Abstract
This study evaluated the in vitro and in vivo hypolipidemic effects of the medicinal mushroom Phellinus pini. The methanol extract (ME) of the fruiting body of Ph. pini was active against pancreatic lipase and cholesterol esterase with 99.14% and 67.23% inhibited activity at 1.0 mg/mL, respectively. It also inhibited 81.81% and 55.33% of α-glucosidase and α-amylase activities, respectively, at 2.0 mg/mL. Hyperlipidemia as induced by feeding rats with a high fat and cholesterol diet (HFC). HFC supplemented with a 5% fruiting body powder of Ph. pini (HFC + PhP) significantly reduced plasma total cholesterol, low-density lipoprotein cholesterol, and triglycerides in rats compared with HFC. The reduced levels were comparable to rats fed the normal control diet (NC). The atherogenic index of HFC + PhP rats was significantly lower than that of the HFC rats. The excretion of fecal total lipid and cholesterol in the HFC + PhP rats was significantly higher than those in the NC and HFC rats. Histopathological examinations demonstrated scant deposition of lipids in the liver of rats fed HFC + PhP. The dietary supplementation with the fruiting body powder provided natural plasma lipid and glucose lowering effects in experimental rats without adverse effects on the plasma biochemical parameters and liver function related enzyme activities. Therefore, the hypolipidemic effects of Ph. pini may be due to the inhibitory effects on pancreatic lipase, cholesterol esterase, α-glucosidase, and α-amylase, and excretion of excess lipids and cholesterol in the feces.
1. Introduction
Epidemiological studies have demonstrated that hyperlipidemia is generally related to increased high levels of plasma total cholesterol, low density lipoprotein (LDL)-cholesterol, and triglycerides [Citation1]. Hyperlipidemia is associated with the development of diseases, including coronary heart disease (CHD), atherosclerosis, and diabetes [Citation2]. CHD is the major cause of death in developing and industrial countries and the number of patients suffering from CHD worldwide is gradually increasing [Citation3]. Methods to control the elevated levels of blood cholesterol include a balance of dietary fats, proper exercise, and a prescription of hydroxymethylglutaryl-CoA (HMG-CoA) reductase (HMGCR) inhibitors (i.e., statins). HMGCR is the main enzyme in the biosynthesis of cholesterol. Suppression of HMGCR activity is the most effective therapy for hyperlipidemia [Citation4]. However, statins, including atorvastatin, lovastatin, pravastatin, and simvastatin, may cause side effects, such as severe muscle pain, liver damage, and kidney failure [Citation5]. Therefore, it is necessary to find new sources of hypolipidemic agents that lack therapeutic complications.
Diabetes mellitus (DM) is a serious chronic metabolic disease caused by insufficient production of insulin in the pancreas or lowered insulin sensitivity to target cells. DM is characterized by high levels of postprandial glucose in the blood, especially in type 2 DM. The major sources of glucose in the blood derive from the digestion of dietary carbohydrates by the hydrolytic enzymes α-amylase and α-glucosidase [Citation6]. An important T2DM management strategy is delaying the digestion of dietary carbohydrates by the inhibition of α-amylase and α-glucosidase, which suppresses the post-prandial glucose levels in the blood. Several drugs, including acarbose, miglitol, and voglibose, are currently available for treating T2DM patients. However, these therapeutic agents also have side effects. Therefore, there is a need to overcome diabetic complications and to discover new anti-diabetic agents [Citation7]. Alpha-amylase produced in the pancreas of humans is the main enzyme for the hydrolysis of starch. The enzyme breaks down starch by successively cleaving the linkages between the glucose molecules of starch to form smaller soluble starches and eventually maltose and dextrin. Then, α-glucosidase breaks down these small molecules to glucose, which can be absorbed in the small intestine and used as an energy source and metabolic pathway [Citation8].
Phellinus pini (currently known as Porodaedalea pini (Brot.) Murrill) belongs to Hymenochaetaceae Basidiomycota. This fungus is a perennial parasite on the trunks of old conifer trees and is widely distributed in the Northern hemisphere. The fruiting body of Ph. pini, commonly termed “pine sanghuang,” has long been utilized as a traditional medicine in far-eastern Asian countries [Citation9,Citation10]. However, its efficacy on diabetes and hyperlipidemia has not been studied.
This study was initiated to assess the effects of the fruiting bodies of Ph. pini in the in vitro inhibition of lipase, cholesterol esterase, α-glucosidase, and α-amylase, and the reduction of plasma lipid-related cholesterol and triglyceride. The in vivo experiments involved rats fed a high fat and cholesterol diet (HFC).
2. Materials and methods
2.1. Mushroom and extract preparation
Phellinus pini fruiting bodies were purchased from the Kyungdong herb market located in Seoul, Korea. Their identity was verified by a mycologist (Dr. Kyung-Rim Lee). The fruiting bodies were fully air-dried and pulverized. Powdered mushroom (20 g) was extracted with 80% methanol for 24 hours at 28 °C in a shaker (150 rpm) and the mixture was filtered through Whatman No. 1 filter papers. The residue was extracted two more times in the same manner. The solvent in the final methanol extract (ME) was evaporated at 40 °C in a rotary evaporator and the remaining water in the ME was removed with a freeze-drier.
2.2. Animals
Sprague–Dawley rats (4 weeks old) were purchased from Dae Han Bio Link Co. Ltd. (Eumseong, Korea). All rats were kept under a constant 12-hour light and dark cycle, with a temperature of 23 ± 1 °C and humidity of 55 ± 5%. The rats were given free access to a basal diet and water and remained in an animal house for one week for adaptation before the experiment. The rats were divided into three groups (n = 8) for the 6-week treatments: normal control diet (NC group); high fat with cholesterol diet (HFC group); and HFC supplemented with 5% fruiting body powder of Ph. pini (HFC + PhP group). The compositions of the diets () were based on AIN93 specifications [Citation11]. The experimental protocol of the rats was approved by the Animal Ethics Committee of Incheon National University.
Table 1. Composition of the experimental diet.
2.3. Alpha-glucosidase inhibitory assay
The inhibitory effect of Ph. pini ME was determined as previously described [Citation12]. One hundred microliters of α-glucosidase enzyme solution (1.0 U/mL) from yeast was preincubated with 50 µL of different ME concentrations (0.125–1.0 mg/mL). Then, 50 µL of p-nitrophenyl-α-D-glucopyranoside (p-NPG) solution (3.0 mM) was dissolved in 20 mM phosphate buffer (pH 6.9) to initiate the reaction. The reaction mixture was incubated at 37 °C for 20 minutes and was terminated by the addition of 0.1 M sodium carbonate. The α-glucosidase inhibitory activity was determined by measuring the NPG released from the p-NPG solution at 405 nm by a spectrophotometer. The inhibition of α-glucosidase was calculated by the following formula:
(1)
Where, A is the absorbance of the control and B is the absorbance of the extract. Acarbose was used as a standard reference.
2.4. Alpha-amylase inhibitory assay
The assay was performed by using a previous method with minor modifications [Citation13]. Two hundred fifty microliters of various ME concentrations (0.125–2.0 mg/mL) were prepared in a tube. The same volume of 20 mM sodium phosphate buffer (pH 6.9) containing the α-amylase solution (0.5 mg/mL) was added. Following preincubation at 25 °C for 10 minutes, 250 µL of 1% starch solution in 20 mM sodium phosphate buffer (pH 6.9) was added and left at 25 °C for 10 minutes. The reaction was stopped by adding 500 µL of dinitrosalicylic acid reagent. Subsequently, the tube was incubated in a water bath (100 °C) for 5 minutes and cooled to 25 °C. The reaction mixture was diluted with distilled water and the absorbance was measured at 540 nm with a spectrophotometer. The inhibition of α-amylase activity was determined by the following formula:
(2)
Where, A is the absorbance of the control and B is the absorbance of the test sample. Acarbose was used for the standard reference.
2.5. Pancreatic lipase inhibitory assay
The pancreatic lipase inhibitory activity of the ME was determined as previously described [Citation14] with some modifications. The reaction volume of 200 µL containing 30 µL of 2.5 mg/mL porcine pancreatic lipase (PPL, type II) and 120 µL of different concentrations of ME in 0.1 M Tris HCl buffer and 5 mM CaCl2 (pH 7.0), were preincubated at 37 °C for 15 minutes. The reaction was started by adding 5 µL of 10 mM p-nitrophenyl butyrate (p-NPB in dimethylformamide) and was allowed to proceed at 37 °C for 30 minutes. The lipase inhibitory activity of the ME was measured at 405 nm using a microplate reader. Lipase inhibition was calculated according to the following formula:
(3)
Where, A is the activity without an inhibitor, a is the negative control without an inhibitor, B is the activity with an inhibitor, and b is the negative control with an inhibitor. Orlistat was used as the positive control.
2.6. Cholesterol esterase inhibitory assay
The pancreatic cholesterol esterase inhibitory activity of the ME of the mushroom was performed as previously described [Citation15] with minor modifications. The reaction volume of 200 µL containing different concentrations of ME was preincubated with 50 µL of 24 mM taurocholic acid, 5 µL of 8 mM p-NPB in acetonitrile in 0.1 M sodium phosphate buffer, and 0.1 M NaCl (pH 7.0) at 25 °C for 10 minutes. The reaction was started by adding 42.5 µL of (1.25 µg/mL) cholesterol esterase, and the change in absorbance was monitored at 405 nm at 25 °C for 6 minutes using a microplate reader. The inhibition of cholesterol esterase was determined by the following formula:
(4)
Where, A is the absorbance of the control and B is the absorbance of the test sample. Simvastatin was used as the positive control.
2.7. Plasma biochemical analysis
After 6 weeks of the diet treatment, the rats were fasted overnight and sacrificed by CO2 asphyxiation. Blood samples were collected by a disposable plastic syringe into tubes and centrifuged at 3000 ×g for 10 minutes at 4 °C to separate the plasma from blood. Plasma glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, (ALP), albumin, creatinine, blood urea nitrogen, uric acid, and total protein were measured using a model 7180 automated analyzer (Hitachi, Tokyo, Japan) with commercial assay reagents (Sekisui Medical Co. Ltd., Tokyo, Japan).
2.8. Analysis of fecal lipid and cholesterol
The feces of rats were collected for 7 days between weeks 6 and 7 of the diet. The collected feces were lyophilized and pulverized. The total lipids and cholesterol in the feces were extracted with chloroform:methanol (2:1, v/v) as previously described [Citation16]. After the extraction, the total amount of lipids was determined as previously described [Citation16], with slight modifications, and cholesterol was measured using an enzymatic cholesterol oxidase kit (Asan Pharmaceutical Co., Seoul, Korea).
2.9. Histopathological analysis of liver
Fresh liver tissues were removed and stored at −80 °C. Five micrometer thick sections of the frozen liver tissues were prepared using a cryo-microtome and stained with Oil red-O [Citation17]. The stained specimens were examined by light microscopy using a model BX51 microscope (Olympus, Tokyo, Japan) and digital photographs were taken to assess structural abnormalities and the presence of lipid droplets in the liver. All images were taken at magnifications of 100× and 400×.
2.10. Statistical analyses
Data are expressed as mean ± SD and were analyzed by one-way analysis of variance using SPSS ver. 11.5 software (SPSS Inc., Chicago, IL) followed by Duncan’s multiple range tests. A p-value ≤.05 was regarded as statistically significant.
3. Results and discussion
3.1. Alpha-glucosidase inhibitory activity
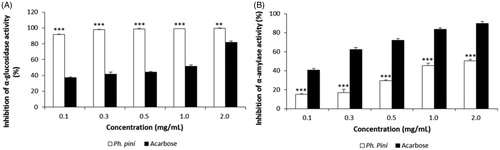
The inhibition of α-glucosidase activity by Ph. pini ME increased gradually as the extract concentration increased (91.79–99.64% at 0.125–2.0 mg/mL). The acarbose reference standard exhibited inhibitory activity that ranged from 37.27 to 81.81% at 0.125–2.0 mg/mL (). The highest inhibitory activity detected in the ME (99.64%) was significantly higher than that of acarbose (81.81%) at the same concentration (2.0 mg/mL). The results indicated that the ME possessed excellent inhibitory activities against α-glucosidase and might be a natural α-glucosidase inhibitor.
3.2. Alpha-amylase inhibitory activity
The α-amylase inhibitory effects of Ph. pini ME increased steadily as the Ph. pini extract concentrations increased (14.94–50.33% at 0.125–2.0 mg/mL). The acarbose positive control displayed excellent inhibition, ranging from 40.61 to 89.91% at 0.125–2.0 mg/mL (). The highest inhibitory activity (50.33%) observed in the ME (2.0 mg/mL) was significantly lower compared with acarbose (89.91%). The α-amylase inhibitory effect of the ME of Ganoderma lucidum fruiting bodies was reported as 21.92–94.46% at concentrations from 0.1 to 1.0 mg/mL [Citation18]. The inhibitory concentration (IC50) of hexane extract from six mushroom species (Grifola frondosa, Hericium erinaceum, G. lucidum, Coriolus versicolor, and Ph. linteus were reported as 2.79, 3.35, 6.90, 2.08, 1.20, and 4.08, respectively [Citation19]. The IC50 value of the Ph. pini ME of 1.67 exceeded the values of the aforementioned mushrooms, with the exception of G. lucidum and C. versicolor. Thus, the Ph. pini ME displayed moderate inhibition of α-amylase at the concentrations tested.
3.3. Pancreatic lipase inhibitory activity
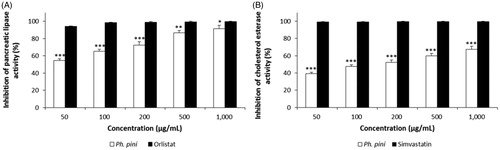
A recent study suggested that delaying dietary fat digestion and absorption is one of the successful strategies for treating hyperlipidemia. Pancreatic lipase is responsible for the digestion of TG into fatty acids and monoglycerides [Citation20]. In the current study, the hypolipidemic activity of the ME of the fruiting body of Ph. pini was investigated by assessing the inhibition of pancreatic lipase. The inhibition of pancreatic lipase is very important for treating diet-induced hyperlipidemia. The Ph. pini ME inhibited pancreatic lipase in a dose-dependent manner (). At 50–1000 µg/mL, the inhibitory effects of the ME ranged from 54.38 to 91.14%. Orlistat used as the standard reference exhibited excellent inhibition, ranging from 93.90 to 99.61% at the same concentrations (). The highest inhibitory activity (91.14%) observed in the ME at 1000 µg/mL was significantly lower than that of orlistat (99.61%). Pancreatic lipase inhibition displayed by the ME of the fruiting bodies of Marasmius stipitarius and Laetiporus sulphureus were 64% and 41% at 25 mg/mL [Citation21], whereas the inhibitory activity of the methanol extract from mycelia of Ph. linteus was 76% at 1000 µg/mL [Citation22], which suggests that the ME of Ph. pini has more pronounced pancreatic lipase inhibitory activity compared to M. stipitarius, L. sulphureus, and Ph. linteus. These results suggest that the ME of the Ph. pini possesses excellent in vitro inhibitory activity for pancreatic lipase compared to ME from the aforementioned mushroom fruiting bodies and mycelia, and compared to orlistat.
Figure 2. The pancreatic lipase and cholesterol esterase inhibitory activities of methanol extract from fruiting bodies of Phellinus pini. (A) Pancreatic lipase inhibitory activity; (B) Cholesterol esterase inhibitory activity. Values are means ± S.D (n = 3). ***p ≤ .001, **p ≤ .001, *p ≤ .05 versus positive controls.
3.4. Cholesterol esterase inhibitory activity
Pancreatic cholesterol esterase is important in the hydrolysis of dietary cholesterol esters. The cholesterol ester is hydrolyzed by pancreatic cholesterol esterase, which liberates free cholesterol in the small intestine. Moreover, pancreatic cholesterol esterase helps regulate the incorporation of cholesterol into mixed micelles [Citation23]. Therefore, the inhibition of cholesterol esterase is crucial to restrict and delay the absorption of dietary cholesterol in the small intestine. Lowering of cholesterol absorption by inhibiting the cholesterol esterase is a good strategy for the treatment of hyperlipidemia and obesity [Citation24]. The inhibitory effects of the ME on cholesterol esterase increased gradually as the extract concentration increased (39.12–67.23% at 50–1000 µg/mL). In contrast, at the same concentrations, the inhibitory activity of the simvastatin positive control ranged from 99.19 to 99.18% (). The highest inhibitory activity of simvastatin (99.68%) detected at 1000 µg/mL was significantly higher than that of the ME (67.23%) at the same concentration. Thus, the ME of Ph. pini fruiting body displayed moderate in vitro inhibitory activity on pancreatic cholesterol esterase compared to simvastatin.
3.5. Effects of Ph. pini on body weight gain and food intake in rats
After 6 weeks of feeding, the three different diets to the rats, the body weight gain of HFC rats (254.7 g) was significantly higher than those of the NC (243.9 g) and HFC + PhP (241.3 g) rats (). Body weight was significantly reduced by 5.6% in the HFC + PhP rats compared to the HFC rats. However, the food intake of the NC rats (741.3 g) was significantly higher than those of the HFC (672.3 g) and HFC + PhP rats (640.6 g), which indicates that the food efficient ratio (FER) of the NC rats were significantly lower than those of the other two diet groups. The lowest body weight gain and a moderate FER observed in the HFC + PhP rats indicate that the powdered fruiting body of Ph. pini may inhibit body weight increase induced by a high fat and cholesterol diet. This finding and the prior observation that rats fed a high cholesterol diet supplemented with 5% fruiting body powder of Pleurotus ostreatus displayed 16.89% reduction in body weight gain compared with rats fed a high cholesterol diet [Citation25] suggest that the supplementation of a high cholesterol diet with mushroom powder could ameliorate obesity. This suggestion is important given that obesity induced by a diet high in fat and cholesterol is related to atherosclerosis, CHD, hyperlipidemia, and diabetes [Citation26].
Table 2. Effect of Phellinus pini on body weight and food intake of rats.
3.6. Effects of Ph. pini on plasma lipid concentrations
Plasma lipid concentrations in the NC, HFC, and HFC + PhP rats after feeding Ph. pini for 6 weeks are presented in . The concentrations of plasma TC, LDL-C, and TG of the HFC rats increased by 20.94%, 82.16%, and 37.21%, respectively, compared to the NC rats. The profiles of the HFC + PhP rats were significantly lower by 39.57%, 53.21%, and 117.14%, respectively, compared to the HFC rats. Thus, feeding 5% Ph. pini to HFC rats significantly suppressed the increase in plasma TC, LDL-C, and TG concentrations. Bobek et al. [Citation27] reported that feeding rats a high cholesterol diet increased plasma TC, LDL-C, and TG levels by 35%, 39%, and 27%, respectively, compared to feeding 5% powdered P. ostreatus fruiting body. This indicates that dietary supplementation with mushroom extract could ameliorate hypercholesterolemia caused by a high cholesterol diet. Presently, feeding 5% Ph. pini to HFC rats significantly repressed the incremental change of plasma TC, LDL-C, and TG. How mushrooms repressed the plasma TC, LDL-C, and TG concentrations in HFC + PhP rats is still unknown. Other studies have demonstrated that the mushroom fruiting bodies of Lentinus edodes and Pleurotus ostreatus contain eritadenine and lovastatin, respectively, which effectively inhibit HMG-CoA reductase, thereby lowering the blood cholesterol level [Citation4,Citation28]. Thus, it is conceivable that feeding rats with Ph. pini mushrooms may inhibit the biosynthesis of cholesterol. Cheung [Citation29] demonstrated that rats fed hypercholesterolemic diets supplemented with 5% fruiting body powder of Auricularia auricula and Tremella fuciformis displayed significantly reduced serum TG, TC, and LDL-C levels compared with rats fed just a hypercholesterolemic diet. The author suggested that the lowered serum concentrations of TG, TC, and LDL-C in rats might be due to several components of the mushroom fruiting bodies, including β-glucans, flavonoids, phenolic compounds, and celluloses. The LDL-C/HDL-C ratio in HFC rats was significantly (8.89%) higher than that of NC rats, whereas the ratio of HFC + PhP (71.43%) was significantly lower than that of HFC (), indicating that feeding rats with 5% fruiting body powder of Ph. pini can significantly improve the atherogenic lipid profiles of the hyperlipidemic rats that were induced by the consumption of an HFC diet.
Figure 3. Effect of Phellinus pini on plasma low density lipoprotein cholesterol (LDL-C)/high density lipoprotein cholesterol (HDL-C) ratio in rats. Results are means ± SD (n = 8). Different symbol indicates significant differences among groups at **p ≤ .01 as determined by Duncan's multiple range tests. NC: normal control diet; HFC: high fat and cholesterol diet; HFC + PhP: high fat and cholesterol diet supplemented with 5% Phellinus pini fruiting body powder.
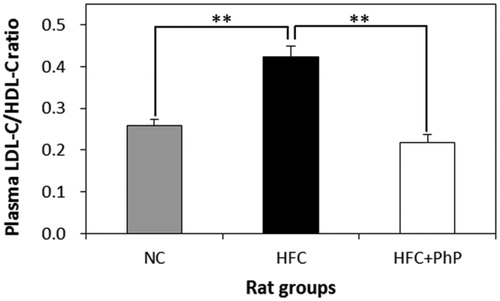
Table 3. Effect of Phellinus pini on plasma lipid profiles in rats.
3.7. Effects of Ph. pini on plasma biochemical parameters
The appropriate concentrations of plasma albumin, creatinine, blood urea nitrogen, uric acid, glucose, and total protein are indicators of normal metabolic conditions of living creatures [Citation30]. The results of these plasma biochemical parameters are presented in . Although the concentrations of albumin, creatinine, blood urea nitrogen, uric acid, and total protein of HFC + PhP rats were lower than the HFC rats, the values were not significantly different among three diet groups. This suggests that feeding Ph. pini did not adversely affect the plasma biochemical parameters. The plasma glucose concentration in the HFC + PhP rats was not significantly different than that of the NC rats, whereas the plasma glucose concentration of the HFC rats was significantly higher than those of the NC and HFC + PhP rats (p ≤ .05). The results suggest that an HFC diet-induced elevation in the level of plasma glucose concentration by feeding rats may be amended by also feeding them powdered fruiting body of Ph. pini.
Table 4. Effect of Phellinus pini on plasma biochemical parameters in rats.
3.8. Effects of Ph. pini on plasma enzyme profiles related to liver function
AST and ALT are typical enzymes within the liver [Citation31]. The enzymes can leak into the bloodstream when liver cells are damaged. Thus, the elevated levels of AST and ALT enzymes in the serum are useful to diagnose conditions and diseases of the liver, such as fatty liver, cirrhosis, and hepatitis [Citation32]. The plasma AST, ALT, and ALP concentrations in the Ph. pini mushroom-fed HFC rats were lower than that of the HFC rats (). No significant difference was observed in the activities of the plasma AST and ALT in the NC, HFC, or HFC + PhP rat groups, while the plasma ALP activity was significantly higher in the HFC rats (p ≤ .05) than those in the NC and HFC + PhP rat groups. Nevertheless, the supplementation of the HFC diet with 5% fruiting body of Ph. pini decreased plasma AST, ALT, and ALP activity by 5.21, 11.64, and 7.84%, respectively. Because the use of hypolipidemic drugs is increasing despite their various side effects, screening and identifying effective natural sources from plants and microorganisms, including mushrooms, are necessary [Citation33]. Therefore, the decreased enzyme activities that result from mushroom treatment may prevent oxidative damage by detoxifying reactive oxygen species in the liver, which could maintain good health in the liver of rats, and hopefully humans.
Table 5. Effect of Phellinus pini on plasma enzyme profiles related to liver function in rats.
3.9. Effects of Ph. pini on fecal total lipid and cholesterol excretion in rats
Fecal total lipids and cholesterol of the 5% Ph. pini mushroom fed HFC rats increased significantly by 2.1- and 3.1-fold compared to the levels in NC and HFC rats, respectively (). Because the plasma TC, LDL-C, and TG concentrations in the HFC + PhP rats were lower than those in the HFC rats, decreased plasma lipid profiles may have contributed to the excretion of lipids and cholesterol in the feces. Alam et al. [Citation34] reported that feeding 5% fruiting body powder of Pleurotus eryngii increased the excretion of total lipids and cholesterol by 2.5- and 3.9-fold, respectively, which resulted in decreased concentrations of plasma TC, LDL-C, and TG. LDL-C is one of the major carriers of cholesterol and transports cholesterol to cells throughout the body and leads to cholesterol accumulation within arteries. The accumulation may eventually lead to arterial blocking and an increased risk of heart disease and stroke [Citation35]. Thus, excessive excretion of TC and TG through feces in rats would decrease the absorption of TC and TG, in turn would reduce cholesterol synthesis in the liver. Therefore, excessive excretion of TC and TG via feces in the rats consuming the HFC + PhP can improve the plasma and liver lipid profiles.
Table 6. Effect of Phellinus pini on fecal total lipid and cholesterol in rats.
3.10. Effects of Ph. pini on the histopathology of liver in rats
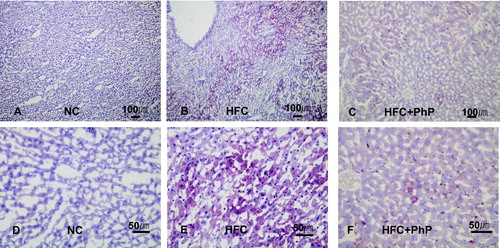
The effect of Ph. pini on hepatocyte cell histopathology of rats is presented in . The hepatic cords of the liver tissues stained with Oil red-O were typically arranged and located in the liver tissue near the central vein of the NC, HFC, and HFC + PhP rats. The abundant deposition of lipid droplets and small amount of lipids in the liver were observed in the HFC and HFC + PhP rats, respectively. Lipid accumulation was not observed in the liver of NC rats. The results suggest that fat liver disease (steatosis) induced by feeding rats a HFC diet was ameliorated by feeding rats 5% Ph. pini fruiting body powder. Why only a scant amount of lipid accumulated in the liver of rats fed a mushroom-supplemented diet is not fully understood, Yoon et al. [Citation36] proposed that diminished steatosis in the liver of rats fed L. edodes fruiting body powder might be due to several medicinal substances contained within fruiting bodies of mushrooms.
Figure 4. Effect of feeding Phellinus pini on hepatocyte cells in rats. (A–C) oil red O stained photomicrographs at 100×; (D–F) photomicrographs of red O stain at 400×. NC: normal control diet; HFC: high fat and cholesterol diet; HFC + PhP: high fat and cholesterol diet supplemented with 5% Phellinus pini fruiting body powder.
In conclusion, the ME of Ph. pini fruiting body inhibited pancreatic lipase and cholesterol esterase activities. The ME also showed comparable inhibitory activities on α-glucosidase and α-amylase. The dietary ingestion of Ph. pini fruiting body could bestow significant health benefits due to the modulation of various physiological functions, including reduced obesity and beneficial alterations in plasma lipid profiles and biochemical parameters, including TC, LDL, TG, and glucose. Our results suggest that the powdered form of Ph. pini fruiting body might be a good natural source of a prophylactic agent against hyperlipidemia and hypercholesterolemia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wahl PW, Walden CE, Knopp RH, et al. Lipid and lipoprotein triglyceride and cholesterol interrelationships: effects of sex, hormone use, and hyperlipidemia. Metab Clin Exp. 1984;33:502–508.
- Esmaillzadeh A, Azadbakht L. Food intake patterns may explain the high prevalence of cardiovascular risk factors among Iranian women. J Nutr. 2009;138:1469–1475.
- Sodjinou R, Agueh V, Fayomi B, et al. Obesity and cardio-metabolic risk factors in urban adults of Benin: relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health. 2008;8:1–13.
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582.
- Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418.
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269–273.
- Ibrahim R. Diabetes mellitus type II: review of oral treatment options. Int J Pharm Pharmaceut Sci. 2010;2:21–30.
- Laoufi H, Benariba N, Adjdir S, et al. In vitro α-amylase and α-glucosidase inhibitory activity of Ononis angustissima extracts. J Appl Pharmaceut Sci. 2017;7:191–198.
- Ying JZ, Mao XL, Ma QM, et al. Icons of medicinal fungi from China. Beijing: Science Press; 1987.
- Pak WH, Lee HD. Illustrated book of Korean medicinal mushrooms. 2nd ed. Seoul: Kyo-Hak Publishing Co. Ltd; 2003.
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951.
- Kim JS, Kwon CS, Son KH. Inhibition of α-glucosidase and amylase by Luteolin, a flavonoid. Biosci Biotech Biochem. 2000;64:2458–2461.
- Dastjerdi MZ, Namjoyan F, Azemi ME. Alpha amylase inhibition activity of some plants extract of Teucrium species. J Biol Sci Opin. 2015;7:26–31.
- Kim YS, Lee YM, Kim HJ, et al. Anti-obesity effect of Morus bombycis root extract: anti-lipase activity and lipolytic effect. J Ethnopharmacol. 2010;130:621–624.
- Pietsch M, Gütschow M. Synthesis of tricyclic 1,3-oxazin-4-ones and kinetic analysis of cholesterol esterase and acetylcholinesterase inhibition. J Med Chem. 2005;48:8270–8288.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
- Bayliss HO. Lipids. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. Edinburgh: Churchill Livingstone; 1990. p. 215–244.
- Santhoshkumar S, Nagarajan N. In vitro antioxidant and antidiabetic activity of methanol extract of wild mushroom Ganoderma lucidum (Curtis) P. Karst. Int J Biosci Nanosci. 2014;1:77–85.
- Su CH, Laib MN, Ng LT. Inhibitory effects of medicinal mushrooms on α-amylase and α-glucosidase-enzymes related to hyperglycemia. Food Funct. 2013;4:644–649.
- Maqsood M, Ahmed D, Atique I, et al. Lipase inhibitory activity of Lagenaria siceraria fruit as a strategy to treat obesity. Asian Pac J Trop Med. 2017;10:305–310.
- Slanc P, Doljak B, Mlinari A, et al. Screening of wood damaging fungi and macrofungi for inhibitors of pancreatic lipase. Phytother Res. 2004;18:758–762.
- Lee JK, Jang JH, Lee JT, et al. Extraction and characteristics of anti-obesity lipase inhibitor from Phellinus linteus. Mycobiology. 2010;38:52–57.
- Lunagariya NA, Patel NK, Jagtap SC, et al. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. Excli J. 2014;13:897–921.
- Chiou SY, Lai GW, Lin G. Kinetics and mechanisms of cholesterol esterase inhibition by cardiovascular drugs in vitro. Indian J Biochem Biophys. 2006;43:52–55.
- Alam N, Yoon KN, Lee TS, et al. Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. Mycobiology. 2011;39:45–51.
- Mori K, Kobayashi C, Tomita T, et al. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake) and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E–deficient mice. Nutr Res. 2008;28:335–342.
- Bobek P, Ozdin L, Kuniak L. Regulation of cholesterol metabolism with addition of oyster mushroom (Pleurotus ostreatus) in rats with hypercholesterolemia. Cas Lek Cesk. 1997;136:186–190.
- Golomb BA, Evans MA, Shimada Y, et al. Eritadenine induced alterations of plasma lipoprotein lipid concentrations and phosphatidylcholine molecular species profile in rats fed cholesterol-free and cholesterol-enriched diets. Biosci Biotechnol Biochem. 2003;67:996–1006.
- Cheung PCK. The hypercholesterolemic effect of two edible mushrooms: Auricularia auricula (tree-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats. Nutr Res. 1996;16:1721–1725.
- Ramachandra SG, Ramesh V, Krishnamurthy HN, et al. Normal hematological and plasma biochemical parameters of the captive bonnet monkey (Maccaca radiata). Primates. 1998;39:127–134.
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223–2230.
- Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Experi Biol. 2013;3:280–284.
- Schwandt P. Drug interactions and side effects of hypolipidemic drugs. Int J Clin Pharmacol Biopharm. 1979;17:351–356.
- Alam N, Yoon KN, Lee JS, et al. Dietary effect of Pleurotus eryngii on biochemical function and histology in hypercholesterolemic rats. Saudi J Biol Sci. 2011;18:403–409.
- Wadhera RK, Steen DL, Khan I, et al. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipid. 2016;10:472–489.
- Yoon KN, Alam N, Lee JS, et al. Antihyperlipidemic effect of dietary Lentinus edodes on plasma, feces and hepatic tissues in hypercholesterolemic rats. Mycobiology. 2011;39:96–102.