Abstract
Okra (Abelmoschus esculentus (L.) Moench) has gained more popularity as an economically significant plant for its nutritional and medicinal value, especially in China. During 2014–2016, the root disease of okra was discovered in four okra commercial fields surveyed in China. A fungul was isolated from the infected tissues, and was identified by Verticillium dahliae based on morphological characteristics. Pathogenicity test demonstrated that the fungus was pathogenic on okra, and fulfilled Koch’s postulates. The analysis of three sequences revealed 99–100% identity with the reported V. dahliae strain in GenBank. Neighbor-joining analysis of the gene sequences revealed that the representative isolates were clustered with V. dahliae. To the best of our knowledge, this is the first report of Verticillium wilt of okra in China.
Okra (Abelmoschus esculentus (L.) Moench) is an annual herb in the family Malvaceae, which is wildly grown in Africa, Asia, America, and Europe. It has high economic value that the whole plant, roots, leaves, flowers, and seeds of okra are commonly consumed as a vegetable or for medicinal purposes [Citation1]. Okra is commonly planted on a large scale worldwide, with 1.78 million ha harvested area and 9.50 million tons in 2012 and 1.83 million ha harvested area and 9.62 million tons in 2014 (http://www.fao.org/faostat/en/#data/QC). In 1914, okra was brought into China [Citation2] and has been commonly cultivated. China became one of the large okra producers in the world.
There are several major pathogens associated with okra production. These include Alternaria alternate, Aplosporella beaumontiana, Cercospora abelmoschi, Choanephora conjuncta, and Fusarium oxysporum worldwide [Citation3–7]. Vascular wilt caused by soil-borne pathogens is one of the most destroying and economically important plant diseases worldwide [Citation8]. Verticillium wilt is one of the most terrible and ubiquitous vascular disease in vegetables, ornamental, and tree crops, which could be caused by many species of Verticillium. Verticillium is a group of fungi and have a long taxonomic history. About 190 species so far have been described [Citation9]. V. dahliae is a highly virulent and phytopathogenic fungus. In China, only three pathogens have been reported on okra, namely Boeremia exigua, Botrytis cinerea, and F. solani, causing Boeremia leaf and fruit spot, grey mold, and Fusarium root rot, respectively [Citation10–12].
From 2014 to 2016, wilt symptoms were observed in four commercial fields of okra the occurrence of this disease was assessed in Yanqing District (115°97′E, 40°47′N), Beijing City, China. The objective of this study was to identify the pathogen associated with this wilt disease.
During a field survey conducted in China in December 2014–October 2016, commercial okra plants exhibiting typical wilt symptoms were observed. In the fields, the plants showed typical wilt symptom (). Leaves became firstly yellow, and then wilted. The black stripes and discoloration were showed in the inside of the infected stems (). Under high humidity condition, the stems developed a layer of white mold (). A total of 39 diseased plants were arbitrarily selected over the okra production area and examined for the possible causes. Diseased vascular system was washed with running tap water and cut into small pieces (5 × 5 mm). The pieces were surface-sterilized for 1 min in 1% NaOCl, 30 s in 75% ethanol, and rinsed with sterilized distilled water, and then placed on potato dextrose agar (PDA) with 0.03% streptomycin sulfate (five pieces per dish). All dishes were incubated at 22 ± 2 °C under darkness in a growth chamber.
Figure 1. (A) Symptoms of Verticillium wilt on okra in the field. (B) Vascular wilt appeared on longitudinal sections of stems. (C) A layer of white mold on stems under high humidity.

Mycelia from some colonies were transferred to new PDA dishes at 22 ± 2 °C under darkness for 20 days. Fungal colonies were purified by single-spore culturing.
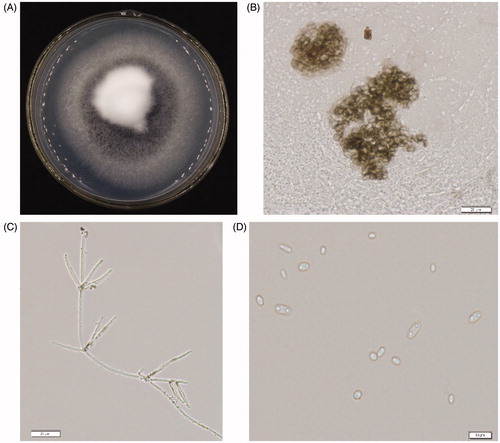
After 10 days of incubation on PDA at 25 °C under darkness, 86 isolates were obtained from 39 diseased plants. Colony texture were off-white with a felt-like surface and a little toothed margin (). On the surface of colonies, a lot of small black spots named microsclerotia were formed. Microsclerotia were nearly spherical, diameter 41.3 μm (). The conidiophore is always transparent, the upper end is composed of 3–4 radial shoots and a bud branch, colorless, with a diaphragm, each layer is separated by 20–45 μm, 3–4 branches per wheel, 13.7–21.4 μm × 2.3–2.7 μm, each branch of the first to a few conidia, full length 110–130 μm × 2.5 μm (). Conidia are long-oval, colorless, monospora, 2.3–9.1 μm × 1.5–3 μm (). Based on these morphological characteristics, all 36 isolates were identified as V. dahliae (Kleb.).
Figure 2. Cultural characteristics and morphological features of Verticillium dahliae. (A) Colony grown on PDA at 25 °C for 20 days. (B) Microsclerotium (Bar = 20 μm). (C) Mycelia and verticillate conidiophores of the isolate (Bar = 20 μm). (D) Conidia of the isolate (Bar = 20 μm).
The pathogenicity tests of the 36 isolates randomly selected were conducted on healthy okra cultivars cv. Jiayuan using the root-dip technique. The conidia were collected from 20-day-old PDA cultures incubated at 22 ± 2 °C under darkness. Spore suspension was prepared by adding autoclaved water and the concentration was adjusted to 1 × 106 spores/ml using a hemocytometer. The seedlings were planted in 6 cm diameter plastic pots in the greenhouse maintained at 25 ± 2 °C with 60% relative humidity (RH) and a 12-h photoperiod. Three-week-old seedlings were inoculated by dipping roots in suspension for 1 min and seedlings treated with sterile distilled water served as a negative control. All inoculated seedlings were transplanted into pots under greenhouse conditions in a growth chamber (25 ± 2 °C with 98% RH 12/12 h day/night cycle). The pathogen was re-isolated from all inoculated seedlings. Morphological and culture characteristics of cultured isolates were compared with the original isolates. The experiment was repeated three times, each time with 15 plants replicates per isolate. Data sets were tested for percent infection using the following formula [Citation13]:
represents the number of chlorotic leaves, while
is the total number of leaves on a tested okra.
The pathogenicity tests revealed that all 36 isolates were pathogenic to okra. The plants inoculated with the spore suspension showed typical vascular wilt symptoms. After 20 days of inoculation, the top of plants showed infection symptoms such as branch dieback on one side of the shoot. The leaves turned yellow subsequently. As the disease expanded, the leaves became wilted (). The stems and roots exhibited the characteristic vascular discoloration of Verticillium infection. Longitudinal sections of the roots displayed browning of vascular tissues (), while the no-incubated plants also showed no symptoms ()). Eventually, the plants wilted and died. Approximately 72% of the inoculated plants showed symptoms of Verticillium wilt ().
Figure 3. Inoculated okra plants. (A) The plant wilt after inoculation (left) and control plants (right). (B) The roots became brown and rotting after inoculation (left) and control plants (right).
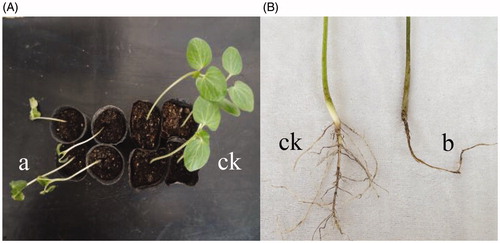
Table 1. Disease incidence of inoculated okra plants.
The 36 isolates randomly selected were grown on PDA at 22 ± 2 °C for 10 days, and then transferred to potato dextrose (PD) at 27 °C for 10 days on a shaker with 120 rpm. Approximately 25 mg mycelium of each isolate was collected for the total genomic DNA extraction using the CTAB method [Citation14]. The internal transcribed spaces rDNA (ITS rDNA), mitochondrial small subunit rDNA (mtSSU rDNA), 5.8S rDNA, and 18S rDNA were amplified using primers ITS1/ITS4, VaF1/VaR1, and VeruniF2/VeruniR3 [Citation15–17]. All PCR mixtures and conditions followed those outlined by White et al. [Citation15–17]. Sequencing was executed by Biomad (BigDye Terminator ready reaction mix v31; Applied Biosystems, Bejing, China). The resulting sequences were compared with the GenBank database using BLASTN in the National Center for Biotechnology Information database (NCBI, http://www.ncbi.nlm.nih.gov). Both similar sequences and the outgroup were aligned using MEGA 6.1 software package, and then phylogenetic analysis was conducted using the Neighbor-Joining (NJ) method with a bootstrap of 1000 replicates based on the ITS sequence. The experiments were conducted twice for confirmation.
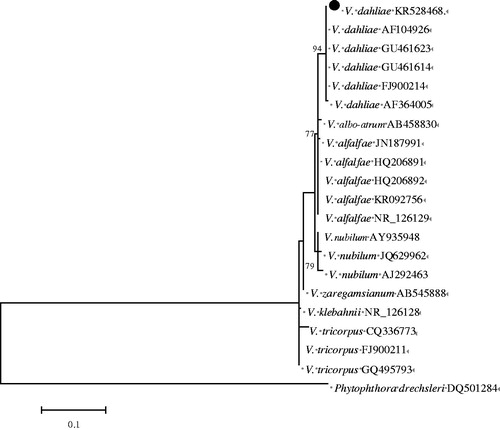
The ITS rDNA, mtSSU rDNA, 5.8S rDNA, and 18S rDNA of all 36 isolates were amplified by PCR. Sequence analysis showed the products were approximately 543, 722, and 699 bp in size. The sequence fragments shared 99–100% identity with published V. dahliae isolates in GenBank (Accession nos. AF104926, FJ900214; GU461423, AF108486; CP010980, CP009075; U33637, KJ443093, respectively). The sequences of the representative isolate QK14120901 have been submitted to NCBI database in GenBank (Accession nos. KR528468, MG572339, MG572340). Phylogenetic analysis revealed that the representative isolate was clustered in a clade with known V. dahliae, while forming a distinct clade from the other Verticillium species ().
Figure 4. Phylogenetic tree constructed using the Neighbour-joining method of MEGA 6.1 program based on the ITS sequences of the tested isolate (KR528468) and 20 isolates retrieved form Genebank. Bootstrap support values resulting from 1000 replicates and Phytophthora drechsleri served as the out group.
V. dahliae can survive in soil for at least 14 years as dormant and persistent microsclerotia [Citation18]. It has the widest host range than other Verticillium species, affecting more than 200 hosts in at least 14 different families [Citation19]. Among the major Verticillium species, V. dahliae and V. albo-atrum are the most important species which could cause economic losses in crops in most studies. V. dahliae could produce microsclerotia while V. albo-atrum produce melanized mycelia and pigmented conidiophores [Citation20]. Verticillium wilt caused by V. longisporum has also been reported in Sweden, Germany, France, Poland, and Japan, which was specifically pathogenic on Brassicacease cabbage and Chinese cabbage. Studies have showed that the molecular phylogenetic relatives of V. dahliae, V. albo-atrum, and V. longisporum are close, whereas they formed a distinct clade from the V. tricorpus [Citation21].
So far, Verticillium wilt has been reported by many countries, such as Israel and Spain on mango, California on artichoke, Greece on sugar beet, Trinidad on pumpkin, and so on. In China, Verticillium wilt is extremely harmful to the widely planted cotton, eggplant, Chinese cabbage, sunflower, tomato. More importantly, Verticillium wilt of okra caused by V. dahliae has been reported only in Brazil, Bulgari, California, and Zimbabwe [Citation3, Citation22–24], whereas there was no detailed description. However, this is the first report of V. dahliae causing Verticillium wilt on okra in China.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Camciuc M, Deplagne M, Vilarem G, et al. Okra-Abelmoschus esculentus, L. (moench.) a crop with economic potential for set aside acreage in France. Ind Crops Prod. 1998;7:257–264.
- Wang RZ. The development and research of the okra composite fruit and vegetable juice. [dissertation]. Sichuan: Xihua University; 2014. p. 45.
- Mendes MAS, da Silva VL, Dianese JC, et al. Fungos em Plants no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia. 1998. p. 555.
- Pande A, Rao VG. The genus Aplosporella Speg. (=Haplosporella Speg.) (Coelomycetes) from India. Nova Hedwigia. 1995;60:79–117.
- Narain U, Mehrotra BS. Some additions to Indian Cercosporae – I. Sydowia. 1970;24:326–330.
- Kobayashi T. Index of fungi inhabiting woody plants in Japan. Host, distribution and literature. Tokyo, Japan: Zenkoku-Noson-Kyoiku Kyokai Publishing Co.; 2007. p. 1227.
- Rao VG. An account of the market and storage diseases of fruits and vegetables in Bombay-Maharashtra India. Mycopathol ET Mycol Appl. 1966;28:165–176.
- Tjamos EC, Beckman CH. Vascular wilt diseases of plants: basic studies and control. Vol. 28, Nato ASI Series H: Cell Biology. Berlin: Springer. 1989. p. 590.
- Zare R, Gams W, Schroers HJ. The type species of Verticillium is not congeneric with the plant-pathogenic species placed in Verticillium and it is not the anamorph of ‘Nectria’ inventa. Mycol Res. 2004;108:576–582.
- Zhao Q, Chai AL, Shi YX, et al. First report of grey mould disease on Abelmoschus esculentus caused by Botrytis cinerea in China. J Phytopathol. 2016;164:354–357.
- Zhao Q, Xie XW, Shi YX, et al. Boeremia leaf and fruit spot of okra caused by Boeremia exigua in China. Can J Plant Pathol. 2016;38:395–399.
- Li BJ, Guo MY, Chai AL. First report of Fusarium solani causing Fusarium root rot on okra (Abelmoschus esculentus) in China. Plant Dis. 2016;100:526–527.
- Uppal AK, Hadrami AE, Adam LR, et al. Pathogenic variability of Verticillium dahliae isolates from potato fields in Manitoba and screening of bacteria for their biocontrol. Can J Plant Pathol. 2007;29:141–152.
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325.
- White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. 1990; 18:315–322.
- Banno S, Saito H, Sakai H, et al. Quantitative nested real-time PCR detection of Verticillium longisporum and V. dahliae in the soil of cabbage fields. J Gen Plant Pathol. 2011;77:282–291.
- Ikeda K, Banno S, Watanabe K, et al. Association of Verticillium dahliae and Verticillium longisporum with Chinese cabbage yellows and their distribution in the main production areas of Japan. J Gen Plant Pathol. 2012;78:331–337.
- Wilhelm S. Longevity of Verticillium wilt fungus in the laboratory and field. Phytopathology. 1955;45:180–181.
- Pegg GF, Brady BL. Verticillium wilts. Wallingford (UK): CABI Publishing; 2002. p. 522.
- Heitefuss R. The genera of Hyphomycetes. J Phypathol. 2012;160:166–166.
- Fahleson J, Hu Q, Dixelius C. Phylogenetic analysis of Verticillium species based on nuclear and mitochondrial sequences. Arch Microbiol. 2004;181:435–442.
- Bobev S. Reference guide for the diseases of cultivated plants. Unknown journal or publisher. 2009. 466 pages.
- French AM. California plant disease host index. California: Calif Dept Food Agric; 1989. p. 394.
- Whiteside JO. A revised list of plant diseases in Rhodesia. Kirkia. 1966;5:87–196.
