Abstract
Certain beneficial microorganisms isolated from rhizosphere soil promote plant growth and induce resistance to a wide variety of plant pathogens. We obtained 49 fungal isolates from the rhizosphere soil of paprika plants, and selected 18 of these isolates that did not inhibit tomato seed germination for further investigation. Based on a seed germination assay, we selected four isolates for further plant tests. Treatment of seeds with isolate JF27 promoted plant growth in pot tests, and suppressed bacterial speck disease caused by Pseudomonas syringae pathovar (pv.) tomato DC3000. Furthermore, expression of the pathogenesis-related 1 (PR1) gene was higher in the leaves of tomato plants grown from seeds treated with JF27; expression remained at a consistently higher level than in the control plants for 12 h after pathogen infection. The phylogenetic analysis of a partial internal transcribed spacer sequence and the β-tubulin gene identified isolate JF27 as Aspergillus terreus. Taken together, these results suggest that A. terreus JF27 has potential as a growth promoter and could be used to control bacterial speck disease by inducing resistance in tomato plants.
1. Introduction
Certain fungal isolates act as plant growth-promoting fungi (PGPF) and biocontrol agents in oilseed rape, cucumber, ginseng, rice, and soybean [Citation1–3]. They can also end seed dormancy [Citation4]. Hyakumachi [Citation5] demonstrated that PGPF enhance plant growth by inducing the production of phytohormones – including gibberellin-like or auxin-like compounds – and by altering phosphate solubilization, volatile compound production, and nutrient supply; they also suppress the activities of deleterious microbes.
The genus Aspergillus comprises filamentous fungi that are usually opportunistic and are common in soil, air, and decaying plant materials; these fungi produce a number of secondary metabolites. Some Aspergillus species inhibit nematode activity, act as biocontrols of plant diseases, and promote plant growth [Citation6]. Aspergillus terreus is industrially and pharmaceutically significant, because it produces secondary metabolites, such as itaconic acid, that are important for the production of fibers and paints, and for the synthesis of the cholesterol-lowering drug lovastatin [Citation7,Citation8]. Waqas et al. [Citation6] reported that the endophytic fungi Penicillium citrinum and A. terreus mitigate stem rot and promote plant growth in sunflowers.
Many PGPF can activate plant defense responses to pathogen attack by inducing resistance in the plants [Citation9]. Induced resistance is triggered through salicylic acid (SA)-dependent and/or jasmonic acid (JA)/ethylene (ET)-dependent pathways and is classified into two main types: systemic acquired resistance (SAR) and induced systemic resistance (ISR). SAR is usually triggered through the SA-dependent pathway after a pathogen attack; ISR is activated through the JA signaling pathway followed by the ET signaling pathway by certain nonpathogenic rhizosphere microorganisms [Citation10,Citation11]. However, these two types of signaling response are connected in a complicated manner, and lead to the systemic induction of disease defense-related genes and proteins.
The aims of the present study were: to identify fungi that promote the growth of tomato plants; to determine whether these PGPF can induce resistance to bacterial speck caused by Pseudomonas syringae pathovar (pv.) tomato DC3000; and to investigate the mechanisms underlying such resistance. To these ends, we examined the expression of marker genes related to SA-dependent and JA/ET-dependent signaling pathways. We also determined the identity of a selected isolate by sequencing and phylogenetic analyses.
2. Materials and methods
To identify potentially beneficial fungi, we tested the germination of tomato seeds in water agar containing various fungi. The tested fungi were initially isolated from the rhizosphere soil of paprika plants sampled in Jinju in 2016. The rhizosphere soil samples were suspended in 10 mM MgSO4 solution for 30 min at 28 °C, and the soil suspensions were then plated onto dichloran rose-bengal chloramphenicol (DRBC) agar. The plates were incubated at 28 °C for 4–7 d, and morphologically distinct colonies were isolated. In total, 49 fungal colonies were picked from the agar plates, and the isolates were stored at 4 °C until required. The isolates were cultured on water agar containing 0.2% glucose at 28 °C for 3 d. Tomato seeds (“Superdotaerang”, Syngenta, Korea), which had been sterilized in 1% NaOCl for 2 min, were placed on the edge of the mycelia and incubated at 28 °C. After incubation for 3–4 d, the seed germination rates were evaluated. This experiment was conducted twice with three replicates of 10 seeds.
To evaluate the effects of fungal isolates on the growth of tomato plants, four isolates (JF02, JF07, JF27, and JF44) were selected based on the results of the tomato seed germination assay and on their distinctive morphologies. We investigated the fungal characteristics of selected isolates: indole acetic acid (IAA) production using Salkowski’s reagent and phosphate solubilization using Pikovskaya’s medium [Citation12]; the production of extracellular enzymes including cellulase on carboxymethyl cellulose (CMC) media, protease on casein medium, and chitinase on colloidal chitin; antifungal activities on potato dextrose agar (PDA) amended with 10% filtered fungal broth; and the antibacterial activities of the filtered fungal broths on tryptic soy agar (TSA) amended with bacterial suspensions (10% of 109 cells/mL, v/v) of P. syringae pv. tomato DC3000 or Ralstonia solanacearum. For the plant assay, spores of these isolates were collected in sterile distilled water containing 0.03% Tween 20 (v/v) and filtered through four layers of sterile cheese cloth to remove the mycelia. The spore suspension was adjusted to a concentration of 107 spores/mL using a hemocytometer. Surface-sterile tomato seeds were soaked in a spore suspension (JF02, JF07, JF27, or JF44), benzothiadiazole (BTH; 0.1 mM, chemical control), or sterile distilled water containing 0.03% Tween 20 (v/v) (negative control) at 28 °C for 3 h. The seeds were then sown onto 9-cm diameter plastic pots containing 25 g of potting mixture (Bunong, Korea). The tomato plants were grown for 4 weeks (5–6-leaf stage) in a greenhouse at room temperature, and shoot length (cm), and fresh weight (g) were measured. The experiment was conducted twice with 10 replicates each.
We tested inoculate-induced resistance to P. syringae pv. tomato DC3000 in the leaves of tomato plants prepared as described above. P. syringae pv. tomato DC3000 was cultured on Luria-Bertani broth (LB) containing rifampicin (50 µg/mL). The cells were then collected and adjusted to a concentration of 104 cells/mL with 10 mM MgSO4 solution. The third leaves of the tomato plants were infiltrated with a suspension of P. syringae pv. tomato DC3000. Leaf discs (diameter, 1.2 cm) were collected 0, 1, 3, and 5 d after inoculation, macerated, and plated on LB containing rifampicin (50 µg/mL). We counted the colony-forming units (CFUs) 3 d after plating. This experiment was conducted twice with five plants in each experiment.
The expression of defense-related genes was analyzed using third leaves sampled at 0 (0–30 min), 6, or 12 h after inoculation with P. syringae pv. tomato DC3000. The following genes were analyzed: phenylalanine ammonia-lyase (PAL1), pathogenesis-related protein (PR1), jasmonic acid-amino synthetase (JAR1), and ethylene signaling protein (EIN2); actin was used as an internal control. The sequences of the gene-specific primer pairs used in this study are listed in . We homogenized leaf tissue samples using a Retsch MM200 mixer model mill (Retsh™, Germany), and total RNA was extracted using a plant RNA extraction kit (iNtRON, Korea) according to the manufacturer’s instructions. For the real-time polymerase chain reaction (PCR), first strand complementary DNA (cDNA) synthesis was performed using TOPscript™ RT DryMix (dT18plus) (Enzyomics, Korea). Real-time PCR was conducted using a reaction mixture with a final volume of 20 µL containing 1 μL of each primer (10 pmol), 3 μL cDNA (20% of the first-strand reaction), and 2 × SYBR reaction buffer (TOPread™ qPCR 2 × PreMIX, Enzynomics, Korea). Amplification was performed in a LightCycler 2.0 (Roche, Germany) under the following conditions: denaturation for 10 min at 95 °C; and 40 cycles for 10 s at 95 °C, 15 s at 57 °C, and 13 s at 72 °C. This experiment was conducted twice with three replicates each.
Table 1. Primer sequences.
We selected isolate JF27 for identification based on the results of the plant growth-promotion and induced-resistance experiments. For molecular identification, mycelia from JF27 isolates cultured in potato dextrose broth at 28 °C for 7 d were collected, and genomic DNA was extracted using a G-spin™ total DNA extraction kit (iNtRON, Korea). We used the following primer pairs for PCR amplification: internal transcribed spacer (ITS): ITS1-F, 5′-TCC GTA GGT GAA CCT GCG G-3′, ITS4-R, 5′-TCC TCC GCT TAT TGA TAT GC-3′; and β-tubulin (Bt2): Bt2a-F, 5′-GGT AAC CAA ATC GGT GCT GCT TTC-3′; Bt2b-R, 5′-ACC CTC AGT GTA GTG ACC CTT GGC-3′). PCR and sequencing were conducted by the Macrogen sequencing service (Daejeon, South Korea). DNA sequences from JF27 were compared to type isolates of Aspergillus species using the basic local alignment search tool (BLAST) network services at the National Center for Biotechnology Information (NCBI). We constructed a phylogenetic tree using the neighbor-joining method with the Molecular Evolutionary Genetics Analysis (MEGA) program (version 6.06; The Biodesign Institute, Tempe, AZ). Statistical analyses were performed using Statistical Analysis System software (SAS Institute, Cary, NC). Pooled data from repeated experiments were subjected to statistical analysis after confirming the homogeneity of variance using Levene’s test. Analyses of variance was performed using the general linear model (GLM) procedure, and differences compared to the control by the least significant difference (LSD) test at p < .05 are represented by an asterisk.
3. Results and discussion
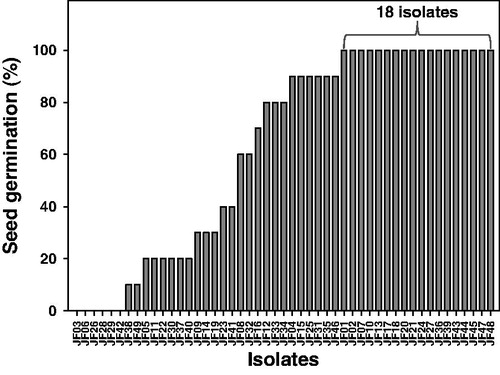
We obtained 49 fungal isolates from rhizosphere soil samples from paprika plants grown in a greenhouse in Jinju, Korea in 2016. The isolates were evaluated to determine their effect on seed germination and radicle formation. We observed different rates of seed germination and radicle growth after treatment with the various isolates (). Of the 49 isolates, 18 allowed seed germination and radicle growth. The remaining 31 isolates either inhibited seed germination or induced rot. Based on their distinctive morphological features, 4 of the 18 isolates were selected for further testing.
Figure 1. Seed germination (%) of tomato plants in water agar grown with the tested fungi. A total of 49 fungal isolates were inoculated on water agar amended with 0.2% glucose, and surface sterile tomato seeds were placed at the edge of the fungal mycelia. The germinated seeds and radicles were evaluated.
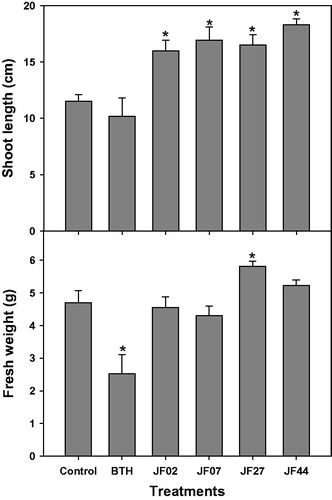
The four isolates (JF02, JF07, JF27, and JF44) were evaluated for the promotion of tomato plant growth. The plants in all groups exhibited significantly increased shoot lengths 4 weeks after seed treatment; in the JF27-treated group, both fresh weight and shoot length increased in comparison to the control group ().
Figure 2. Growth promotion of tomato plants by four fungal isolates (JF02, JF07, JF27, and JF44). Surface sterile tomato seeds were treated with a fungal spore suspension (107 spores/mL) at 28 °C for 3 h. Tomato seeds were grown in pots containing a potting mixture for 4 weeks (5–6 leaf stage), then the shoot length (cm) and fresh weight (g) were measured. Asterisks on the bars indicate significant differences (p < .05) compared with the control according to the least significant difference test.
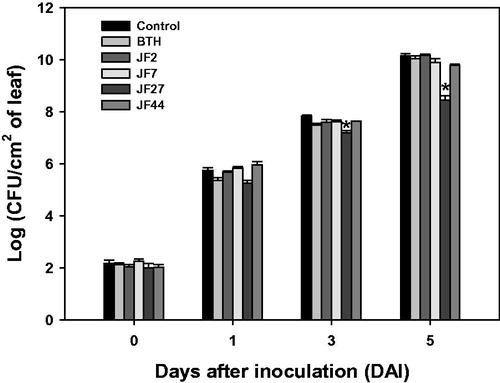
In addition to promoting plant growth, JF27 treatment reduced pathogen infection and the spread of P. syringae pv. tomato DC3000 compared to the control group at 3 and 5 d after inoculation (). However, JF02, JF07, and JF44 did not reduce disease rates in the tomato plants. Overall, we found that the JF27 isolate promoted growth and induced disease resistance in tomato plants.
Figure 3. Resistance of tomato plants to Pseudomonas syringae pv. tomato DC 3000 induced by the fungal isolates. Seeds were treated with a fungal spore suspension, and 4 weeks after seeding, the bacterial pathogen (104 cells/mL) was infiltrated into the third leaf of each tomato plant. The infiltrated leaf was macerated and plated onto LB amended with rifampicin (50 μg/mL). Colony-forming units (CFUs) were counted. Asterisks on the bars indicate significant differences (p < .05) compared with the control according to the least significant difference test.
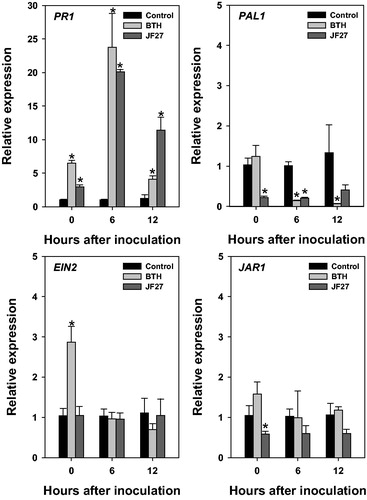
We also investigated the expression of marker genes related to specific signaling pathways to determine the molecular mechanisms involved in inducing resistance to P. syringae pv. tomato DC3000 in tomato plants (). The SA-, JA- and ethylene-mediated signaling pathways regulate the expression of particular defense marker genes: the SA-mediated pathway regulates PAL1 and PR1; the JA-mediated signaling pathway regulates JAR1; and the ethylene-mediated pathway regulates EIN2. Therefore, we determined the relative expression levels of these genes at 0, 6, and 12 h after inoculation. Overall, the expression of PR1 was higher in the tomato plants treated with the JF27 isolate than in the control plants. At 6 h after inoculation, PR1 expression was highest in the BTH-treated group (the positive control) and the JF27-treated group; in contrast, at 12 h after inoculation, the relative expression of PR1 was reduced in the BTH-treated group, but remained higher in the JF27-treated groups than in the control group. There were no differences in the expression levels of the other tested marker genes – PAL1, EIN2, and JAR1 – compared to the control.
Figure 4. Relative gene expression in tomato plants after infection with Pseudomonas syringae pv. tomato DC3000. Expression analysis of genes PR1, PAL1, EIN2, and JAR1 at 0, 6, and 12 h after inoculation. Actin was used as an internal control. Asterisks on the bars indicate significant differences (p < .05) compared with the control according to the least significant difference test.
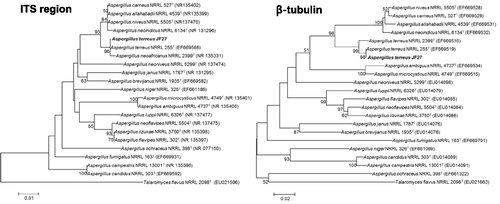
A partial ITS region (533 bp) and the β-tubulin gene (423 bp) were sequenced in the JF27 isolate. Based on a neighbor-joining analysis of the ITS and β-tubulin regions, isolate JF27 clustered with A. terreus NRRL255T, with 100% similarity in both regions. A maximum likelihood analysis yielded similar results to those of the neighbor-joining analysis (). Therefore, JF27 was renamed A. terreus JF27.
Figure 5. Phylogenetic trees created using the neighbor-joining method showing the relationships between isolate JF27 and the other members of the genus Aspergillus, based on a phylogenetic analysis of the partial nuclear ribosomal internal transcribed spacer (ITS) region and the β-tubulin sequence. Bootstrap values of 1000 analyses are shown at the branching points. The scale bar represents numbers of nucleotide substitutions per 100 nucleotides of the sequence. The type isolate is indicated as “T”; accession numbers deposited at GenBank are shown in parentheses.
The use of microorganisms to promote plant growth and suppress disease offers a promising alternative to the application of chemical fertilizers, and may lead to sustainable agricultural systems. PGPF affect plant phytohormones – such as gibberellin-like and auxin-like hormones – and alter the levels of endogenous plant hormones related to plant growth and phosphate production [Citation14,Citation15]. Auxin-like phytohormones, such as indoleacetic acid (IAA), can induce morphological and architectural changes in plants, and contribute to overall plant growth and development [Citation16]. Isolate JF27 also caused the production of IAA (17.5 µg/mL), but did not induce phosphate production (). Therefore, IAA production induced by the JF27 isolate might be one of the mechanisms by which plant growth is promoted.
Table 2. Characterization of fungi isolates JF02, JF07, JF27, and JF44.
The present study also revealed that JF27-mediated resistance to P. syringae pv. tomato DC3000 stimulated upregulation of the SAR-marker PR1 in tomato plants [Citation17–19]. SAR is dependent on the SA-pathway, and leads to the activation of pathogenesis-related proteins by NPR1 and WRKY transcription factors. Interestingly, in the present study, treatment with the JF27 isolate did not induce expression of the PAL gene. Mathys et al. [Citation20] reported that in the ISR system of Arabidopsis thaliana, SA was synthesized via the chorismate pathway rather than the phenylalanine pathway, which is generally considered the main route of SA-synthesis. The chorismate pathway has been postulated as an alternative route for the production of SA-mediated defense responses [Citation21]. Induction of the mitogen-activated protein kinases (MAPKs) pathway might lead to activation of transcription factors for defense genes, which then participate in the induction of WRKY and MYB or MYC proteins. WRKY proteins play a pivotal role in chromatin modification for gene expression, and MYB and MYC proteins are known activators of pathogenesis-related genes [Citation22,Citation23]. Therefore, defense responses after JF27 treatment, especially those involving changes to PR1 expression, could affect diverse and complex signaling pathways.
Although the mechanism by which fungi induce resistance has not been fully elucidated, it is known that defense resistance is activated by elicitors released by fungal agents that might interact with plant receptors and reinforce defense responses. Based on research into Trichoderma–plant interactions, elicitors released from Trichoderma hyphae appear to be involved in the induction of resistance [Citation23]. The elicitors released by fungi are proteins (small extracellular cysteine-rich proteins), low-molecular-weight peptides [Citation24], enzymes (serine protease, xylanase, chitinases, cellulases, and proteases) [Citation25], indole compounds [Citation26], lipids and their derivatives (glycosphingolipids) [Citation27], polysaccharides, and oligosaccharides [Citation20]. In this study, the JF27 isolate induced the release of indole compounds, but did not induce the release of extracellular cellulase, chitinase, or protease. Indoles or unidentified compounds produced by JF27 might act as elicitors for the induction of resistance as well as plant growth promotion. Recent studies have demonstrated that novel elicitors – including low-molecular-weight peptides and nonpolar compounds – released by PGPF or biocontrol fungi are able to trigger defense responses [Citation18,Citation28]. Aspergillus species also produce a large number of bioactive metabolites such as penicillin, viridivatin, mevinolin, and cyclopiazonic acid [Citation29,Citation30]. A. terreus can produce many biological compounds, including polyketides, sulochrin, terretonin, butyrolactone, lavostatin analogs, and other metabolites [Citation31–35]. Although these compounds have not been investigated as elicitors of the induction of plant defenses, they warrant further study.
In conclusion, in this study, we showed that isolate JF27 can improve plant growth and reduce bacterial speck infection caused by P. syringae pv. tomato DC3000. To alleviate disease and produce healthy plants, an investigation of fungal elicitors and the diverse and complex pathways by which they function is required.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang Q, Zhang J, Yang L, et al. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control. 2014;72:98–108.
- Park Y-H, Chung JY, Ahn DJ, et al. Screening and characterization of endophytic fungi of Panax ginseng Meyer for biocontrol activity against ginseng pathogens. Biol Control. 2015;91:71–81.
- Syamsia Kuswinanti T, Syam’un E, et al. The potency of endophytic fungal isolates collected from local aromatic rice as indole acetic acid (IAA) producer. Procedia Food Sci. 2015;3:96–103.
- Delgado-Sánchez P, Ortega-Amaro MA, Jiménez-Bremont JF, et al. Are fungi important for breaking seed dormancy in desert species? Experimental evidence in Opuntia streptacantha (Cactaceae). Plant Biol. 2011;13:154–159.
- Hyakumachi M. Research on biological control of plant diseases: present state and perspectives. J Gen Plant Pathol. 2013;79:435–440.
- Waqas M, Khan AL, Hamayun M, et al. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrnum and Aspergillus terreus. J Plant Interact. 2015;10:280–287.
- Saha BC, Kennedy GJ. Ninety six well microtiter plate as microbioreactors for production of itaconic acid by six Aspergillus terreus strains. J Microbiol Methods. 2018;144:53–59.
- Raina S, De Vizio D, Palonen EK, et al. Is quorum sensing involved in lovastatin production in the filamentous fungus Aspergillus terreus? Process Biochem. 2012;47:843–852.
- Jogaiah S, Abdelrahman M, Tran L-SP, et al. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J Exp Bot. 2013;64:3829–3842.
- Van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–483.
- Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209.
- Løvda T, Lillo C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem. 2009;387:238–242.
- Munoz-Espinoza V, López-Climent MF, Casaretto JA, et al. Water stress responses of tomato mutants impaired in hormone biosynthesis reveal absicisic acid, jasmonic acid and salicylic acid interactions. Front Plant Sci. 2015;6:1–13.
- Priyadharsini P, Muthukumar T. The root endophytic fungus Curvularia geniculate from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017;27:69–77.
- Khan AL, Hamayun M, Kim Y-H, et al. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011;46:440–447.
- Wani ZA, Mirza DN, Arora P, et al. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: an insight into the microbiome of Crocus sativus Linn. Fungal Biol. 2016;120:1509–1524.
- Murali M, Sudisha J, Amruthesh KN, et al. Rhizosphere fungus Penicillium chrysogenum promotes growth and induces defense-related genes and downy mildew disease resistance in pearl millet. Plant Biol. 2013;15:111–118.
- Nawrocka J, Małolepsza U. Diversity in plant systemic resistance induced by Trichoderma. Biol Control. 2013;67:149–156.
- Liu S-I, Wu J, Zhang P, et al. Response of phytohormones and correlation of SAR signal pathway genes to the different resistance levels of grapevine against Plasmopara viticola infection. Plant Physiol Biochem. 2016;107:56–66.
- Mathys J, De Cremer K, Timmermans P, et al. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 against Botrytis cinerea infection. Front Plant Sci. 2012;3:1025–1081.
- Pikovskaya RI. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370.
- Conrath U. Molecular aspects of defense priming. Trends Plant Sci. 2011;16:524–531.
- López-Mondéjar R, Ros M, Pascual JA. Mycoparasitism-related genes expression of Trichoderma harzianum isolates to evaluate their efficacy as biological control agent. Biol Control. 2011;56:59–66.
- Salas-Marina MA, Silva-Flores MA, Uresti-Rivera EE, et al. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur J Plant Pathol. 2011;131:15–26.
- Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43.
- Gravel V, Antoun H, Tweddell RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem. 2007;39:1968–1977.
- Mukherjee PK, Horwitz BA, Kenerley CM. Secondary metabolism in Trichoderma-a genomic perspective. Microbiology (Reading, Engl.). 2012;158:35–45.
- Baker SE, Perrone G, Richardson NM, et al. Phylogenomic analysis of polyketide synthase-encoding genes in Trichoderma. Microbiology. 2012;158:147–154.
- Frisvad JC, Larsen TO. Chemodiversity in the genus Aspergillus. Appl Microbiol Biotechnol. 2015;99:7859–7877.
- Wang Q, Yang Y-B, Yang X-Q, et al. Lovastatin analogues and other metabolites from soil-derived Aspergillus terreus YIM PH30711. Phytochemistry. 2018;145:146–152.
- Guo CJ, Wang CCC. Recent advances in genome mining of secondary metabolites in Aspergillus terreus. Front Microbiol. 2014;5:717.
- Nesha R, Siddiqui ZA. Effects of Paecilomyces lilacinus and Aspergillus niger alone and in combination on the growth, chlorophyll contents and soft rot disease complex of carrot. Sci Hortic. 2017;218:258–264.
- Shoresh M, Harman GE. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: a proteomic approach. Plant Physiol. 2008;147:2147–2163.
- Strawn MA, Marr SK, Inoue K, et al. Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem. 2007;282:5919–5933.
- Zhang Y, Shi X, Li B, et al. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiol Biochem. 2016;106:64–72.
