Abstract
Two-hundred and fifty-five strains of actinomycetes isolated from soil samples were screened for their antagonistic activities against four well-known wood decay fungi (WDF), including a brown rot fungus, Gloeophyllum trabeum and three white rot fungi Donkioporia expansa, Trametes versicolor, and Schizophyllum commune. A dual culture assay using culture media supplemented with heated or unheated culture filtrates of selected bacterial strains was used for the detection of their antimicrobial activity against four WDF. It was shown that Streptomyces atratus, S. tsukiyonensis, and Streptomyces sp. greatly inhibited the mycelial growth of the WDF tested compared with the control. To evaluate the biocontrol efficacy of S. atratus, S. tsukiyonensis, and Streptomyces sp., wood blocks of Pinus densiflora inoculated with three selected Streptomyces isolates were tested for weight loss, compression strength (perpendicular or parallel to the grain), bending strength, and chemical component changes. Of these three isolates used, Streptomyces sp. exhibited higher inhibitory activity against WDF, especially G. trabeum, as observed in mechanical and chemical change analyses. Scanning electron microscopy showed that cell walls of the wood block treated with Streptomyces strains were thicker and collapsed to a lesser extent than those of the non-treated control. Taken together, our findings indicate that Streptomyces sp. exhibits the potential to be used as a biocontrol agent for wood decay brown rot fungus that causes severe damage to coniferous woods.
1. Introduction
Increasing efforts have been made to improve disease management for sustainable production systems with a reduced chemical input in agriculture and forestry due to the growing awareness that integrated pest management strategies may provide more environmentally sound and economically feasible alternatives. The uncontrolled use of chemical pesticides has led to side effects, including residual toxicity and environmental pollution [Citation1], demanding immediate replacement of some of the chemical fungicide treatments with biocontrol agents [Citation2].
It is well known that actinomycetes, especially Streptomyces species, are saprophytic bacteria that decompose organic matters such as lignocellulose, starch, and chitin in soil [Citation3–5] and are capable of producing a wide range of antibiotics as secondary metabolites [Citation6–Citation8]. Although the role of Streptomyces species in mediating soil processes is less studied, the fact that these bacteria stimulate plant growth and protect plant roots against invasion by root pathogenic fungi provides a clear evidence that these are an important part of the rhizosphere microbiome [Citation9,Citation10].
Wood decay fungi (WDF) can be mainly classified into either brown rot or white rot fungi. This is based on the enzymatic capabilities to rapidly depolymerize cellulose through oxidative mechanisms; however, modified lignin remains as a polymeric residue in brown rot infection [Citation11–14]. In contrast, all components of plant cell walls, including cellulose, hemicellulose, and lignin are degraded during white rot infection [Citation15]. Although only a few groups of decay fungi are directly responsible for tree mortality as primary invaders [Citation16], the decay caused by wood rot fungi leads to the structural deterioration of woody tissues and cause significant economic losses [Citation17–19].
A biological control agent should essentially exert sufficient levels of antagonistic activity against a wide variety of pathogens. There are many cases where the goal of sustainable disease control against fungal pathogens is achieved by a wide range of microorganisms [Citation20]. This includes bacteria belonging to the genus Bacillus [Citation21,Citation22], Xanthomonas, and Serratia [Citation23], as well as fungal species belonging to the genus Trichoderma [Citation24]. Of these, Streptomyces species have received increased attention as biocontrol agents, given their exceptional abilities to produce secondary metabolites such as antibiotics [Citation6–8] and fungal cell wall-degrading enzymes produced, such as cellulases, hemicellulases, chitinases, and glucanases [Citation25,Citation26], as well as their potential to suppress the growth of a wide variety of fungal pathogens [Citation25,Citation27–29]. In this regard, the main aim of this study was to isolate actinomycetes, particularly Streptomyces species, from soil samples and characterize and evaluate the antifungal activities of these isolated species for their application as biocontrol agents against wood rotting fungi.
2. Materials and methods
2.1. Isolation of Streptomyces spp. from soil
Soil samples were obtained from the oak forest in Cheonggye Mountain, Korea, in 2013 to selectively isolate Streptomyces spp. Soil samples collected were placed in a plastic bag and transported to the laboratory for further analyses. A total of 10 g sieved soil sample was placed in a crucible dish and heated in an oven at 45 °C for 24 h until dried. The samples were suspended in 100 mL distilled water (DW) and subsequently serially diluted ranging from 10−3 up to 10−7. From each dilution, 0.1 mL suspension was spread evenly onto the surface of humic acid-vitamin (HV) agar media [Citation30] and the plates were incubated at 30 °C for 2 weeks. Colonies produced from each serially diluted plate were purified using 2% potato dextrose agar (PDA; Difco, Detroit, MI) and incubated at 30 °C for 2 weeks. Purified isolates presumed to be Streptomyces spp. were selected based on their morphologies [Citation31].
2.2. Isolates of WDF
To test the antifungal activity of Streptomyces spp. against WDF, four WDF reported causing damages to trees, timbers, and wood structures were selected [Citation19]. Of these, Trametes versicolor was isolated from Seokbyung mountain, Gangneung, Korea, and Donkioporia expansa, Gloeophyllum trabeum, and Schizophyllum commune were obtained from Centraalbureau voor Schimmelcultures (CBS, Utrecht, the Netherlands) (). All isolates obtained in this study were transferred to 2% PDA and incubated at 25 °C for 10 days for further analyses.
Table 1. Wood decay fungi used for testing the antifungal activity of Streptomyces spp.
2.3. Screening of Streptomyces spp. with antifungal activity
To evaluate the antifungal activity of 255 Streptomyces spp. isolated from soil against WDF, a dual culture assay was performed as a primary screening method. The fungal inoculum was prepared from the actively growing edge of the plate, excised using a cork borer and transferred onto the center of Petri dishes (90 mm) containing 2% PDA. Four different Streptomyces strains were applied to the edge of Petri dishes in the direction opposite to one another. These were subsequently incubated at 25 °C for 10 days and the antagonistic activity of Streptomyces spp. against WDF was inspected.
Based on the result from the primary screening, Streptomyces spp. that exhibited high-antagonistic activity against WDF were selected for the secondary screening of antifungal activity using the dual culture assay (one Streptomyces sp. against one wood decay fungus). The antifungal activity of Streptomyces spp. was scored based on the extent of suppression (inhibition zone) of WDF growth (45–65 mm: +, 25–45 mm: ++, ∼25 mm: +++).
2.4. Identification of the selected strains with strong antifungal activity against WDF
To ensure the identity of selected Streptomyces spp. with strong antifungal activity against WDF, these were subjected to DNA sequence comparisons based on the 16S rDNA region using primers, 27 F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1525 R (5′-AAG GAG GTG WTC CAR CC-3′) [Citation32]. Genomic DNAs were extracted using DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions. PCR amplification was performed using the T100TM thermal cycler (Bio-Rad, Hercules, CA). DNA sequence analysis was carried out following the techniques described by Lee et al. [Citation33]. All isolates obtained in this study were deposited at the culture collection of the Tree Pathology and Mycology Laboratory (TPML), Kangwon National University ().
Table 2. Identity of five Streptomyces spp. isolated in this study.
2.5. Assay using culture filtrates of the selected Streptomyces strains
For preparing culture filtrates for each selected Streptomyces spp., each strain was grown on 2% potato dextrose broth (PDB; Difco, Detroit, MI) by incubating at 30 °C with constant shaking at 140 rpm for 7 days. To decant the supernatant, suspensions of each Streptomyces spp. were centrifuged for 15 min at 9000 rpm. The supernatant was filtered with 0.4 μm nucleopore membranes. The filtered suspension was added to the media. Two types of media, heated and unheated media, were used for the assay to evaluate the antifungal activity against WDF. For the heated media (PHM), filtered suspensions of each strain were added after autoclave sterilization of PDA supplemented with 100 mg/L streptomycin sulfate (Sigma-Aldrich, Steinheim, Germany), whereas the filtered suspensions of each strain were directly added to PDA supplemented with 100 mg/L streptomycin sulfate without autoclaving for the unheated media (PUHM).
Agar plugs were excised from the growing edge of WDF isolates grown on PDA at 25 °C for 10 days and transferred onto either PHM or PUHM and incubated at 25 °C for 7 days to measure the rate of suppression (S) of the fungal growth. Controls were prepared by adding DW to PDA. The rate of suppression was calculated as below:
where C is the mycelial growth for control (PDA + DW), T is the mycelial growth for treatment (either PHM or PUHM).
2.6. Evaluation of Streptomyces spp. as a biocontrol agent using wood blocks
2.6.1. Preparing wood blocks
Sample blocks were prepared from Pinus densiflora harvested from the experiment forest in Hongcheon, Korea (belonging to Kangwon National University), as per the Korean Industrial Standards (KS F). Hexahedron wood blocks were prepared as 340 (l) × 20 (w) × 20 (h) mm for the analysis of the bending strength (KS F 2208), 20 (l) × 20 (w) × 30 (h) mm for the analysis of the compression strength (parallel to the grain) (KS F 2206), and 30 (l) × 20 (w) × 20 (h) mm for the analysis of the compression strength (perpendicular to the grain) (KS F 2206). All wood blocks were measured for the weight to evaluate the reduction ratio in the weight owing to WDF-mediated decay before their autoclave sterilization with ethylene oxide at 55 °C for 15 h.
2.6.2. Pre-inoculation of Streptomyces spp. on wood blocks
Selected Streptomyces spp. with high-antifungal activity against WDF were transferred to 2% PDB and incubated at 30 °C with shaking at 160 rpm for 4 days. To stimulate the colonization of Streptomyces spp. into wood blocks, wood blocks were immersed in the 4-day-old broth cultures of Streptomyces spp. and placed in a stainless-steel container inside plastic bags, which were sealed to prevent contamination. These were incubated at 30 °C for 10 days. Controls were prepared using sterile 2% PDB.
2.6.3. Preparation and inoculation of WDF onto Streptomyces-treated wood blocks (STW)
The isolate of WDF that showed maximum growth suppression in the dual culture assay in the presence of selected Streptomyces spp. was subjected to the inoculation test on Streptomyces-treated wood blocks (STW). WDF isolate was grown on 2% PDA at 25 °C for 7 days and 7-mm agar disc plugs excised from the growing edge of WDF isolate were inoculated onto STW and incubated in the dark at 25 °C for 120 days. All STW inoculated with WDF isolate were evaluated for weight loss, and mechanical and histological changes.
2.6.4. Mechanical test of STW
Changes in the weight of STW caused by WDF were demonstrated in accordance with KS F 2213. To calculate the weight loss, the oven-dry weight of STW (W1) was measured prior to the inoculation. The oven-dry weight of STW inoculated with WDF (W2) that was washed twice with sterilized water was measured 120 days after incubation at 25 °C. The weight loss of each wood block was calculated by the equation as follows:
A universal testing machine (Model: 4482; Instron, Norwood, MA) was used to test changes in mechanical properties of STW in accordance with KS F (compression strength and perpendicular or parallel to the grain: KS F 2206 and bending strength: KS F 2208). Measurements were made at a constant loading speed of 1.5 mm/min and span length of 200 mm. For the bending strength test, the modulus of rupture and modulus of elasticity were determined.
2.6.5. Determination of changes in the chemical components after treatment
To determine the changes in the chemical components of STW treated with WDF isolate, the wood flour (WF) was prepared with a size of 40 mesh from treated STW using a cutter mill (KF-20; KoreaMedi Co. Ltd., Daegu, Korea). The chemical properties were determined following the Soxhlet method (Technical Association of Pulp and Paper Industry, 1994).
Four grams of oven-dried WF in a thimble filter was extracted with 210 mL of ethanol/benzene mixture (1:2 v/v) using a Soxhlet extractor fitted with a reflux condenser at 80 °C for 6 h. The extracted solution was evaporated under reduced pressure. The extract was subsequently dried at 105 °C for 3 h and weighed. The extract-free sample left in the thimble filter was subjected to analyses for lignin and holocellulose contents.
The content of lignin and holocellulose was determined as the delignified residue using sodium chlorate (NaClO2) [Citation34]. A total of 1.5 g of the extract-free sample in 250 mL flask was treated with 75 mL of DW, 0.6 g of NaClO2, and 0.09 mL of acetic acid (CH3COOH) at 70–80 °C for 1 h. This procedure was repeated thrice. The solution was filtered using a glass filter (Iwaki Glass Co. Ltd., Tokyo, Japan) and successively washed with 300 mL of cold DW and 30 mL of acetone. The filtrated residue was dried at 105 °C for 2 h and weighed. The content of lignin and holocellulose was determined according to the equations below:
where L is the content of lignin (%), H is the content of holocellulose (%), W1 is the weight of the extract-free sample, and W2 is the weight of the treated sample.
2.6.6. Analysis of histological changes
The treated STWs incubated at 25 °C for 120 days were cut on a freezing microtome and subjected to scanning electron microscopy (SEM) analysis to determine histological changes. Wood specimens were mounted on aluminum, sputter-coated with gold–palladium, and observed by a scanning electron microscope (FE-SEM, SUPRA 55VP; Carl Zeiss, Oberkochen, Germany) at 15 kV.
2.7. Statistical analyses and graphs
One-way analysis of variance (ANOVA) and Tukey’s honestly significance difference (Tukey’s HSD) test were used to determine significant differences in the changes in mechanical, chemical, and histological properties of treated STWs based on a p value computed using R v.2.5.1 [http://www.r-project.org/: Citation35]. All the graphs produced in this study were generated using SigmaPlot v.10.0 (SPSS Inc., Chicago, IL).
3. Results and discussion
3.1. Isolation of Streptomyces spp. from soil and WDF
In total, 255 Streptomyces spp. were successfully isolated from the soil sample. These were used for screening strains with strong antifungal activities against WDF. In addition to Streptomyces spp. obtained in this study, six WDF isolates were successfully obtained and deposited at the culture collection of TPML, Kangwon National University, Korea ().
3.2. Screening and identification of Streptomyces spp. with antifungal activity
To screen and identify the strains of Streptomyces spp. with strong antifungal activity against WDF, 255 strains of Streptomyces spp. isolated from the soil were used for the dual culture assay. All Streptomyces spp. with strong antifungal activity against WDF were sequenced and the identities of the strains were confirmed based on the 16S rDNA region. Sequences were subjected to BLASTn analysis against the nucleotide database of NCBI (http://blast.st-va.ncbi.nlm.nih.gov/Blast.cgi). All sequence data produced in this study were deposited at NCBI (MG923820–923824, MG972195) (). In addition, all strains of Streptomyces spp. obtained in the study were deposited at the culture collection of TPML, Kangwon National University, Korea ().
On the basis of the primary screening, six strains of Streptomyces spp. were selected for the secondary screening using the dual culture assay. Of the strains used, three strains, including Streptomyces sp. 1 (TPML 13102), Streptomyces sp. 4 (TPML 13106), and Streptomyces sp. 6 (TPML 13094) showing high-antifungal activity against G. trabeum (TPML 12104) were eventually selected. This was followed by testing for their activities against T. versicolor (TPML 11015), D. expansa (TPML 13119), and S. commune (TPML 13123) ().
Table 3. Antifungal activity of Streptomyces spp. against four WDF.
3.3. Assay using culture filtrates of the selected strains of Streptomyces spp
Three strains of Streptomyces spp. (TPML 13102, TPML 13106, and TPML 13094) with strong antifungal activities against four WDF based on the dual culture assay were used to prepare culture filtrates. Five fungal isolates used as biocontrol agents against WDF [Citation36,Citation37] were also used as controls. These included Trichoderma atroviride (TPML 13118), T. viride (TPML 13117), T. harzianum (TPML 13115), T. virens (TPML 13116), and Gliocladium roseum (TPML 13114).
Of all stains, Streptomyces sp. 1 (TPML 13102) showed the highest suppression (∼50%) of the growth of WDF. The growth of G. trabeum (TPML 12104) was most suppressed in the presence of Streptomyces sp. 1 in the culture media, followed by S. commune (TPML 13123), T. versicolor (TPML 11015), and D. expansa (TPML 13119) ().
Table 4. Suppression rate (%) of the growth of WDF.
Culture filtrate is widely used to determine or screen potential biocontrol agents, especially Streptomyces spp., with antagonistic activities against pathogens [Citation38–41]. Our results based on the culture filtrate of Streptomyces spp. successfully identified the strain of Streptomyces sp. 1 that exhibited the potential as a biocontrol agent, given its ability to suppress the mycelial growth of WDF at a higher rate than the controls.
Our results showed that the selected Streptomyces sp. 1 strain exhibited higher levels of inhibition against WDF in the culture filtrate assay compared with five fungal isolates used as controls, including T. atroviride (TPML 13118), T. viride (TPML 13117), T. harzianum (TPML 13115), T. virens (TPML 13116), and G. roseum (TPML 13114) (), although these were shown to exhibit suppressive activities against wood rot fungi [Citation41,Citation42]. Thus, Streptomyces sp. 1 screened using the culture filtrate assay is more effective as a biocontrol agent than other fungi tested against WDF.
3.4. Evaluation of Streptomyces spp. as a biocontrol agent using wood blocks
3.4.1. Changes in mechanical properties
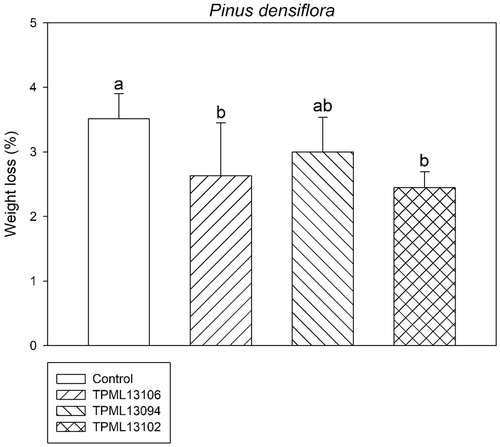
The weight loss caused by G. trabeum in P. densiflora wood blocks treated with the selected Streptomyces spp. is presented in . The weight loss was least for samples treated with Streptomyces sp. 1 (TPML 13102) (p = .02 at 95% confidence interval) compared with the control (3.5% weight loss). No significant difference was observed between samples treated with Streptomyces sp. 1 (TPML 13102, 2.4% weight loss) and Streptomyces sp. 6 (TPML 13094, 2.6% weight loss) (p = .523 at 95% confidence interval).
Figure 1. Comparison of the weight loss (%) of Pinus densiflora wood blocks treated with the selected Streptomyces spp. at 120 days after inoculation with Gloeophyllum trabeum. Means with the same letter on the bar are not significantly different, as analyzed with Tukey’s honestly significance difference test (p = .017 at 95% confidence interval).
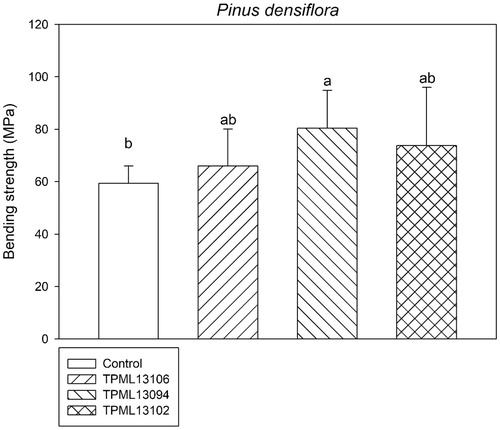
The compression strength (perpendicular or parallel to the grain) and bending strength of P. densiflora wood blocks treated with the selected Streptomyces spp. are shown in and Citation3, respectively. In analyses determining the compression strength (perpendicular or parallel to the grain) of P. densiflora wood blocks, the smallest decrease was observed for samples treated with Streptomyces sp. 1 (TPML 13102; 56.03 Mpa and 2.99 Mpa in compression strength perpendicular to the grain and parallel to the grain, respectively). This tendency was also observed in the analysis of the bending strength of wood blocks, wherein the second lowest decrease was reported for samples treated with Streptomyces sp. 1 (TPML 13102; 73.73 Mpa).
Figure 2. Comparison of the compression strength (perpendicular or parallel to the grain) of Pinus densiflora wood blocks subjected to treatment with selected Streptomyces spp. for 120 days after inoculation with Gloeophyllum trabeum. Means with the same letter on the bar are not significantly different based on Tukey’s honestly significance difference test (p = 1.708e−05 and 2.536e−05 at 95% confidence interval for compression strength perpendicular and parallel to the grain, respectively).
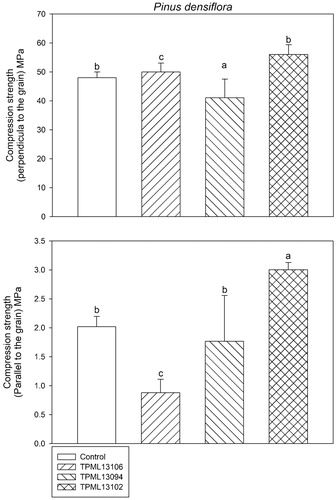
Figure 3. Comparison of the bending strength of Pinus densiflora wood blocks treated with the selected Streptomyces spp. for 120 days after inoculation with Gloeophyllum trabeum. Means with the same letter on the bar are not significantly different, based on Tukey’s honestly significance difference test (p = .017 at 95% confidence interval).
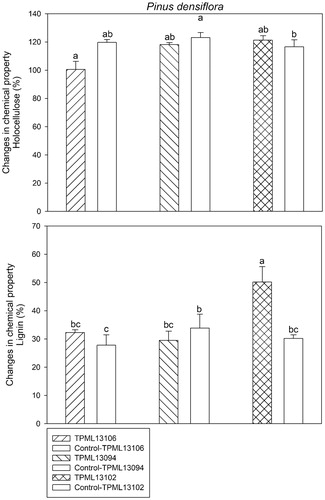
3.4.2. Changes in chemical properties
Cellulose deconstruction rate was higher in wood blocks treated with the selected Streptomyces spp. except for those treated with Streptomyces sp. 1 (TPML 13102), as observed in the analyses of changes in chemical properties, including lignin and holocellulose, at 120 days after the inoculation of wood blocks with G. trabeum (). The average amounts (%) of lignin and holocellulose retained in wood blocks were 50.1% and 121.2%, respectively, after treatment with Streptomyces sp. 1 (TPML 13102) compared with 30.2% and 116.5% in the control.
3.4.3. Analysis of histological changes
The wood blocks treated with Streptomyces sp. 1 that showed high-antifungal activity against G. trabeum were subjected to histological evaluation using SEM. The cell wall fiber of the wood blocks from the control group became thinner due to fungal attack, indicative of the degradation of the cell wall polymers by the inoculum. In addition, the tracheid collapsed in wood blocks from the control group. However, this tendency was not observed from the wood blocks treated with Streptomyces sp. 1 (TPML 13102) ().
Figure 5. Scanning electron micrographs (×150, ×1000, and ×5000) of cross-sections of Pinus densiflora specimens at 120 days after its inoculation with Gloeophyllum trabeum. Blocks treated with Streptomyces sp. 1 (A–C) and the non-treated controls (D–F). (A, D) bar 200 μm; (B, E) bar 20 μm; (C, F) bar 10 μm.
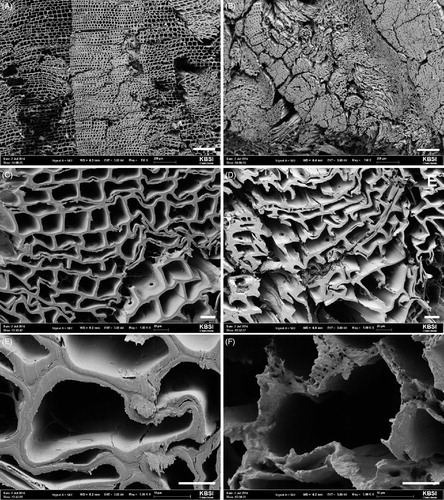
G. trabeum is one of the brown rot fungi of ecological importance, as it plays a significantly important role in biomass recycling and soil fertility in forest ecosystems [Citation43,Citation44]. In addition, it is an economic concern, as it causes the most prevalent and destructive type of wood deterioration arising from the rapid structural failure [Citation45]. We observed that the selected strain, Streptomyces sp. 1, successfully protected the wood from decay inflicted by the brown rot fungus, G. trabeum, as observed through mechanical, chemical, and histological properties.
Efforts are underway to explore alternatives for the use of chemical biocides due to increased concern over the environmental side-effects, as well as associated legislative constraints with the use of some chemical treatments. Our results suggest that Streptomyces sp. 1 is a promising biological control agent given the fact that it exhibits strong antagonism toward WDF, especially G. trabeum. Although modes of antagonism of Streptomyces sp. 1 against G. trabeum are remained uncovered, this strain may be a likely source of a large number of bioactive compounds.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wellman RH. Problems in development, registration, and use of fungicides. Annu Rev Phytopathol. 1977;15:153–163.
- Spadaro D, Gullino ML. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop Prot. 2005;24:601–613.
- Lechevalier MP. Actinomycetes in agriculture and forestry. In: Goodfellow M, Williams ST, Mordarski M, editors. Actinomycetes in biotechnology. New York: Academic Press; 1989. p. 327–358.
- Srnivasan MC, Laxman RS, Despharde MV. Physiology and nutritional aspects of actinomycetes: an overview. World J Microbiol Biotechnol. 1991;7:171–184.
- Zimmerman W. Degradation of lignin by bacteria. J Biotechnol. 1990;13:199–130.
- Franklin TJ, Snow GA, Barrett-Bee KJ, et al., editors. Antifungal, antiprotozoal and antiviral agents. 4th ed. Biochemistry of antimicrobial action. New York: Chapman & Hall Ltd; 1989. p. 137–161.
- Waksman SA, Lechevalier HA. The actinomycetes. Vol. III, Antibiotics of actinomycetes. Baltimore: The Williams & Wilkins Co; 1962. p. 248–252.
- Lechevalier MP. Actinomycetes in agriculture and forestry. In: Goodfellow M, Williams ST, Mordarski M, editors. Actinomycetes in biotechnology. New York: Academic Press; 1989. p. 327–358.
- Miller JJ, Liljeroth E, Henken G, et al. Fluctuations in the fluorescent pseudomonad and actinomycete populations of rhizosphere and rhizoplane during the growth of spring wheat. Can J Microbiol. 1990;36:389–391.
- Miller JJ, Liljeroth E, Willemsen-de Klein MJEIM, et al. The dynamics of actinomycetes and fluorescent pseudomonads in wheat rhizoplane and rhizosphere. Symbiosis. 1990;9:389–391.
- Blanchette R. Degradation of the lignocellulose complex in wood. Can J Bot. 1995;73:999–1010.
- Worrall JJ, Anagnost SE, Zabel RA. Comparison of wood decay among diverse lignicolous fungi. Mycologia. 1997;89:199–219.
- Yelle DJ, Ralph J, Lu F, et al. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol. 2008;10:1844–1849.
- Niemenmaa O, Uusi-Rauva A, Hatakka A. Demethoxylation of [O14CH3]-labelled lignin model compounds by the brown-rot fungi Gloeophyllum trabeum and Poria (Postia) placenta. Biodegradation. 2008;19:555–565.
- Martinez D, Challacombe J, Morgenstern I, et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA. 2009;106:1954–1959.
- Gilbertson RL. Wood-rotting fungi of North America. Mycologia. 1980;72:1–49.
- Rayner AD, Boddy L. Fungal decomposition of wood. Its biology and ecology. Wiley: John Wiley & Sons; 1988.
- Paterson RR. Ganoderma disease of oil palm – a white rot perspective necessary for integrated control. Crop Prot. 2007;9:1369–1376.
- Schmidt O. Indoor wood-decay basidiomycetes: damage, causal fungi, physiology, identification and characterization, prevention and control. Mycol Progress. 2007;6:261–279.
- Butt TM, Copping LG. Fungal biological control agents. Pestic Outlook. 2000;11:186–191.
- Ashwini N, Srividya S. Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. 2014;4:127–136.
- Solanki MK, Kumar S, Pandey AK, et al. Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Techn. 2012;22:203–117.
- Kobayashi DY, Guglielmoni M, Clarke BB. Isolation of the chitinolytic bacteria Xanthomonas maltophilia and Serratia marcescens as biological control agents for summer patch disease of turfgrass. Soil Biol Biochem. 1995;27:1479–1487.
- Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 2003;7:4–10.
- Yuan WM, Crawford DL. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl Environ Microbiol. 1995;61:3119–3128.
- Trejo-Estrada SR, Sepulveda IR, Crawford DL. In vivo antagonism of Streptomyces violaceusniger YCED9 a fungal pathogen of turfgrass. World J Microbiol Biotechnol. 1998;14:865–872.
- Crawford DL, Lynch JM, Whipps JM, et al. Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl Environ Microbiol. 1993;59:3899–3905.
- El-Abyad MS, El-Sayed MA, El-Shanshoury AR, et al. Towards the biological control of fungal and bacterial diseases of tomato using antagonistic Streptomyces spp. Plant Soil. 1993;149:185–193.
- Trejo-Estrada SR, Paszczynski A, Crawford DL. Antibiotics and enzymes produced by biocontrol agent Streptomyces violaceusniger YCED9. J Ind Microbiol Biotechnol. 1998;21:81–90.
- Hayakawa M, Nonomura H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Tech. 1987;65:501–509.
- Shirling ET, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol. 1966;16:313–340.
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: Wiley; 1991. p. 115–175.
- Lee DH, Lee SK, Lee SH, et al. Accurate detection of chestnut ink disease causing Phytophthora katsurae by nested PCR. Australasian Plant Pathol. 2012;41:535–539.
- Wise LE, Murphy M, D Adieco AA. A chlorite holocellulose, its fractionation and bearing on summative wood analysis and studies on the hemicelluloses. Paper Trade J. 1946;122:35–43.
- R Core Team. 2014. R: a language and environment for statistical computing. 2012.
- Kumar D, Gupta RK. Biocontrol of wood-rotting fungi. Indian J. Biotechnol. 2006;5:20–25.
- Susi P, Aktuganov G, Himanen J, et al. Biological control of wood decay against fungal infection. J. Environ Manage. 2011;92:1681–1689.
- Li Q, Jiang Y, Ning P, et al. Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Control. 2011;58:139–148.
- Aldesuquy HS, Mansour FA, Abo-Hamed SA. Effect of the culture filtrates of Streptomyces on growth and productivity of wheat plants. Folia Microbiol. 1998;43:465–470.
- Prapagdee B, Kuekulvong C, Mongkolsuk S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci. 2008;4:330–337.
- Bruce A, Highley TL. Control of growth of wood decay Basidiomycetes by Trichoderma spp. and other potentially antagonistic fungi. Forest Prod J. 1991;41:63–67.
- Pellegrini A, Prodorutti D, Pertot I. Use of bark mulch pre-inoculated with Trichoderma atroviride to control Armillaria root rot. Crop Prot. 2014;64:104–109.
- Gilbertson RL, Ryvarden L. North American polypores. Vol. 1 and 2. Oslo, Norway: Fungiflora; 1986.
- McFee WW, Stone EL. The persistence of decaying wood in the humus layers of northern forests. Soil Sci Soc Am J. 1966;30:513–516.
- Morrell JJ, Zabel RA. Wood microbiology: decay and its prevention. San Diego: Academic Press; 1992.