia; 2005.
- Hesler LR, Smith AH. North American species of Lactarius. Ann Arbor: University of Michigan Press; 1979.
- Heilmann-Clausen J, Verbeken A, Vesterholt J. The Genus Lactarius: fungi of northern Europe. Vol. 2. Mundelstrup: Svampetryk; 1998.
- Parladé J, Pera J, Luque J. Evaluation of mycelial inocula of edible Lactarius species for the production of Pinus pinaster and P. sylvestris mycorrhizal seedlings under greenhouse conditions. Mycorrhiza. 2004;14:171–175.
- Rochet J, Moreau PA, Manzi S, et al. Comparative phylogenies and host specialization in the alder ectomycorrhizal fungi Alnicola, Alpova and Lactarius (Basidiomycota) in Europe. BMC Evol Biol. 2011;11:40–53.
- Yamashita S, Hijii N. The role of fungal taxa and developmental stage of mushrooms in determining the composition of the mycophagous insect community in a Japanese forest. Eur J Entomol. 2007;104:225–233.
- Fogel R, Trappe JM. Fungus consumption (mycophagy) by small animals. Northwest Sci. 1978;52:1–31.
- Guo W. Resources of wild edible fungi in Tibet, China. Zhongguo Shiyongjun. 1992;11:33–34.
- Mooibroek H, Cornish K. Alternative sources of natural rubber. Appl Microbiol Biotechnol. 2000;53:355–365.
- Buyck B, Hofstetter V, Eberhardt U, et al. Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers. 2008;28:15–40.
- Lebel T, Dunk CW, May TW. Rediscovery of Multifurca stenophylla (Berk.) T. Lebel, CW Dunk & TW May comb. nov. (Russulaceae) from Australia. Mycol Progress. 2013;12:497–504.
- Verbeken A, Nuytink J, Buyck B. New combinations in Lactifluus. 1. L. subgenera Edules, Lactariopsis and Russulopsis. Mycotaxon. 2011;118:447–453.
- Verbeken A, Van de Putte K, De Crop E. New combinations in Lactifluus. 3. L. subgenera Lactifluus and Piperati. Mycotaxon. 2012;120:443–450.
- Stubbe D, Verbeken A. Lactarius subg. Plinthogalus: the European taxa and American varieties of L. lignyotus re-evaluated. Mycologia. 2012;104:1490–1501.
- Buyck B, Hofsetter V, Verbeken A, et al. Proposal 1919: to conserve Lactarius nom. Cons. (Basidiomycota) with avconserved type. Taxon. 2010;59:295–296.
- Verbeken A, Nuytinck J. Not every milkcap is a Lactarius. Scripta Botanica Belgica. 2013;51:162–168.
- Schoch CL, Seifert KA, Huhndorf S, Fungal Barcoding Consortium, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA. 2012;109:6241–6246.
- Lee H, Park MS, Jung PE, et al. Lactarius cucurbitoides (Russulales, Basidiomycota), a new species from South Korea supported by molecular and morphological data. Phytotaxa. 2015;205:168–176.
- Lee H, Park MS, Jung PE, et al. Re-evaluation of the taxonomy and diversity of Russula section Foetentinae (Russulales, Basidiomycota) in Korea. Mycoscience. 2017;58:351–360.
- Park MS, Fong JJ, Lee H, et al. Delimitation of Russula subgenus Amoenula in Korea using three molecular markers. Mycobiology. 2013;41:191–201.
- Park WH, Lee JH. New wild fungi of Korea. Seoul: Kyo-Hak Publishing Co.; 2011.
- Verbeken A, Walleyn R. Fungus flora of tropical Africa. Vol. 2, monograph of Lactarius in tropical Africa. Meise: National Botanic Garden of Belgium; 2010.
- Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London: Eyre Methuen Ltd.; 1978.
- Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoort RA, editors. Plant molecular biology manual. Boston (MA): Kluwer Academic Publishers; 1994. p. 183–190.
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. p. 315–322.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software versions 7: improvement in performance and usability. Mol Biol Evol. 2013;30:772–780.
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690.
- Darriba D, Taboada GL, Doallo R, et al. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772.
- Ronquist F, Huelsenbeck JP. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574.
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees. SC10 workshop on gateway computing environments (GCE10); 2010 Nov 13–19; New Orleans (LA): IEEE Computer Society; 2010. p. 1–8.
- Verbeken A, Horak E. Lactarius (Basidiomycota) in Papua new Guinea 2. Species in tropical-montane rainforests. Aust Syst Bot. 2000;13:649–707.
- Le HT, Stubbe D, Verbeken A, et al. Lactarius in Northern Thailand: 2. Lactarius subgenus Plinthogali. Fungal Divers. 2007;27:61–94.
- Le HT, Nuytinck J, Verbeken A, et al. Lactarius in Northern Thailand: 1. Lactarius subgenus Piperites. Fungal Divers. 2007;24:173–224.
- Wisitrassameewong K, Nuytinck J, Le HT, et al. Lactarius subgenus Russularia (Russulaceae) in South-East Asia, 3: new diversity in Thailand and Vietnam. Phytotaxa. 2015;207:215–241.
- Van de Putte K, Nuytinck J, Stubbe D, et al. Lactarius volemus sensu lato (Russulales) from northern Thailand: morphological and phylogenetic species concepts explored. Fungal Divers. 2010;45:99–130.
- Van de Putte K, Nuytinck J, De Crop E, et al. Lactifluus volemus in Europe: three species in one-revealed by a multilocus genealogical approach, Bayesian species delimitation and morphology. Fungal Biol. 2016;120:1–25.
- Le HT, Verbeken A, Nuytinck J, et al. Lactarius in Northern Thailand: 3. Lactarius subgenus Lactoriopsis. Mycotaxon. 2007;102:281–291.
- Lee YS, Lim YW, Kim JJ, et al. National list of species of Korea: basidiomycota. Incheon: National Institute of Biological Resources; 2015.
- Bok JD, Shin GC. Taxonomical studies on the genus Lactarius in Korea (I). Kor J Mycol. 1985;13:249–262.
- Cho DH. Notes on the Korean Higher Fungi (XVII). Kor J Plant Res. 2002;5:51–58.
- Cho HJ, Lee H, Park JY, et al. Seven new recorded species in five genera of the strophariaceae in Korea. Mycobiology. 2016;44:137–145.
- Stubbe D, Nuytinck J, Verbeken A. Critical assessment of the Lactarius gerardii species complex (Russulales). Fungal Biol. 2010;114:271–283.
- Methven AS. North American and European Species of Lactarius. Scripta Botanica Belgica. 2013;51:91–105.
- Barge EG, Cripps CL. New reports, phylogenetic analysis, and a key to Lactarius Pers. in the Greater Yellowstone Ecosystem informed by molecular data. MycoKeys. 2016;15:1–58.
- Nuytinck J, Verbeken A, Miller SL. Worldwide phylogeny of Lactarius section Deliciosi inferred from ITS and glyceraldehyde-3-phosphate dehydro-genase gene sequences. Mycologia. 2007;99:820–832.
- Wisitrassameewong K, Looney BP, Le HT, et al. Lactarius subgenus Russularia (Basidiomycota, Russulales): novel Asian species, worldwide phylogeny and evolutionary relationships. Fungal Biol. 2016;120:1554–1581.
- Kim MJ, Kim BY, Kim JS, et al. Two unrecorded species of the snapper (Perciformes: lutjanidae) collected from Jeju Island, Korea. Fish Aquatic Sci. 2012;15:313–316.
- Shah M, Mahfuzur R, An SJ, et al. Presence of benthic dinoflagellates around coastal waters of Jeju Island including newly recorded species. J Ecol Environ. 2013;36:347–370.
- Lee YM, Park SH, Jung SY, et al. Study on the current status of naturalized plants in South Korea. Kor. Korean J Pl Taxon. 2011;41:87–101.
- Dolezal J, Altman J, Kopecky M, et al. Plant diversity changes during the postglacial in East Asia: insights from forest refugia on Halla Volcano, Jeju Island. PLoS One. 2012;7:e33065.
- Pickles BJ, Egger KN, Massicotte HB, et al. Ectomycorrhizas and climate change. Fungal Ecol. 2012;5:73–84.
- Münzenberger B, Metzler B, Kottke I, et al. Morphologische und anatomische Charakterisie-rung der Mykorrhiza Lactarius deterrimus-Picea abies in vitro. Z Mykol. 1986;52:407–422.
- Gernandt DS, López GG, García SO, et al. Phylogeny and classification of Pinus. Taxon. 2005;54:29–42.
