Abstract
Arbuscular mycorrhizal fungi (AMF) are well-known for their ability to improve plant growth and help plants withstand abiotic stress conditions. Unlike other fungi and bacteria, AMF cannot be stored, as they are obligate biotrophs. Long-term preservation of AMF spores is challenging and may lead to the loss of viability and efficiency. This study aimed to understand the effect of prolonged subculture of AMF species on the growth and glomalin-related soil protein (GRSP) from red pepper (Capsicum annuum L.). AMF spores were mass-produced using different techniques and subcultured in pots with sorghum sudangrass as the host plant for 3 years. Experimental soil samples were collected from natural grassland. Five different AMF inocula were used in triplicate as treatments. After 70 days of growth, red pepper plants were harvested and plant dry weight, plant nutrient content, mycorrhizal colonization, AMF spore count, and soil glomalin content were determined. AMF-treated plants displayed higher dry weight than controls, with only fruit dry weight being significantly different. Similarly, significant differences in phosphorous and potassium contents of the above-ground plant parts were observed between mycorrhizal and control treatments. In addition, soil GRSP content was significantly higher in plants inoculated with Rhizophagus sp. and Gigaspora margarita. The increased plant growth and GRSP content suggest that AMF can be maintained for 3 years without losing their efficiency if subcultured regularly with different symbiotic host plants.
1. Introduction
Most plants require microbial symbiosis for a more efficient uptake of nutrients from the soil. Arbuscular mycorrhizal fungi (AMF) are microorganisms that form symbiotic associations with plants and facilitate efficient plant uptake of nutrients from the soil [Citation1]. Most AMF species positively influence plant growth, even under adverse environmental conditions [Citation2]. AMF also improve plant nutrient uptake by extending their hyphae in the soil. In addition, glomalin protein released from AMF hyphae aggregates soil and improves soil fertility [Citation3]. Glomalin refers to a group of soil proteins that are proposed to be partially produced by AMF [Citation4]. Production of glomalin proteins varies depending on the function of the AMF species and the particular host plant [Citation5]. Previous reports have proposed that AMF hyphae indirectly affect soil aggregation [Citation4,Citation6]. Schreiner et al. [Citation7] reported that Funneliformis mosseae (formerly Glomus mosseae) significantly improves water-stable aggregation compared to Claroideoglomus etunicatum (formerly G. etunicatum) and Gigaspora rosea, suggesting that each AMF species varies in its soil aggregation ability.
The efficiency of mycorrhizal association with plant roots varies depending on the plant species and AMF involved [Citation8]. Although the application of AMF is encouraged to improve the sustainability of organic agriculture, the amount of AMF inoculum required for large-scale application cannot be achieved in a cost-effective manner using current mass-production techniques [Citation9]. For instance, aeroponic [Citation10] and root organ culture [Citation11] methods are limited to the laboratory scale and are costly for AMF mass production.
AMF inoculation significantly improves the growth of various plants including maize, cotton, tomato, orange, and pepper [Citation12–14]. However, maintenance and storage techniques of this obligate biotroph over the long-term remain challenging. Varga et al. [Citation15] proposed a cold storage technique for maintaining AMF over an extended period of time. Although the spores remain intact during the storage period, the viability of AMF spores may be reduced during cold storage. Ruiz-Lozana and Azcon [Citation16] maintained AMF spores in soils with different water potentials and found that the number of spores was dramatically reduced when the substrate dried. These findings suggest that an effective alternative method is required to maintain AMF spores for extended times without reducing their viability and efficiency.
Long-term subculture of AMF spores in pots with continuous crop rotation has recently attracted attention, as it is less artificial and closer to the field condition. The present study aimed to assess the effect of long-term subcultured AMF on the growth of red pepper (Capsicum annuum L.) and on the content of red pepper glomalin-related soil protein (GRSP).
2. Materials and methods
2.1. Strain details and initial spore count
AMF strains, method of propagation, storage period used in this study, and culture collection center deposit numbers are provided in . AMF spores were mass-produced using different techniques including the slide method and monosporic culture. The mass-produced spores were regularly subcultured and maintained in pots with continuous crop rotation for 3 years. Sorghum sudangrass was used as the host plant. The initial spore count of the soil samples was assessed using wet sieving and a decanting method as previously described [Citation17], followed by sucrose centrifugation.
Table 1. Arbuscular mycorrhizal strains used in this study.
2.2. Soil sample collection and initial soil analysis
Experimental soil samples were collected from natural grasslands at Wanju-gun, South Korea. No agricultural practices were conducted in these fields. The natural grassland was rich in hairy vetch (Vicia villosa) and other common weeds. The collected soils were subjected to initial soil analysis. The initial soil properties of the natural grassland soil were as follows: pH 5.54, 0.17 dS/cm electrical conductivity, 2.70 mg/kg available phosphorus, 0.03% total nitrogen, 0.18 cmol/kg potassium, 1.98 cmol/kg calcium, 2.25 cmol/kg magnesium, and 0.08 cmol/kg sodium.
2.3. Pot preparation and inoculum application
Collected bulk soil was consecutively sterilized for 3 days at 121 °C for 15 min to kill all microorganisms. The amount of fertilizer and compost mixed with the soil was determined according to the recommendation of the Rural Development Administration of Korea, except for phosphorus (P). Only 10% of the recommended P was added to promote AMF colonization. The sterilized soils (4 kg) were added to the pots. Red pepper seeds were surface-sterilized with 70% ethanol for 1 min, followed by 2% sodium hypochlorite for 1 min, and washed 5–7 times with sterile distilled water. Sterilized seeds were sown in seedling trays containing sterilized commercial nursery soil. After 5 days of growth, the seedlings were transplanted to pots containing sterilized natural grassland soil. Except for the control, each treatment received 100 g of the AMF inoculum (each inoculum containing approximately 250 spores and 35 root bits). Controls received no AMF inoculation. The AMF inoculum was placed 1 cm below the root zone of the red pepper seedlings. The potted red pepper plants were maintained in the greenhouse for 70 days.
2.4. AMF inoculation effect on red pepper growth
During the growth period, the numbers of leaves and fruits were checked every week. Plants were harvested 70 days after sowing and the fresh weight and dry weight were measured separately for the shoot, root, and fruits. Total nutrient (T-N) in the plant tissues was analyzed using the Kjeldahl method. Other nutrients including phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES).
2.5. Mycorrhizal parameters
Mycorrhizal spore count was determined using a wet sieving and decanting method. AMF colonization in red pepper roots was assessed as previously described [Citation18]. Briefly, the roots were first washed with 10% KOH for 10 min in a water bath at 90 °C. Next, the roots were washed with tap water. Root fragments were softened in 2% HCl for 10 min at room temperature (18–22 °C). After discarding the HCl, root fragments were stained with 0.5% Trypan blue in lactoglycerol at 90 °C for 10 min. The staining solution was discarded and stained roots were washed with tap water. To remove excess stain, the roots were immersed in a destaining solution (lactoglycerol) overnight and then checked for colonization. Lactoglycerol solution was prepared using lactic acid, glycerol, and water in a ratio of 875:62.5:62.5. The stained root fragments (1 cm) were arranged on glass slides and observed under a microscope for the presence of hyphae, vesicles, and arbuscules. Scoring was conducted based on the intensity of colonization (0–5) and based on arbuscule intensity (A0–A3) as previously described [Citation19].
2.6. Post-experiment soil properties and soil glomalin content
After the experiment, soil properties of all treatments were determined using standard laboratory protocols. Easily extractable GRSP (EE-GRSP) and total extractable GRSP (TE-GRSP) contents were determined. For EE-GRSP, 1 g of soil sample was mixed with 8 mL of 20 mM citrate solution (pH 7.0) and autoclaved for 30 min at 121 °C. After cooling to room temperature, samples were centrifuged at 8000 ×g for 20 min and the supernatant was collected and stored at 4 °C until use. For TE-GRSP, the remaining soil pellet was dissolved in 50 mM citrate solution (pH 8.0) and autoclaved for 1 h at 121 °C and centrifuged as described for EE-GRSP. The supernatant was collected and this step was repeated another 3–4 times until the reddish-brown color in the supernatant disappeared. Supernatants collected by TE-GRSP extraction were pooled and protein was measured using the Bradford method [Citation20]. Bovine serum albumin was used as a standard.
2.7. Data analysis and statistical analysis
The relationships between soil properties and soil glomalin content were analyzed by Pearson’s correlation coefficient analysis with SPSS software (SPSS, Inc., Chicago, IL). Data were subjected to analysis of variance and the mean significant differences were compared by Tukey’s range test at p < .05. All data were analyzed using SAS package, Version 9.2 (SAS, Inc., Cary, NC).
3. Results
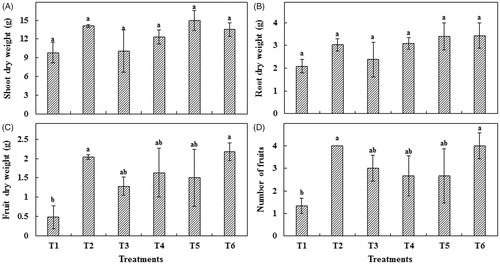
Mycorrhizal inoculation had a positive effect on red pepper plant growth in terms of dry weight. All AMF-treated plants had higher shoot and root dry weights than non-inoculated plants. However, the difference was not significantly different (). Mycorrhizal plants showed increased fruit dry weight. Inoculation with Rhizophagus sp. and G. margarita significantly increased fruit dry weight compared to non-inoculated plants (). Similarly, mycorrhizal plants showed a significantly higher number of fruits than non-mycorrhizal plants ().
Figure 1. Mycorrhizal inoculation effect on red pepper plant growth. (A) Shoot dry weight; (B) Root dry weight; (C) Fruit dry weight; and (D) Number of fruits. Each value represents the mean of three replicates ± standard error. T1: Control; T2: C. etunicatum; T3: Rhizophagus sp.; T4: F. mosseae; T5: G. margarita; T6: C. lamellosum.
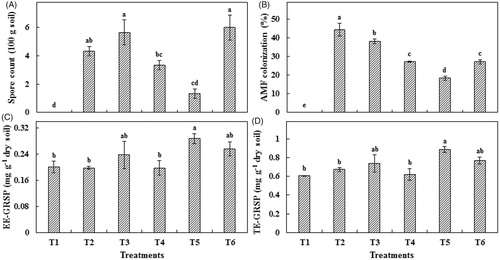
After harvest, pots inoculated with Rhizophagus sp. and C. lamellosum had produced the highest number of spores (). C. etunicatum showed significantly higher root colonization, followed by Rhizophagus sp. and C. lamellosum (). Although mycorrhizal pots showed higher amounts of EE-GRSP, only G. margarita showed a significant difference from non-mycorrhizal pot soil (). The same trend in EE-GRSP was observed for TE-GRSP where G. margarita-inoculated pot soils showed significantly higher TE-GRSP () compared to non-mycorrhizal pot soil.
Figure 2. Mycorrhizal inoculation effect on spore production and glomalin content. (A) Spore count; (B) Mycorrhizal root colonization; (C) EE-GRSP content; and (D) TE-GRSP content. Each value represents the mean of three replicates ± standard error. T1: Control; T2: C. etunicatum; T3: Rhizophagus sp.; T4: F. mosseae; T5: G. margarita; T6: C. lamellosum.
Mycorrhizal plants showed higher nutrient contents than non-mycorrhizal plants in shoots (). However, the differences were not significantly high, except for P. Although high amounts of P, K, and Ca were observed in mycorrhizal roots, the differences were not significant compared to non-mycorrhizal plants (). The high amounts of P in red pepper shoots and roots suggest that mycorrhizal inoculation effectively improved P uptake by plants.
Table 2. Inoculation effect of AMF strains on nutrient accumulation in red pepper shoot and root tissues.
To understand the relationship between glomalin production and soil properties, post-experiment soil properties were correlated with spore count, colonization, and glomalin content. Soil P and Ca contents were significantly influenced by glomalin production (). Mycorrhizal spore production and mycorrhizal colonization showed no correlation with the soil properties studied.
Table 3. Relationship between soil properties and soil glomalin content according to Pearson’s correlation coefficient analysis.
4. Discussion
Microorganisms play a vital role in enhancing plant nutrient uptake and maintaining soil biological activities. Although soil contains both beneficial and pathogenic microbes, beneficial microbes such as plant growth-promoting bacteria and AMF have received attention because they are environmentally friendly bio-fertilizers. AMF are recognized for their ability to improve plant growth and help plants withstand harsh environmental conditions [Citation21]. In addition, AMF improve soil fertility by producing glomalin-related soil proteins. Despite the many beneficial roles proposed for AMF, commercialization of this obligate biotroph remains limited because of the lack of cost-efficient mass-production techniques and possible effects of preservation techniques on efficiency. The current study examined the effects of long-term subculture of AMF spores on red pepper plant growth and glomalin production.
The AMF spores used in this study were previously mass-produced and were reported to significantly improve plant growth [Citation22,Citation23]. AMF inoculation was shown to significantly increase pepper growth [Citation24,Citation25], even under harsh environmental conditions [Citation26]. The use of the same crop in the present study may improve the understanding of the effect of long-term subcultured AMF on plant growth. Mycorrhizal plants enhanced red pepper plant growth compared to non-mycorrhizal plants. Ortas et al. [Citation27] reported that mycorrhizal spores inoculated with pepper plants had significantly higher shoot dry weight and root dry weight and even flowered earlier than non-mycorrhizal plants. We also observed that mycorrhizal plants had a significantly higher number of dry fruits and heavier dry fruits than non-mycorrhizal plants. In addition, the mycorrhizal effect on plant nutrient accumulation was also considerably higher than on non-mycorrhizal plants.
Mycorrhizal colonization is an important parameter in studies on the efficiency of AMF, as it is involved in nutrient transport in plants. AMF colonization efficiency was higher when fresh spores were used, although a slight decrease in AMF colonization was evident after long-term subculture. In our previous study [Citation9], freshly mass-produced G. margarita and C. lamellosum spores had higher colonization percentages, although the colonization efficiency decreased by nearly twofold after long-term subculture. Ruiz-Lozano and Azcon [Citation16] also reported that the viability and infection rate declined dramatically after long-term storage of AMF spores. Varga et al. [Citation15] reported that even after prolonged cold storage of AMF spores, most spores were viable. However, when these spores were introduced in the experimental plots, no root colonization was observed even after 21 months of inoculation because the spores did not germinate. These results suggest that cold storage techniques may preserve the viability of spores for an extended time, but it reduces AMF spore germination and colonization efficiency. A previous study by Trejo-Aguilar et al. [Citation28] showed that the use of a single host for continuous crop rotation in trap cultures decreased the diversity of AMF spores. The spores used in this study were maintained in a trap culture with continuous crop rotation using a single host plant, which may have influenced the efficiency of AMF spores.
Glomalin plays an important role in aggregating and increasing the water holding capacity of soil. We found that different AMF species produced different amounts of glomalin. Furthermore, there was no correlation between glomalin levels and the percentage of AMF colonization. Soil nutrient contents may alter glomalin production by AMF [Citation29,Citation30]. In the present study, a positive correlation was demonstrated between glomalin production and soil chemical properties such as P and Ca, suggesting that higher P and Ca contents in soil influence glomalin production.
The use of AMF spores that have been subcultured for an extended period of time can increase plant growth, particularly fruit dry weight and the number of fruit produced. AMF inoculation also increases plant nutrient uptake and glomalin-related soil protein content. Our results and those of Trejo-Aguilar et al. [Citation28] suggest that long-term subculture of AMF spores without a loss of viability and efficiency can be achieved if the host plant is changed during every crop rotation to increase AMF colonization.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Smith SE, Read DJ. Mycorrhizal symbiosis, 2nd ed. San Diego, CA: Academic press; 1997.
- Colla G, Rouphael Y, Cardarelli M, et al. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorous concentration. Biol Fertil Soils. 2009;44:501–509.
- Piotrowski JS, Denich T, Klironomos JN, et al. The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species. New Phytol. 2004;164:365–373.
- Rillig MC. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci. 2004;84:355–363.
- Wright SF, Franke-Synder M, Morton JB, et al. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil. 1996;181:193–203.
- Rillig MC, Wright SF, Eviner VT. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant Soil. 2002;238:325–333.
- Schreiner RP, Mihaa KL, McDaniel H, et al. Mycorrhizal fungi influence plant and soil functions and interactions. Plant Soil. 1997;188:199–209.
- Feldmann F, Hutter I, Niemann P, et al. Integration of the mycorrhizal technology into plant production process of medicinal and ornamental plants as well as commercialization. In: Backhaus GF, Feldmann F, editors. Arbuscular mycorrhiza in plant production: Examples and perspectives for practical application. Mitteilungen aus der Biologischen Bundesanstalt fur Land- and Forsteirtschaft, vol 363. Berlin-Dahlem: Biologische Bundesanstalt; 1999. p. 6–38.
- Selvakumar G, Krishnamoorthy R, Kim K, et al. Propagation technique of arbuscular mycorrhizal fungi isolated from coastal reclamation land. Eur J Soil Biol. 2016;74:39–44.
- Mohammad A, Khan AG, Kuek C. Improved aeroponic culture of inocula of arbuscular mycorrhizal fungi. Mycorrhiza. 2000;9:337–339.
- Bidondo LF, Pergola M, Silvani V, et al. Continuous and long-term monoxenic culture of the arbuscular mycorrhizal fungus Gigaspora decipiens in root organ culture. Fungal Biol. 2012;16:729–735.
- Tian CY, Feng G, Li XL, et al. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl Soil Ecol. 2004;26:143–148.
- Kaya C, Ashraf M, Sonmez O, et al. The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hortic. 2009;121:1–6.
- Hajiboland R, Aliasgharzadeh N, Laiegh SF, et al. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil. 2010;331:313–327.
- Varga S, Finozzi C, Vestberg M, et al. Arctic arbuscular mycorrhizal spore community and viability after storage in cold conditions. Mycorrhiza. 2015;25:335–343.
- Ruiz-Lozana JM, Azcon R. Viability and infectivity of mycorrhizal spores after long term storage in soils with different water potentials. Appl Soil Ecol. 1996;3:183–186.
- Daniels BA, Skipper HD. Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC, editor. Methods and principles of mycorrhizal research. St Paul, Minnesota: American Phytopathological Society; 1982. p. 244.
- Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–161.
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. Mesure du taux de mycorhization VA dun systeme radiculaire. Recherche de methodes destimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S, editors. Physiological and genetical aspects of Mycorrhizae, Paris: INRA Press; 1986. p. 217–221.
- Cornejo P, Meier S, Borie G, et al. Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci Total Environ. 2008;406:154–160.
- Estrada B, Aroca R, Maathuis FJM, et al. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013;36:1771–1782.
- Lee Y, Krishnamoorthy R, Selvakumar G, et al. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J Korean Soc Appl Biol Chem. 2015;58:533–540.
- Krishnamoorthy R, Kim K, Subramanian P, et al. Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agric Ecosyst Environ. 2016;231:233–239.
- Regvar M, Vogel-Mikus K, Severkar T. Effect of AMF inoculum from field isolates on the yield of green pepper, parsley, carrot, and tomato. Folia Geobot. 2003;38:223–234.
- Ortas I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop Res. 2012;125:35–48.
- Turkmen O, Sensoy S, Demir S, et al. Effects of two different AMF species on growth and nutrient content of pepper seedlings grown under moderate salt stress. Afr J Biotechnol. 2008;7:392–396.
- Ortas I, Sari N, Akpinar C, et al. Screening mycorrhiza species for plant growth, P and Zn uptake in pepper seedling grown under greenhouse conditions. Sci Hortic. 2011;128:92–98.
- Trejo-Aguilar D, Lara-Capistran L, Maldonada-Mendoza IE, et al. Loss of arbuscular mycorrhizal fungal diversity in trap cultures during long-term subculturing. IMA fungus. 2013;4:161–167.
- Wright SF, Starr JL, Paltineanu IC. Changes in aggregate stability and concentration of glomalin during tillage management transition. Soil Sci Soc Am J. 1999;63:1825–1829.
- Borie F, Rubio R, Rouanet JL, et al. Effects of tillage systems on soil characteristics, glomalin and mycorrhizal propagules in a Chilean Ultisol. Soil Tillage Res. 2006;88:253–261.
