Abstract
The distribution and occurrence of rare ascomycete fungi within freshwater samples in Korea was investigated. Three rare fungal strains, CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1, were isolated using serial dilution method. On the basis of their morphological characteristics and phylogenetic analysis of their internal transcribed spacer regions and 28S rDNA sequences, the three isolates were identified as Acrostalagmus luteoalbus, Bartalinia robillardoides, and Collariella carteri, respectively. To our knowledge, these are the first records of rare genera Acrostalagmus, Bartalinia, and Collariella from Korea, and the first reports of A. luteoalbus, B. robillardoides, and C. carteri from freshwater samples.
1. Introduction
Freshwater fungi have an important role in the decomposition of organic matter in ecological systems, by virtue of producing enzymes that break down wood and producing bioactive compounds against pathogenic bacteria, fungi, and nematodes [Citation1–4]. There are more than 622 species (170 genera) of Ascomycetes; more than 226 species of Hyphomycetes (55 genera); and 183 species of Trichomycetes (3 orders), while little is known about the Basidiomycetes and Zygomycetes [Citation3].
Sordariomycetes is one of the largest classes of Ascomycetes, with six subclasses, 32 orders, 105 families, and 1331 genera [Citation5]. Its species are characterized by nonlichenized, flask-shaped fruiting bodies (perithecia) and unitunicate asci [Citation6]. They can be found in soil, dung, leaf litter, fresh water, plants, arthropods, and mammals [Citation3,Citation5,Citation7,Citation8].
The genus Acrostalagmus, which belongs to the class Sordariomycetes, order Hypocreales, and family Hypocreaceae, was established by Corda (1838) [Citation9], with the type species A. cinnabarinus Corda. The species belonging to this genus are characterized as verticillate conidiophores, with hyaline, egg-shaped conidia formed singly [Citation10]. Members of Acrostalagmus are found in saffron soil, needle mushroom, vermicompost, and branches of cacao [Citation11–14]. They are also known for their ability to produce a variety of secondary metabolites. To date, 27 species belonging to this genus are known, according to the Index Fungorum (www.indexfungorum.org).
The genus Bartalinia, which belongs to the class Sordariomycetes, order Amphisphaeriales, and family Bartaliniaceae, was established by Tassi (1900) [Citation15], with the type species Bartalinia robillardoides. It is characterized by the production of fusiform conidia with an acute or blunt apex and having three to four septate. Members of the genus have frequently been isolated from the leaves, stems of medicinal plants, or dead aerial spines of Rosa canina L. [Citation16–19]. B. robillardoides has been reported to produce taxol, an anticancer drug [Citation20]. Several types of compounds have been investigated from B. robillardoides strain LF550, such as three new chloroazaphilones named helicusin E, isochromophilone X, and isochromophilone XI, which expressed antimicrobial activity towards Bacillus subtilis, Staphylococcus lentus, Candida albicans, Trichophyton rubrum, and Septoria tritici, and also inhibited PDE4 and AChE [Citation21]. Currently, there are 17 accepted species in this genus [Citation22].
The genus Collariella, which belongs to the class Sordariomycetes, order Sordariales, and family Chaetomiaceae, was introduced by Wang et al. [Citation23]. Based on phylogenies derived from ITS, LSU rDNA, rpb2, and tub2 sequence data combined with morphological observations, seven species have been recognized [Citation23]. Currently, nine species have been registered in Index Fungorum. The species belonging to this genus are characterized by the production of a dark collar-like apex around the ostiolar pore of the ascomata [Citation23]. They are found in soil, dust, and air [Citation23–25]. Some of them have been reported to produce a variety of metabolites, including chaetoquadrin E, cochliodinol B, prenisatin, and SB236049/SB236050/SB238569 [Citation23].
In Korea, many studies on the fungal diversity of various habitats have been conducted, although the occurrence of freshwater fungi remains poorly understood. Until now, only five new species and eight new records from freshwater habitat have been reported in Korea [Citation19,Citation26–30].
During our collection from freshwater samples, three rare species were found in Korea: A. luteoalbus, B. robillardoides, and C. carteri.
2. Materials and methods
2.1. Isolation of fungal strains from freshwater samples
Freshwater samples were collected from a branch stream of the Nakdong river located in Gyeongsangbuk-do, the Yeongsan river located in Gwangju, and a pond located in the Chonnam National University Arboretum, Gwangju, Korea. The samples were transported in sterile 50-mL Falcon tubes and stored at 4 °C until examination. Serial dilution methods were employed using potato dextrose agar (PDA), malt extract agar (MEA), and corn meal agar (CMA). The media and method used were based on the protocol described by Wanasinghe et al. [Citation19] and Nguyen et al. [Citation31]. The plates were incubated at 25 °C for 3–7 days. To isolate pure cultures, individual colonies with various morphologies were picked up, transferred to PDA, and subcultured until pure mycelia were obtained. All pure isolates, including those of A. luteoalbus, B. robillardoides, and C. carteri, were stored in 20% glycerol at −80 °C at the Chonnam National University Fungal Collection (CNUFC), Gwangju, Korea. A. luteoalbus, B. robillardoides, and C. carteri strains isolated in our study were designated CNUFC-YR537-1 and CNUFC-YR537-2, CNUFC-CNUP1-1 and CNUFC-CNUP1-2, CNUFC-NDR3-1 and CNUFC-NDR3-2, respectively. Strains CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1 were also deposited at the Culture Collection of the Nakdonggang National Institute of Biological Resources (NNIBR, Sangju, Korea).
2.2. Morphological studies
For detailed morphological studies, CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1 strains were cultured on PDA, MEA, and CMA. The plates were incubated at 25 °C in the dark for 7 d. Samples were mounted in lactophenol solution (Junsei Chemical Co. Ltd., Tokyo, Japan) and observed under an Olympus BX51 microscope with DIC optics (Olympus, Tokyo, Japan). For scanning electron microscopy (SEM), samples were prepared as described previously [Citation32].
2.3. DNA extraction, polymerase chain reaction (PCR), and sequencing
Strains CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1 were grown on PDA, covered with cellophane at 25 °C for 4–5 days. Genomic DNA was extracted using the Solg TM Genomic DNA prep. Kit (Solgent Co. Ltd., Daejeon, South Korea). The internal transcribed spacers (ITS1, and ITS2) and 5.8S region of the ribosomal DNA were amplified with the primer pair ITS1 and ITS4 [Citation33]. The large subunit of 28S rDNA was amplified with the primer pair LROR and LR5F [Citation34]. The PCR amplification mixture (total volume, 20 μL) was comprised of 2 μl of the fungal DNA template (10 ng), 1.5 μl of each primer (5 pM/μL), 1 μl Accupower® PCR premix (Bioneer Corp., Daejeon, South Korea) containing Taq DNA polymerase, dNTPs, buffer, and tracking dye, and 14 μl sterile water. The amplification parameters were as follows: an initial denaturation step at 95 °C for 5 min, followed by 35 thermal cycles with denaturation at 94 °C for 30 s, annealing at 55 °C (ITS) or 52 °C (28S) for 30 s, and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 10 min. The PCR products were purified using the Accuprep® PCR Purification Kit (Bioneer Corp.). DNA sequencing was performed in an ABI 3700 Automated DNA sequencer (Applied Biosystems Inc., Foster City, CA, USA).
2.4. Phylogenetic analysis
The fungal sequences obtained from the GenBank database () were aligned using Clustal_X v.1.83 [Citation35] and edited with Bioedit v.5.0.9.1 [Citation36]. Phylogenetic trees based on the ITS rDNA and 28S sequences were constructed using the neighbor-joining method in MEGA 6 [Citation37]. Colletotrichum boninense, Glomerella cingulate, Microascus trigonosporus, and Pestalotiopsis malayana were used as outgroups. The reliability of internal branches was assessed using the p-distance substitution model with 1000 bootstrap replications. Sequence data were compared with similar sequences available in the GenBank databases using BLASTn.
Table 1. Sequences used in this study and GenBank accession numbers.
3. Results
3.1. Phylogenetic analysis
A BLAST search of ITS sequences via the NCBI database indicated that the isolates CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1 most closely resembled A. luteoalbus (GenBank accession no. KP050692), B. robillardoides (GenBank accession no. KJ710460), and C. carteri (GenBank accession no. KX976747), with 99.6% (542/544 bp), 99.6% (539/541 bp), and 99.8% (499/500 bp) homology, respectively. The 28S sequences of A. luteoalbus (GenBank accession no. KJ443145), B. robillardoides (GenBank accession no. KJ710438), and C. carteri (GenBank accession no. KX976742) showed 100% (850/850 bp), 99.8% (871/873 bp), and 100% (487/487 bp) homology with the 28S sequence of the isolates CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1, respectively.
On the basis of the ITS and 28S sequence analysis, the isolates CNUFC-YR537-1, CNUFC-CNUP1-1, and CNUFC-NDR3-1 were identical to A. luteoalbus, B. robillardoides, and C. carteri, respectively ().
Figure 1. Phylogenetic tree based on neighbor-joining analysis of internal transcribed spacers (ITS rDNA) and 28S rDNA sequences of A. luteoalbus CNUFC-YR537-1 and A. luteoalbus CNUFC-YR537-2. Colletotrichum boninense and Glomerella cingulata were used as outgroups. Bootstrap support values of ≥50% are indicated at the nodes. The bar indicates the number of substitutions per position.
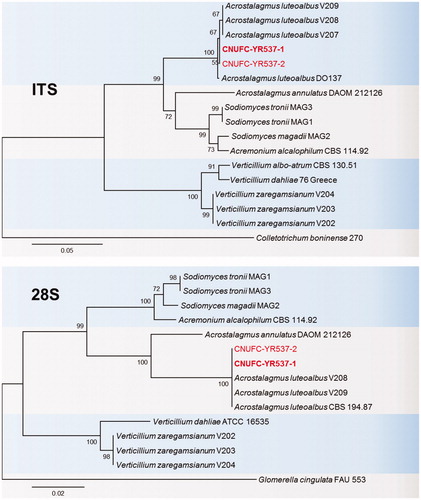
Figure 2. Phylogenetic tree based on neighbor-joining analysis of internal transcribed spacers (ITS rDNA) and 28S rDNA sequences of B. robillardoides CNUFC-CNUP1-1 and B. robillardoides CNUFC-CNUP1-2. Pestalotiopsis malayana and Phlogicylindrium uniforme were used as outgroups. Bootstrap support values of ≥50% are indicated at the nodes. The bar indicates the number of substitutions per position.
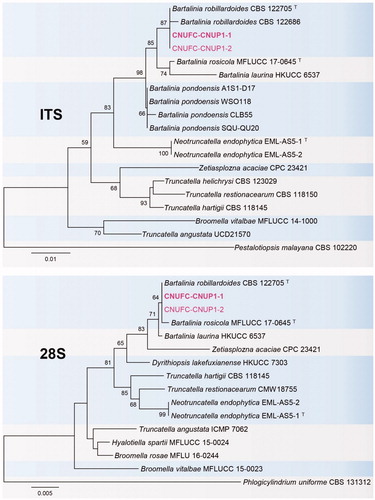
Figure 3. Phylogenetic tree based on neighbor-joining analysis of internal transcribed spacers (ITS rDNA) and 28S rDNA sequences of C. carteri CNUFC-NDR3-1 and C. carteri CNUFC-NDR3-2. Microascus trigonosporus was used as an outgroup. Bootstrap support values of ≥50% are indicated at the nodes. The bar indicates the number of substitutions per position.
3.2. Taxonomy
3.2.1. Taxonomy of CNUFC-YR537-1
A. luteoalbus (Link) Zare, W. Gams & Schroers, Mycological Research 108 (5): 581 (2004) (, ).
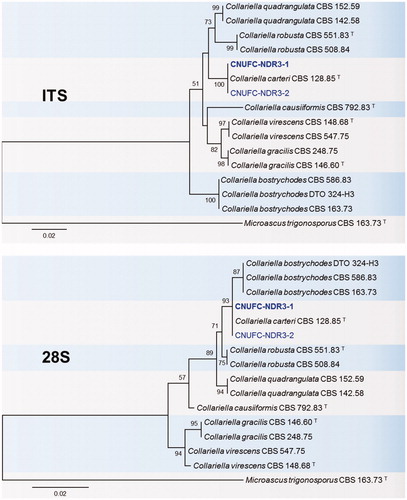
Figure 4. Morphology of A. luteoalbus CNUFC-YR537-1. (A), Colonies in corn meal agar (CMA). (B), Colonies in potato dextrose agar (PDA). (C–E), Conidiophores bearing conidia. (F,G), Conidiophores and phialides. (H), Conidia (scale bars D–H = 20 µm).
Table 2. Morphological characteristics of CNUFC-YR537-1 compared to A. luteoalbus reference strain.
=Sporotrichum luteoalbum (Link), Magazin der Gesellschaft Naturforschenden Freunde Berlin 3 (1): 13 (1809).
=Verticillium luteoalbum (Link) Subram., Hyphomycetes: an account of Indian species, except Cercosporae: 649 (1971).
=Verticillium tenerum Nees, System der Pilze und Schwämme: 57, t. 4:55 (1817).
=Sporotrichum lateritium Ehrenb., Sylvae mycologicae Berolinenses: 22 (1818).
=Sporotrichum hippocastani Corda, Icones fungorum hucusque cognitorum 1: 10, t. 2:159 (1837).
=Acrostalagmus cinnabarinus Corda, Icones fungorum hucusque cognitorum 2: 15, t. 10:66 (1838).
=Sporotrichum luteoalbum Thüm., Fungi pomicoli. Monographische Beschreibung der auf den Obstfrüchten der gemässigten Klimate vorkommenden Pilze: 21 (1879).
=Sporotrichum vile P. Karst., Hedwigia 30: 303 (1891).
Description: Colonies of the strain grew slowly on PDA, appearing dull orange to orange-brown, and reaching 24–26 mm in diameter after 7 days of incubation at 25 °C. The reverse of the colonies were orange, appearing a lighter orange in the centers. Conidiophores were erect, pale orange-brown, measured 33–121 × 2.0–4.0 µm, and repeatedly verticillately branched in one to several orders. Phialides were flask-shaped, hyaline, and measured 10–29 × 2.0–3.5 µm. Conidia were oval, pale reddish, and measured 3.5–6.2 × 2.2–2.9 µm.
3.2.2. Taxonomy of CNUFC-CNUP1-1
B. robillardoides Tassi, Bollettino del Laboratorio de Orto Botanico Reale Universita Siena 3: 5 (1900) (, ).
Figure 5. Morphology of B. robillardoides CNUFC-CNUP1-1. (A), Colonies in potato dextrose agar (PDA). (B), Colonies in malt extract agar (MEA). (C,E), Conidiogenous cells with developing conidia. (D,F), Conidia. (C, D: observed under light microscope; E, F: observed under SEM) (scale bars C, D = 20 µm; E = 15 µm, F = 10 µm).
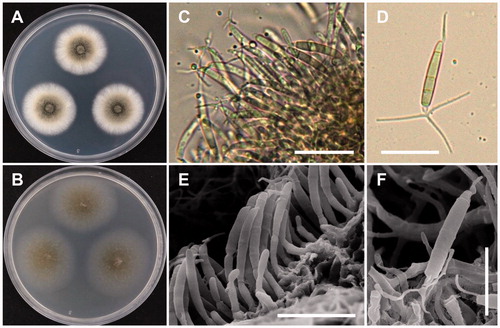
Table 3. Morphological characteristics of CNUFC-CNUP1-1 compared to B. robillardoides reference strain.
≡Seimatosporium robillardoides (Tassi) Arx, The genera of fungi sporulating in pure culture: 224 (1981).
Description: Colonies of the strain grew rapidly on PDA, were greyish, and reached 80–83 mm in diameter after 14 d culture at 25 °C. Conidiomata were globose or subglobose, appearing black to brownish black, and measured 276.7–482.2 × 228.1–364.8 μm. Conidiophores were reduced to conidiogenous cells, which were hyaline, cylindrical to subcylindrical, ampuliform, measured 8.4–11.3 × 2.5–3.8 μm, and were formed from the inner cells of the peridial wall. Conidia were subcylindrical to slightly curved fusoid, basal cell obconic with a truncate base, 4-septate, and measured 18.3–24.2 × 3.0–3.8 μm. The apical appendage was 12.4–18.2 μm long and three-branched. The basal appendage was 3.8–7.3 μm long, single, unbranched, filiform, and excentric.
3.2.3. Taxonomy of CNUFC-NDR3-1
C. carteri X. Wei Wang, Houbraken & Samson, Studies in Mycology 84: 179 (2016) (, ).
Figure 6. Morphology of C. carteri CNUFC-NDR3-1. (A), Colonies in potato dextrose agar (PDA). (B), Colonies in malt extract agar (MEA). (C), Ascomata mounted in lactophenol. (D), Tip of terminal hairs. (E,F), Asci containing ascospores. (G,H), Ascospores. (C– F: observed under light microscope; G, H: observed under SEM) (scale bars E, F = 20 µm; G, H = 5 µm).
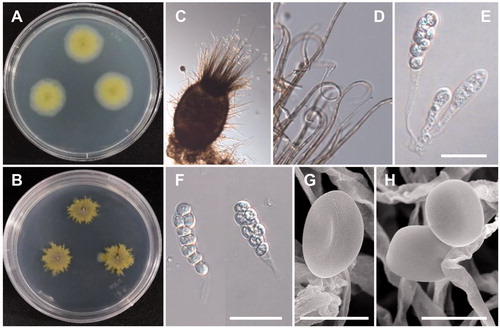
Table 4. Morphological characteristics of CNUFC-NDR3-1 compared to C. carteri reference strain.
Description: Colonies of this strain grew moderately slowly on PDA, reaching 35–37 mm in diameter after 7 d incubation at 25 °C. The colony color was pale yellowish and then became greenish/olivaceous with age. Ascomata were globose to subglobose, and measured 81–364.5 × 78–247.9 µm. Ascomatal hair were 3–5 µm diameter at the base, tapering and fading towards the tip. Asci were fasciculate, clavate, or slightly fusiform, contained seven to eight ascospores, and measured 16.5–31.5 × 7.8–10.8 µm. Ascospores were brown, limoniform, usually biapiculate at both ends, and measured 5.0–6.7 × 4.5–5.5 µm.
4. Discussion
The use of DNA sequences has dramatically increased the number of fungal species being recognized and identified [Citation38,Citation39]. Especially the ITS-5.8S rDNA sequences have become important features for the rapid identification of fungi [Citation40,Citation41]. The LSU region on its own or in combination with the ITS region is also valuable in identifying fungi at the intermediate taxonomic level [Citation41,Citation42].
Phylogenetic analysis based on ITS and 28S sequences showed that CNUFC-YR357-1 and CNUFC-YR357-2 strains were clustered within the same clade as A. luteoalbus from NCBI (). The results of our molecular data analysis were consistent with the phylogeny presented by Grum-Grzhimaylo et al. [Citation43]. The morphological characteristics of the A. luteoalbus isolate studied were generally similar to those previously described by Zare et al. [Citation10], who transferred this species to genus Acrostalagmus from genus Verticillium, based on their molecular studies. Species of A. luteoalbus have been reported to produce two new indole diketopiperazines, namely luteoalbusins 1 and 2, along with eight previously known ones (3–10). These compounds were evaluated for their cytotoxic activities against SF-268, MCF-7, NCI-H460, and HepG-2 cell lines, and compounds 1–5 showed significant cytotoxicity against all four cancer cell lines [Citation44]. Two new epipolythiodioxopiperazines, named chetracins E and F (1 and 2), along with the previously known chetracin C, exhibited cytotoxicity against the five tested cancer cell lines at the low-micromolar or nanomolar IC50 values isolated from A. luteoalbus [Citation45]. A novel alkali-tolerant rhamnosidase was isolated from the soil fungus A. luteoalbus from Argentina [Citation46].
Our analyses of ITS and 28S sequences showed that the strains CNUFC-CNUP1-1 and CNUFC-CNUP1-2 clustered with B. robillardoides CBS 122705 (type species), with well-supported branches, in full agreement with previous studies by Crous et al. [Citation16] and Wanasinghe et al. [Citation19] (). The CNUFC-CNUP1-1 isolate was morphologically most similar to B. robillardoides, as described by Crous et al. [Citation16]. B. robillardoides, B. laurina, and B. rosicola have 4-septate conidia. However, there is significant morphological variation in the conidial measurement; B. robillardoides has a conidia length/width ratio of 6.4 [Citation16]; B. laurina has a conidia length/width ratio of 7.4 [Citation47]; B. rosicola has a conidia length/width ratio of 3.8 [Citation19]. Some Bartalinia species have been recorded as plant pathogens that cause diseases on economically important crops [Citation22]; while some species, such as B. robillardoides, are well known for their secondary metabolites used in pharmaceutical industry. Hence, it is necessary to explore the biodiversity of Bartalinia species in Korea.
Phylogenetic analysis based on ITS and 28S sequences showed that CNUFC-NDR3-1 clustered within the same clade as C. carteri CBS 128.85 (type species) (). The morphological features of our isolates corresponded well with the description of C. carteri by Wang et al. [Citation23]. However, our observations showed that the CNUFC-NDR3-1 isolate produces seven to eight ascospores per ascus, whereas the ascus of C. carteri CBS 128.85 produces eight ascospores, as described by Wang et al. [Citation23]. Our results suggest that different isolates of the same species of C. carteri have different number of ascospores. Eight ascospores in each ascus are frequently occurring in comparison to seven ascospores (rare). This species is also showed to produce metabolite such as cochliodinol B and prenisatin [Citation23].
This study significantly improved our understanding of the rare ascomycete genera Acrostalagmus, Bartalinia, and Collariella in freshwater from Korea. There are numerous unknown freshwater derived species still awaiting description. In addition, three species obtained from this study may potentially be highly valuable. Thus, the potential biological activities of A. luteoalbus, B. robillardoides, and C. carteri should be further studied.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shearer CA. The freshwater Ascomycetes. Nova Hedwigia. 1993;56:1–33.
- Bucher VVC, Pointing SB, Hyde KD. Production of wood decay enzymes, loss of mass, and lignin solubilization in wood by diverse tropical freshwater fungi. Microb Ecol. 2004;48:331–337.
- Jones EBG, Hyde KD, Pang KL. Freshwater fungi and fungal-like organisms. Boston: De Gruyter; 2014.
- Hyde KD, Fryar S, Tian Q, et al. Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol. 2016;19:190–200.
- Maharachchikumbura SSN, Hyde KD, Jones EBG, et al. Families of Sordariomycetes. Fungal Divers. 2016;79:1–317.
- Hongsanan S, Maharachchikumbura SNN, Hyde KD, et al. An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers. 2017;84:25–41.
- Zhang N, Castlebury LA, Miller AN, et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia. 2006;98:1076–1087.
- Maharachchikumbura SN, Hyde KD, Jones EBG, et al. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015;72:199–301.
- Corda ACJ. Abbildungen der Pilze und Schwaemme. Icones Fungorum Hucusque Cognitorum. 1838;2:1–43.
- Zare R, Gams W, Schroers HJ. The type species of Verticillium is not congeneric with the plant-pathogenic species placed in Verticillium and it is not the anamorph of ‘Nectria' inventa. Mycol Res. 2004;108:576–582.
- Rubini MR, Silva-Ribeiro RT, Pomella AWV, et al. Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of witches’ broom disease. Int Biol Sci. 2005;1:24–33.
- Grantina-levina L, Andersone U, Berkolde-Pīre D, et al. Critical tests for determination of microbiological quality and biological activity in commercial vermicompost samples of different origins. Appl Microbiol Biotechnol. 2013;97:10541–10554.
- Mohammadi A, Amini Y. Molecular characterization and identification of A. luteoalbus from saffron in Iran. Agric Sci Dev. 2015;4:16–18.
- Zhang GZ, Tang CY. First report of Acrostalagmus luteo-albus causing red rust of needle mushroom (Flammulina velutipes) in China. Plant Dis. 2015;99:158.
- Tassi F, Bartalinia Fl. Tassi Nuovo genere di Sphaeropsidaceae. Bull Lab Ort Bot Siena. 1990;3:3–5.
- Crous PW, Giraldo A, Hawksworth DL, et al. The genera of Fungi: fixing the application of type species of generic names. IMA Fungus. 2014;5:141–160.
- Tejesvi MV, Tamhankar SA, Kini KR, et al. Phylogenetic analysis of endophytic Pestalotiopsis species from ethnopharmaceutically important medicinal trees. Fungal Divers. 2009;38:167–183.
- Wang Y, Jin L, Lin L, et al. New hosts for Bartalinia and Chaetopyrena in China. Mycotaxon. 2016;131:1–6.
- Wanasinghe DN, Phukhamsakda C, Hyde KD, et al. Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018;89:1–236.
- Gangadevi V, Muthumary J. Taxol, an anticancer drug produced by an endophytic fungus B. robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biotechnol. 2008;24:717–724.
- Jansen N, Ohlendorf B, Erhard A, et al. Helicusin E, isochromophilone X and isochromophilone XI: new chloroazaphilones produced by the fungus B. robillardoides strain LF550. Mar Drugs. 2013;11:800–816.
- Wijayawardene NN, Hyde KD, Wanasinghe DN, et al. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers. 2016;77:1–316.
- Wang XW, Houbraken J, Groenewald JZ, et al. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud Mycol. 2016;84:145–224.
- Crous PW, Wingfield MJ, Burgess TI, et al. Fungal planet description sheets: 625–715. Persoonia. 2017;39:270–467.
- Zhang ZF, Liu F, Zhou X, et al. Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia. 2017;39:1–31.
- Hyde KD, Hongsanan S, Jeewon R, et al. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;80:1–270.
- Mun HY, Goh J, Oh Y, et al. New records of three aquatic fungi isolated from freshwater in Samcheok and Yeongju, Korea. Kor J Mycol. 2016;44:247–251.
- Goh J, Mun HY, Oh Y, et al. Four species of Montagnulaceae unrecorded in Korea and isolated from plant litter in freshwater. Kor J Mycol. 2016;44:263–270.
- Nguyen TTT, Choi Y-J, Lee HB. Isolation and characterization of three unrecorded Zygomycete fungi in Korea: Cunninghamella bertholletiae, Cunninghamella echinulata, and Cunninghamella elegans. Mycobiology. 2017;45:318–318.
- Tibpromma S, Hyde KD, Jeewon R, et al. Fungal diversity motes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017;83:1–261.
- Nguyen TT, Lee SH, Bae S, et al. Characterization of two new records of Zygomycete species belonging to undiscovered taxa in Korea. Mycobiology. 2016;44:29–37.
- Nguyen TT, Paul NC, Lee HB. Characterization of Paecilomyces variotii and Talaromyces amestolkiae in Korea based on the morphological characteristics and multigene phylogenetic analyses. Mycobiology. 2016;44(4):248–259.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In. Innis M.A., Gelfand DH, Sninsky JJ, White TJ., editors. PCR protocols: a guide to methods and applications. New York, United States; Academic Press: 1990; p. 315–322.
- Lee HB. Molecular phylogenetic status of Korean strain of Podosphaera xanthii, a causal pathogen of powdery mildew on Japanese thistle (Cirsium japonicum) in Korea. J Microbiol. 2012;50:1075–1080.
- Thompson JD, Gibson TJ, Plewniak F, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Kelly LJ, Hollingsworth PM, Coppins BJ, et al. DNA barcoding of lichenized fungi demonstrates high identification success in a floristic context. New Phytol. 2011;191:288–300.
- Dai YC, Cui BK, Si J, et al. Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol Prog. 2015;14:62.
- Kiss L. Limits of nuclear ribosomal DNA internal transcribed spacer (ITS) sequences as species barcodes for Fungi. Proc Natl Acad Sci USA 2012;109:E1811.
- Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA. 2012;109:6241–6246.
- Porras-Alfaro A, Liu KL, Kuske CR, et al. From genus to phylum: large-subunit and internal transcribed spacer rRNA operon regions show similar classification accuracies influenced by database composition. Appl Environ Microbiol. 2014;80:829–840.
- Grum-Grzhimaylo AA, Georgieva ML, Bondarenko SA, et al. On the diversity of fungi from soda soils. Fungal Divers. 2016;76:27–74.
- Wang FZ, Huang Z, Shi XF, et al. Cytotoxic indole diketopiperazines from the deep sea-derived fungus A. luteoalbus SCSIO F457. Bioorg Med Chem Lett. 2012;22:7265–7267.
- Yu G, Wang Y, Yu R, et al. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus A. luteoalbus HDN13-530. RSC Adv. 2018;8:53–58.
- Rojas NL, Voget CE, Hours RA, et al. Purification and characterization of a novel alkaline α-L-rhamnosidase produced by A. luteoalbus. J Ind Microbiol Biotechnol. 2011;38:1515–1522.
- Nag Raj TR. Coelomycetous anamorphs with appendage-bearing conidia. Waterloo, Canada; Mycologue Publications: 1993; p. 1101.
