Abstract
In this study, we evaluated the effect of different temperatures (10, 20, 30, and 40 °C) and relative humidities (RHs; 12, 44, 76, and 98%) on populations of predominant grain fungi (Aspergillus candidus, Aspergillus flavus, Aspergillus fumigatus, Penicillium fellutanum, and Penicillium islandicum) and the biocontrol activity of Pseudomonas protegens AS15 against aflatoxigenic A. flavus KCCM 60330 in stored rice. Populations of all the tested fungi in inoculated rice grains were significantly enhanced by both increased temperature and RH. Multiple linear regression analysis revealed that one unit increase of temperature resulted in greater effects than that of RH on fungal populations. When rice grains were treated with P. protegens AS15 prior to inoculation with A. flavus KCCM 60330, fungal populations and aflatoxin production in the inoculated grains were significantly reduced compared with the grains untreated with strain AS15 regardless of temperature and RH (except 12% RH for fungal population). In addition, bacterial populations in grains were significantly enhanced with increasing temperature and RH, regardless of bacterial treatment. Higher bacterial populations were detected in biocontrol strain-treated grains than in untreated control grains. To our knowledge, this is the first report showing consistent biocontrol activity of P. protegens against A. flavus population and aflatoxin production in stored rice grains under various environmental conditions of temperature and RH.
Rice (Oryza sativa L.) is a primary grain crop that is cultivated worldwide. In Korea, harvested rice in the form of unhulled grains is often stored for extended periods [Citation1]. During storage, fungal contamination is one of the most important problems related to the decreased quality of the rice grains. Fungi can contaminate rice grains before harvest, during harvesting, or even at the storage facility [Citation2]. However, storage conditions, particularly temperature and relative humidity (RH), may determine the type of fungi that develop in the stored grains [Citation3]. Unlike field fungi, the growth and development of xerophilic fungi, such as Aspergillus and Penicillium spp., are enhanced during storage because these fungi are relatively tolerant to lower RH [Citation4–7].
Aspergillus and Penicillium spp. are common contaminants of stored food products, particularly cereal grains, and can deteriorate grain quality. More importantly, these fungi can produce mycotoxins that are hazardous secondary metabolites. Mycotoxins, including aflatoxins B1, B2, G1, and G2 are mainly produced by A. flavus and A. parasiticus [Citation8]. Aflatoxin B1 is one of the most carcinogenic compounds known, with several adverse effects on human and animal health by direct or indirect consumption of contaminated food or feed [Citation9,Citation10]. Several control methods, including chemical measures, have been used to inhibit mycotoxigenic fungi. Increased awareness of the adverse health effects of agricultural chemicals and the possible emergence of fungicide resistance have prompted researches on safer alternatives for the management of fungal contamination of stored grains [Citation11]. The biological control of deleterious fungi using antagonistic microorganisms is a potentially feasible and environment-friendly alternative to chemical control during grain storage [Citation11]. However, previous studies have inadequately considered the influence of temperature and RH on the biocontrol of microorganisms capable of suppressing fungal contamination or aflatoxin production in stored rice grains.
In our previous studies, A. candidus AC317, A. flavus AF57, A. fumigatus AF8, Penicillium fellutanum KU53, and Penicillium islandicum KU101 were identified as the predominant fungi in stored rice grains [Citation12–14]. Furthermore, a total of 460 bacterial strains were isolated from rice grains and screened for their potential as biocontrol agents for the fungal contaminants. Among the tested strains, Pseudomonas protegens AS15, which produces volatile compounds, exhibited the most effective antifungal activity, with inhibition of A. flavus growth in unhulled rice grains and biodegradation of aflatoxins produced by the fungus in a liquid medium [Citation15–17]. In this study, therefore, we evaluated the effect of temperature and RH on the growth of the predominant Aspergillus and Penicillium spp., and the biocontrol activity of P. protegens AS15 against A. flavus population and aflatoxin production in stored rice grains.
A. candidus AC317, A. flavus AF57, A. fumigatus AF8, P. fellutanum KU53, and P. islandicum KU101 isolated from our previous studies [Citation12–14] were used in this study. The fungal isolates were stored on potato dextrose agar (PDA) at 4 °C until use. To prepare fungal inocula, conidia from cultures grown on PDA at 25 °C for 5 days were harvested in sterile distilled water (SDW) containing 0.03% Tween 20 and then adjusted to 107 conidia/ml using a hemocytometer. P. protegens AS15 was used in this study as it exhibited significant biocontrol activity against A. flavus and aflatoxin production in our previous study [Citation16]. This strain was stored in nutrient broth (NB) containing 20% glycerol at −70 °C until use. To prepare the bacterial suspension, P. protegens AS15 was streaked on nutrient agar (NA) and incubated for 48 h at 28 °C. Single colonies appearing on NA were transferred to NB and incubated at 28 °C in a rotary shaker (160 rpm) for 48 h. Bacterial cells were harvested by centrifugation as described by Mannaa et al. [Citation16]. The harvested cells were suspended in MgSO4 solution and adjusted to 108 cells/ml (OD600 = 0.5) using a spectrophotometer.
Four saturated salt solutions (LiCl, K2CO3, NaCl, and K2SO4) were prepared and adjusted to four different RH levels (12, 44, 76, and 98%, respectively), as described by Greenspan [Citation18]. One hundred milliliters of each solution were aseptically poured into plastic containers (15 cm in width ×11 cm in length ×5 cm in height; Daiso, Higashihiroshima, Japan) disinfected with 99% ethanol [Citation19] to create an internal atmosphere with the designed RH, in which RH (%) = water activity (aw) × 100 at equilibrium. These containers containing each saturated salt solution were stored at different temperatures (10, 20, 30, and 40 °C). HOBO® temperature/RH data-logging units (Onset Computer Corporation, Bourne, MA) and HOBOware for Windows version 3.7.11 (Onset Computer Corporation) were used to monitor temperatures and RHs in the plastic containers containing the solutions (Supplementary Table S1). At equilibrium, RH in the atmosphere of the containers was maintained, with slight fluctuations (±1% in the target RH adjusted by each saturated salt solution), by temperature changes [Citation20]. The designed RH conditions showed almost the expected water activity when rice grains were measured using an AquaLab water activity meter (Model 4TE; Decagon Devices, Pullman, WA) (Supplementary Table S2).
Two grams of stored unhulled rice (cv. “Ilpum”) grains obtained from Korea University Farm (Namyangju, Korea) were surface-sterilized with 1% sodium hypochlorite for 3 min and 70% ethanol for 5 min [Citation21]. Grains were then washed three times in SDW and blotted on Whatman No. 1 filter paper. The surface-sterilized grains were inoculated with 200 µl of a conidial suspension (107 conidia/ml) of each tested fungus, which was equivalent to 106 conidia/g dry weight of rice grains. Inoculated grains were placed in a Petri plate (60 mm in diameter) and the plates were put in a plastic container (replicate) containing each of the saturated salt solutions prepared as described above. These containers sealed with Uniwrap® (Uniwrap Co., Hwaseong, Korea) were tightly closed using lids. They were then incubated in the dark at 10, 20, 30, and 40 °C. After 2 weeks of incubation, fungal populations in inoculated rice grains were determined, as described previously [Citation16]. Briefly, rice grains from a Petri plate from each container were finely ground with an analytical mill (A11 basic; IKA Works, Wilmington, DE). One gram of ground grains was suspended in 10 ml of SDW and incubated in a rotary shaker (160 rpm) at 28 °C for 30 min. After serial dilution, aliquots (200 µl) of the dilutions were spread on dichloran 18% glycerol agar (DG18; Fluka 40587; Sigma-Aldrich, St. Louis, MO) amended with 50 mg/ml chlortetracycline [Citation22]. Smeared plates (three plates per replicate) were incubated in the dark at 28 °C for 4 days and the colony-forming units (CFUs) per g dry weight of rice grains was assessed.
The biocontrol activity of P. protegens AS15 against A. flavus and aflatoxin production in unhulled rice (cv. “Ilpum”) grains was examined at different temperatures (10, 20, 30, and 40 °C) and RHs (12, 44, 76, and 98%). In this experiment, the aflatoxigenic strain, A. flavus KCCM 60330, obtained from the Korean Culture Center of Microorganisms (Seoul, Korea) was used because A. flavus AF57 produces a low level of aflatoxins [Citation16]. Briefly, two grams of surface-sterilized unhulled rice (cv. “Ilpum”) grains were immersed in 10 mM MgSO4 solution (untreated control) or a bacterial suspension of P. protegens AS15 for 3 h. The rice grains, bacterial suspensions, and fungal inocula were prepared as described above. Treated rice grains were inoculated with 106 conidia of A. flavus KCCM 60330/g dry weight of grains. Inoculated grains were then placed in plastic containers prepared as described above and these containers were incubated at different temperatures. Populations of A. flavus KCCM 60330 in treated rice grains were evaluated after 2 weeks of incubation as described above. In addition, total bacteria of the same inoculated grains were assessed on NA supplemented with 50 mg/l cycloheximide. Fungal and bacterial CFUs were assessed on smeared plates (three plates per replicate) after 4 and 2 days of incubation at 28 °C, respectively. In addition, total aflatoxins, including aflatoxin B1 and B2, produced by A. flavus KCCM 60330 were analyzed using a competitive direct enzyme-linked immunosorbent assay with Veratox® HS (Neogen Co., Lansing, MI), as described by Mannaa et al. [Citation16].
The experiments were established with factorial designs to observe the effect of temperature and RH with four different levels on fungal and bacterial populations and mycotoxin production in rice grains. All experiments were performed twice with three replicates per treatment except for RH measurement (10 replicates) in plastic containers. Statistical analyses were performed using the Statistical Analysis Systems software (SAS Institute, Cary, NC). Data from repeated experiments were combined after confirmation of the homogeneity of variances using Levene’s test [Citation23]. The fungal and bacterial population data were analyzed after logarithmic transformation. Multiple linear regression analysis was conducted to detect the effect of both temperature and RH on fungal populations. Analysis of variance was conducted using general linear model procedures and means were separated using Tukey’s HSD test at p < .05.
When growth on unhulled rice grains inoculated with the tested fungi (A. candidus AC317, A. flavus AF57, A. fumigatus AF8, P. fellutanum KU53, and P. islandicum KU101) was assessed at different temperatures and RHs, growth of all fungi was significantly (p < .0001) enhanced by both increased temperature and RH (; Supplementary Figure S1 and Supplementary Table S3). There were significant (p < .0001) temperature × RH interactions in all the tested fungal populations (Supplementary Table S3). In general, populations of A. flavus AF57 and A. fumigatus AF8 were higher than the other tested fungi, regardless of temperature and RH (). Multiple linear regression analysis to observe the effect of both temperature and RH on fungal populations revealed significantly (p < .0001) positive relationships in all the tested fungi (). The increase in all the fungal populations was higher in response to one unit temperature increase than RH according to the regression coefficients ().
Figure 1. Effect of different temperatures (10, 20, 30, and 40 °C) and relative humidities (RHs; 12, 44, 76, and 98%) at equilibrium on fungal populations 2 weeks after inoculation of rice grains with (A) Aspergillus candidus AC317, (B) Aspergillus flavus AF57, (C) Aspergillus fumigatus AF8, (D) Penicillium fellutanum KU53, or (E) Penicillium islandicum KU101. Surface-sterilized rice grains were inoculated with 106 fungal conidia/g dry weight of rice grains. Fungal populations were evaluated after 4 days of incubation on dichloran 18% glycerol agar amended with 50 mg/ml of chlortetracycline. Different lowercase and uppercase letters on bars (n = 6) are significantly different between different temperatures for a given RH and RHs for a given temperature, respectively, according to Tukey’s HSD test at p < .05. CFU: colony-forming unit.
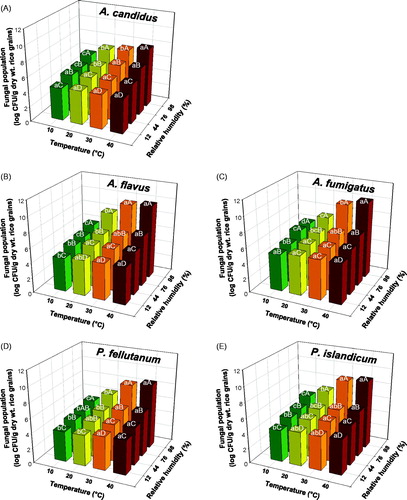
Table 1. Multiple linear regression analysis (fungal populationa = intercept + β1 × temperature + β2 × relative humidity) relating the effects of both temperature (10, 20, 30, and 40 °C) and relative humidity (RH; 12, 44, 76, and 98%) on the population of Aspergillus candidus AC317, Aspergillus flavus AF57, Aspergillus fumigatus AF8, Penicillium fellutanum KU53, and Penicillium islandicum KU101.
When rice grains treated with or without the biocontrol strain, P. protegens AS15, were inoculated with A. flavus KCCM 60330, fungal populations in the inoculated grains were significantly (p < .0001) enhanced by increasing temperature from 10 to 40 °C and by increasing RH from 12 to 98% (; Supplementary Figure S2 and Supplementary Table S4). Treatment with P. protegens AS15 significantly (p < .01) inhibited fungal populations in rice grains compared with untreated control grains, regardless of the temperature and RH (; Supplementary Table S4). There were significant (p < .0001) temperature × RH interactions in the fungal populations, regardless of the biocontrol strain treatment (Supplementary Table S4). The biocontrol strain, P. protegens AS15, significantly (p < .01) suppressed A. flavus KCCM 60330 populations in the grains from 10 to 40 °C, compared with control grains not treated with strain AS15, at 44–98% RH (). However, at 12% RH, no significant differences were detected in fungal populations between rice grains that were untreated or treated with the biocontrol strain at the tested temperatures, except at 30 °C ().
Figure 2. Effect of different temperatures (10, 20, 30, and 40 °C) and relative humidities (RHs; 12, 44, 76, and 98%) at equilibrium on (A) fungal population and (B) aflatoxin production in rice grains treated with (+) or without (−) the biocontrol strain, Pseudomonas protegens AS15, followed by inoculation with the aflatoxigenic strain, Aspergillus flavus KCCM 60330. Fungal population and total aflatoxin amount were assessed 2 weeks after inoculation with 106 conidia/g dry weight of rice grains. Different lowercase and uppercase letters on bars with error bars (standard deviations, n = 6) indicate significant differences between different temperatures of rice grains treated with and without P. protegens AS15, respectively, according to Tukey’s HSD test at p < .05. Asterisks (*, **, and ***) on bars at a given temperature indicate significant differences between rice grains treated with or without P. protegens AS15 at p < .05, .01, and .001, respectively. ns, not significant; CFU, colony-forming unit.
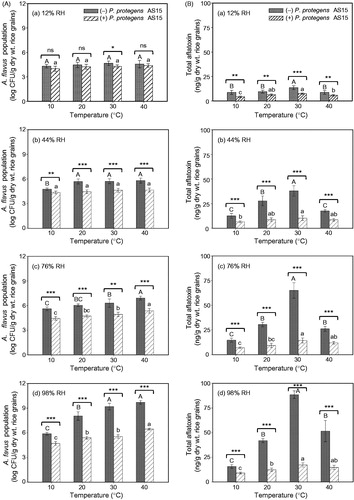
In biocontrol strain-treated and untreated rice grains inoculated with the aflatoxigenic strain, A. flavus KCCM 60330, total aflatoxin production was significantly (p < .0001) enhanced with increased temperature and RH (; Supplementary Table S4). Significant (p < .001) temperature × RH interactions in aflatoxin production were detected regardless of biocontrol strain treatment (Supplementary Table S4). In general, biocontrol strain, P. protegens AS15, consistently suppressed aflatoxin production by A. flavus KCCM 60330 in rice grains from 10 to 40 °C, regardless of RH (). However, aflatoxin production by A. flavus KCCM 60330 was significantly (p < .01) higher in the control grains not treated with strain AS15 than in the treated grains at all temperatures and RHs. In addition, the highest levels of aflatoxin production were detected in grains at 30 °C among the tested temperatures, regardless of biocontrol bacterial treatment ().
In rice grains treated with or without P. protegens AS15 prior to inoculation with A. flavus KCCM 60330, total bacterial populations were significantly (p < .0001) enhanced by increased temperature and RH, regardless of whether the grains were treated with the biocontrol strain or not (Supplementary Table S4; ). However, bacterial populations in grains not treated with the biocontrol strain at 12 and 44% RH could not be assessed, because the natural bacterial populations were below detectable levels (). Significant (p < .0001) temperature × RH interactions were observed in bacterial populations in rice grains treated with the biocontrol strain, but not in grains that were not treated with the biocontrol strain (Supplementary Table S4). High bacterial populations (5.85–10.06 log CFU/g dry weight of rice grains) were detected in biocontrol strain-treated grains at 98% RH, regardless of temperature (). However, bacterial populations in untreated grains remained at low levels (3.30–4.03 log CFU/g dry weight of rice grains) at 76 and 98% RH, regardless of temperature ().
Table 2. Effect of different temperatures (10, 20, 30, and 40 °C) and relative humidities (RHs; 12, 44, 76, and 98%) at equilibrium on total bacterial populations 2 weeks after rice grains treated with (+) or without (–) the biocontrol strain, Pseudomonas protegens AS15.
The growth of storage grain fungi is generally dependent on environmental conditions such as water activity and temperature [Citation6,Citation24]. The xerophilic nature of these fungi allows them to tolerate relatively low water activity, unlike field fungi, which need higher water activity for spore germination and growth [Citation7]. Although several previous studies have assessed the effect of temperature and water activity on storage fungi, the environmental effect on rice grains has been often examined with a narrow range of temperature and RH [Citation25]. Therefore, in this study, we examined the effect of various temperatures and RHs, ranging from 10 to 40 °C and 12 to 98%, respectively, on populations of predominant storage rice fungi (A. candidus, A. flavus, A. fumigatus, P. fellutanum, and P. islandicum). We also examined the biocontrol activity of P. protegens AS15 against aflatoxigenic A. flavus KCCM 60330 in rice grains under these conditions. The growth of all the tested fungi was significantly enhanced in rice grains by increased temperature and RH. P. protegens AS15 significantly inhibited fungal population and aflatoxin production in grains inoculated with A. flavus KCCM 60330 under the same conditions.
Holmquist et al. [Citation26] reported that water activity had a greater effect than temperature on the growth of A. flavus and A. parasiticus. Furthermore, they found that fungal growth at low temperatures occurred only at high water activity. In another study, 11 out of 20 fungi, including A. flavus and A. fumigatus, showed visible growth at 20 and 30 °C and RHs of 60–80% [Citation27]. Similarly, in this study, varying degrees of both temperature and RH significantly affected the populations of all tested fungi. In particular, temperature (i.e., one unit increase) had a greater influence than RH on fungal populations. In addition, significantly positive effects of both temperature and RH on fungal populations were evident in the multiple linear regression analysis. Although low RH is considered a limiting factor for fungal growth in rice grains, the observed populations would be related to conditions within individual rice grains, which may provide a micro-environment favorable for fungal growth [Citation28].
P. protegens AS15 displayed effective biocontrol activity by reducing fungal growth and aflatoxin production in rice grains inoculated with the aflatoxigenic strain, A. flavus KCCM 60330. Strain AS15 significantly suppressed fungal populations in the grains compared with bacterium-untreated control grains regardless of temperature and RH. Interestingly, aflatoxin production in the grains was consistently inhibited to a low level by the biocontrol strain regardless of temperature and RH. These results indicate that the biocontrol strain can suppress aflatoxin production by A. flavus, even in unfavorable environmental conditions. Moreover, the consistent inhibitory activity of P. protegens AS15 indicates that it can effectively compete with A. flavus KCCM 60330 and survive in its original habitat, rice grains. This survival ability is further supported by the observation of bacterial populations in rice grains. In addition, the effective colonization of grains by this biocontrol strain was demonstrated in our previous study [Citation16]. Although the bacterial population was negatively affected by the environmental conditions, P. protegens AS15 could exist at high levels in rice grains, regardless of temperature and RH. However, natural bacterial populations in the untreated grains remained undetectable (at 12–44% RH) or at low (at 76–98% RH) levels, regardless of temperature. Recently, Punja et al. [Citation29] observed a similar phenomenon, in which the biocontrol activity of Bacillus subtilis persisted at a low temperature. However, even though its population was dramatically affected, B. subtilis was still able to minimize postharvest disease development in tomatoes. Previously, Lahlali et al. [Citation30] reported the efficient biocontrol activity of an antagonistic yeast strain against Penicillium italicum, the causal agent of blue mold, under controlled environmental conditions, in which the biocontrol activity was not affected by different temperatures and RHs.
In this study, we observed that aflatoxin production in rice grains was highly influenced by temperature and RH. Mislivec et al. [Citation31] previously demonstrated that active fungal growth of mycotoxigenic Aspergillus and Penicillium spp., which are essential for toxin production, is controlled by ecological factors, such as RH, temperature, and substrate. Rapid growth and subsequent aflatoxin production by A. parasiticus has been positively related to high storage RH [Citation24,Citation28]. In the present study, high RH was also positively related with higher levels of aflatoxin accumulation and fungal population in rice grains. However, unlike the fungal population, aflatoxin production was suppressed at 40 °C. Maximum aflatoxin production, among the tested temperatures was observed at 30 °C. Gqaleni et al. [Citation32] reported that the optimum temperature for maximum aflatoxin production by A. flavus was 30 °C, with further temperature increases resulting in a gradual suppression of toxin production. Similarly, other studies [Citation33,Citation34] have shown that aflatoxin production is reduced at temperatures >30 °C. Although the optimum temperature for aflatoxin production may vary between A. flavus isolates, Kheiralla et al. [Citation35] showed that most aflatoxins are produced by A. flavus isolates between 25 and 30 °C. The mycelial dry weight in that study, as an indicator of the fungal growth, was continuously enhanced with increasing temperature, with maximum growth at 35 °C; however, aflatoxin production was not related to fungal growth at temperatures >30 °C, as observed in our present study.
The characterization of biosynthetic and regulatory genes after the complete sequencing of aflatoxin genes has facilitated a better understanding of the biosynthetic pathway and the roles of different genes governing aflatoxin production [Citation36,Citation37]. Expression of aflatoxin biosynthetic genes depends on temperature. Using microarray analysis, O’Brian et al. [Citation38] reported reduced expression of aflatoxin biosynthetic genes at 37 °C and induced expression at 28 °C coupled with suppression and induction of aflatoxin production, respectively. Subsequently, Schmidt-Heydt et al. [Citation39] showed that reduced expression of aflS and aflJ (regulatory genes of the aflatoxin production pathway) at temperatures >37 °C was related to reduced aflatoxin production, unlike the observations of O’Brian et al. [Citation38] regarding the expression levels of these two genes at high temperature. Using RNA-sequencing technology, Yu et al. [Citation40] observed the expression of >50% of aflatoxin production-related rRNA at 30 °C, which was associated with higher levels of aflatoxin production. Additionally, the transcriptional regulatory genes, aflR and aflS, were expressed at higher levels at 30 °C than at 37 °C. These findings may explain the observed significant reduction in aflatoxin production by A. flavus in rice grains at 40 °C in our present study.
Taken together, our results suggest that different temperatures and RHs affect the growth of Aspergillus and Penicillium spp. predominant in rice grains. Although these environmental factors similarly affected A. flavus KCCM 60330 growth and aflatoxin production, P. protegens AS15 consistently inhibited fungal population and aflatoxin production in stored rice grains under the same conditions. To our knowledge, this is the first report showing the consistent biocontrol activity of P. protegens against A. flavus population and aflatoxin production in stored rice grains under various environmental conditions of temperature and RH.
Supplemental Material
Download MS Power Point (46.6 MB)Supplemental Material
Download MS Word (105.5 KB)Acknowledgments
This work was supported by a Korea University Grant. Mohamed Mannaa was supported by the Korean Government Scholarship Program (KGSP) during his Ph.D. study at Korea University, Seoul, Korea.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- KOSTAT. Annual Trends of Food Grain Consumption in 2016. Korean Statistical Information Service, [WWW document]. Available from: http://kostat.go.kr/portal/eng/pressReleases/2/11/index.board [accessed 2 March 2017]; 2017.
- Kabak B, Dobson ADW, Var I. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit Rev Food Sci Nutr. 2006;46:593–619.
- Magan N, Lacey J. Effect of temperature and pH on water relations of field and storage fungi. Trans Br Mycol Soc. 1984;82:71–81.
- Christensen CM, Kaufmann HH. Grain storage: the role of fungi in quality loss. Minneapolis (MN): University of Minnesota Press; 1969.
- Hocking AD, Pitt JI. Water relations of some Penicillium species at 25 °C. Trans Br Mycol Soc. 1979;73:141–145.
- Magan N, Lacey J. Ecological determinants of mould growth in stored grain. Int J Food Microbiol. 1988;7:245–256.
- Mannaa M, Kim KD. Influence of temperature and water activity on deleterious fungi and mycotoxin producing during grain storage. Mycobiology. 2017;45:240–254.
- Klich MA. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycoscience. 2007;48:71–80.
- Squire RA. Ranking animal carcinogens: a proposed regulatory approach. Science. 1981;214:877–880.
- Sweeney MJ, Dobson ADW. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol. 1998;43:141–158.
- Mannaa M, Kim KD. Microbe-mediated control of mycotoxigenic grain fungi in stored rice with focus on aflatoxin biodegradation and biosynthesis inhibition. Mycobiology. 2016;44:67–78.
- Oh JY, Kim EN, Ryoo MI, et al. Morphological and molecular identification of Penicillium islandicum isolate KU101 from stored rice. Plant Pathol J. 2008;24:469–473.
- Oh JY, Sang MK, Oh JE, et al. Microbial population, aflatoxin contamination and predominant Aspergillus species in Korean stored rice. Plant Pathol J. 2010;26:121–129.
- Oh JY, Sang MK, Lee HJ, et al. First detection of Penicillium fellutanum from stored rice in Korea. Res Plant Dis. 2011;17:216–221.
- Mannaa M, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: study II. Mycobiology. 2018;46:52–63.
- Mannaa M, Oh JY, Kim KD. Microbe-mediated control of Aspergillus flavus in stored rice grains with a focus on aflatoxin inhibition and biodegradation. Ann Appl Biol. 2017;171:376–392.
- Mannaa M, Oh JY, Kim KD. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology. 2017;45:213–219.
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stan Sect A. 1977;81A:89–96.
- Pardo E, Marín S, Sanchis V, et al. Impact of relative humidity and temperature on visible fungal growth and OTA production of ochratoxigenic Aspergillus ochraceus isolates on grapes. Food Microbiol. 2005;22:383–389.
- Wexler A, Hasegawa S. Relative humidity-temperature relationships of some saturated salt solutions in the temperature range 0 to 50 °C. J Res Natl Bur Stan. 1954;53:19–26.
- Gu Q, Han N, Liu J, et al. Expression of Helicobacter pylori urease subunit B gene in transgenic rice. Biotechnol Lett. 2006;28:1661–1666.
- Hocking AD, Pitt JI. Dichloran-glycerol medium for enumeration of xerophilic fungi from low-moisture foods. Appl Environ Microbiol. 1980;39:488–492.
- Levene H. Robust tests for equality of variances. In: Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB, editors. Contributions to probability and statistics: essays in honor of Harold hotelling. Stanford (CA): Stanford University Press; 1960. p. 278–292.
- Marín S, Companys E, Sanchis V, et al. Effect of water activity and temperature on competing abilities of common maize fungi. Mycol Res. 1998;102:959–964.
- Choi S, Jun H, Bang J, et al. Behaviour of Aspergillus flavus and Fusarium graminearum on rice as affected by degree of milling, temperature, and relative humidity during storage. Food Microbiol. 2015;46:307–313.
- Holmquist GU, Walker HW, Stahr HM. Influence of temperature, pH, water activity and antifungal agents on growth of Aspergillus flavus and A. parasiticus. J Food Sci. 1983;48:778–782.
- Misra N. Influence of temperature and relative humidity on fungal flora of some spices in storage. Z Lebensm Unters Forch. 1981;172:30–31.
- Boller RA, Schroeder HW. Influence of relative humidity on production of aflatoxin in rice by Aspergillus parasiticus. Phytopathology. 1974;64:17–21.
- Punja ZK, Rodriguez G, Tirajoh A. Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 2016;84:98–104.
- Lahlali R, Hamadi Y, El Guilli M, et al. Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biol Control. 2011;56:217–224.
- Mislivec PB, Dieter CT, Bruce VR. Effect of temperature and relative humidity on spore germination of mycotoxic species of Aspergillus and Penicillium. Mycologia. 1975;67:1187–1189.
- Gqaleni N, Smith JE, Lacey J, et al. Effects of temperature, water activity, and incubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Appl Environ Microbiol. 1997;63:1048–1053.
- Mousa W, Ghazali FM, Jinap S, et al. Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. J Food Sci. 2013;78:M56–M63.
- Ogundero VW. Temperature and aflatoxin production by Aspergillus flavus and A. parasiticus strains from Nigerian groundnuts. J Basic Microbiol. 1987;27:511–514.
- Kheiralla ZH, Hassanin NI, Amra H. Effect of incubation time, temperature and substrate on growth and aflatoxin production. Int Biodeterior Biodegr. 1992;30:17–27.
- Georgianna DR, Payne GA. Genetic regulation of aflatoxin biosynthesis: from gene to genome. Fungal Genet Biol. 2009;46:113–125.
- Yu J, Bhatnagar D, Cleveland TE. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004;564:126–130.
- OBrian GR, Georgianna DR, Wilkinson JR, et al. The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia. 2007;99:232–239.
- Schmidt-Heydt M, Abdel-Hadi A, Magan N, et al. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int J Food Microbiol. 2009;135:231–237.
- Yu J, Fedorova ND, Montalbano BG, et al. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol Lett. 2011;322:145–149.
