Abstract
Fungal perylenequinones have photodynamic activity and are promising photosensitizers for photodynamic therapy (PDT). Here, we investigated the bactericidal and antitumor activities of phleichrome from the fungal perylenequinone family in vitro. Photodynamic bactericidal activity of phleichrome was analyzed by agar-well diffusion method under dark and illuminated conditions. The photodynamic antitumor activity of phleichrome was analyzed in MCF-7, HeLa, SW480, and HepG2 human cancer cell lines using in vitro cytotoxicity assays. Photodynamic bactericidal activities against Gram-negative and Gram-positive bacteria were species-specific. Antitumor activity against all tumor cell lines increased under the illuminated condition. Depending on the results of the analyses, Phleichrome has potential for further drug development related to its antibacterial and antitumor activities.
Photodynamic therapy (PDT) is an innovative and attractive method to treat cancer and other obstinate diseases [Citation1,Citation2]. PDT requires both a selective photosensitizer and a light source, which matches the absorption spectrum of the photosensitizer. PDT is considered a promising alternative approach to treat antibiotic-resistant bacterial strains [Citation3–5]. Phleichrome, a fungal perylenequinone with photodynamic activity, has been isolated from the phytopathogenic fungus Cladosporium phlei. Phleichrome has a structure of 1,12-bis-(2-hydoxypropyl)-2,6,7,11-tetramethoxy-4,9-dihydroxyperylene-3,10-quinone, a derivative of 4,9-dihydroxy-3,10-quinone, and the spectrum of phleichrome has absorption maxima at 226, 260, 274, 474, 540, and 584 nm [Citation6,Citation7]. In our previous study, we reported that phleichrome converted oxygen into reactive oxygen species (ROS) when illuminated [Citation7]. Photosensitizer is activated to generate short-lived toxic species upon absorption of the certain wavelength of light during PDT treatment. Therefore, in this study, we measured the in vitro photodynamic activities of phleichrome in the presence or absence of light to investigate the in vitro photodynamic activity of phleichrome against representative human pathogenic bacteria and cancer cell lines.
Thirteen human pathogenic bacteria strains, including six Gram-positive and seven Gram-negative strains were tested for photodynamic bactericidal activity ( and ). All strains were grown on Luria–Bertani agar medium (1% tryptone, 0.5% yeast-extract, 1% sodium chloride, and 1.5% agar) and incubated at 37 °C for 16–18 h. The bacterial suspensions were prepared in Luria–Bertani broth at a final concentration of approximately 1.0 × 108 CFU/mL. The photodynamic bactericidal activity of phleichrome was analyzed using the agar-well diffusion method under dark and illuminated conditions, as described previously [Citation8]. Purified phleichrome was dissolved in absolute ethanol and used for further analysis. Briefly, paper discs (Advantec, Tokyo, Japan), containing 5, 10, 25, and 50 μg of purified phleichrome, were prepared. Discs containing absolute ethanol were used as a control. All culture plates were incubated under 3.8 × 106 lx irradiation for 10 h, and the diameters of the inhibition zones were measured to compare the strains.
Table 1. Photoinactivation results for the different bacterial strains under the dark condition.
Table 2. Photoinactivation results for the different bacterial strains under the illuminated condition.
As a result of analysis of photodynamic bactericidal activity, all other bacterial strains, except two Bacillus strains, showed normal growth compared to that of the control disk under the dark condition. Growth of the two Bacillus strains was inhibited dose dependently in the dark (). However, growth was inhibited in a dose-dependent manner in all Gram-positive bacterial strains, including Bacillus cereus, B. infantis, Enterococcus faecium, E. hirae, Staphylococcus saprophyticus, and S. xylosus under the illuminated condition (). Six of the seven Gram-negative bacterial strains, Cronobacter sakazakii, Enterobacter cowanii, Escherichia coli, E. hermannii, Pantoea agglomerans, and P. anthophila were not sensitive to phleichrome under the illuminated condition. Interestingly, growth was inhibited in a dose-dependent manner by phleichrome in one Gram-negative bacteria strain, P. ananatis, under the illuminated condition ().
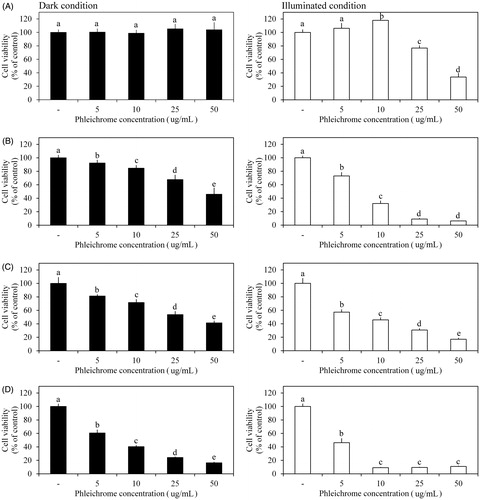
Additionally, we examined the antitumor efficacy of phleichrome in four human cancer cell lines (HeLa cervical cancer cells, HepG2 liver cancer cells, MCF-7 breast cancer cells, and SW480 colon cancer cells) using cytotoxicity assays, as described previously [Citation9]. Briefly, 1.0 × 105 cells/mL were seeded in 96-well plates and incubated under 5% CO2 at 37 °C for 24 h. Fresh medium supplemented with various concentrations (0, 5, 10, 25, and 50 μg/mL) of phleichrome was added to each well and further incubated for 4 h. Then, one group was illuminated for 20 min at 2.3 × 106 lx and another group remained in the dark. All treated cell lines were incubated for another 24 h followed by the addition of 10 μL WST solution (EZ-Cytox, Daeillab, Seoul, Korea) and further incubation for 4 h. The cell viability index was determined by measuring formazan production using a microplate reader (Benchmark Plus; Bio-Rad, Hercules, CA) at 480 nm with a reference wavelength of 650 nm, which is used to quantify the basal absorption of the plate.
Evaluation of the antitumor efficacy showed that viability of the HeLa, SW480, and MCF-7 cancer cell lines decreased significantly and dose dependently in response to 5–50 μg/mL phleichrome under the dark condition (). The HeLa, SW480, and MCF-7 cell lines had their lowest viability with 50 μg/mL treatment. No significant difference in viability was observed in the HepG2 cell line in response to phleichrome under the dark condition (). However, the three phleichrome-sensitive cell lines showed more rapidly reduced viability under the illuminated condition (). The lowest viability in the HeLa and MCF-7 cell lines was detected in the 25- and 10-mM treatments under the light compared to the dark condition, respectively. The SW480 cell line was 29.5–58.7% more sensitive to phleichrome in the presence of light than in the absence of light. Interestingly, HepG2 showed dose-dependent sensitivity to phleichrome only under the illuminated condition. Among all of the cell lines, MCF-7 cells were the most sensitive to phleichrome.
Figure 1. Effects of phleichrome on cancer cell viability. Cell viability was measured in (A) HepG2, (B) HeLa, (C) SW480, and (D) MCF-7 cell lines under dark (black bar) and illuminated conditions (white bar). Results are the means ± standard deviation (SD) of triplicate independent experiments. Statistical analyses were performed using ANOVA at p = .05 using SPSS software (version 23.0, IBM Corp., Armonk, NY).
Photodynamic activity is widely used in various fields, including biology, chemistry, and medicine [Citation10,Citation11]. The antimicrobial applications of PDT are rapidly growing compared to traditional anti-cancer applications [Citation12]. Although many significant advances have been made in photosensitizers, there are several limitations, such as skin photosensitivity, pain experienced by patients during irradiation, and limited treatment depth [Citation13]. Therefore, it is important to discover and develop new photosensitizers. In this study, the photodynamic activity of phleichrome, a new fungal perylenequinone, was investigated.
Gram-positive bacterial strains were more sensitive to phleichrome than Gram-negative bacterial strains, i.e. all Gram-positive bacterial strains were photoinactivated, whereas only one Gram-negative bacteria, P. ananatis, was photoinactivated by phleichrome. These results are consistent with those of other studies showing that Gram-positive bacteria are remarkably sensitive to the photosensitizing action. Photoinactivation process of Gram-type bacteria is dependent on presence of the outer membrane, the number of positive charges, charge distribution, chemical structure, and the nature of meso-substituent groups present on the macrocycle periphery, but the specific mechanism related to Gram type has not yet been completely elucidated [Citation14–Citation17]. Therefore, further studies are required to understand the species-specific photodynamic mechanisms of photoinactivation.
Phleichrome showed cytotoxic effects on HeLa, MCF-7, and SW480 cell lines under the dark condition. However, pheichrome was more toxic to those cancer cells under the illuminated condition than under the dark condition. Interestingly, HepG2 liver cancer cells were only sensitivity to phleichrome under the illuminated condition. These results indicate the broad-spectrum antitumor efficacy of phleichrome regardless of the cell line. Considering the specific photoinactivation effect on HepG2 cells using phleichrome, it may be more suitable for treating liver tumors than for other tumors.
In this study, we investigated the bactericidal and antitumor activities of phleichrome from the phytopathogenic fungus C. phlei. We observed that phleichrome had species-specific photoinactivation activity against bacteria. In addition, the photoinactivation activity of phleichrome was effective in all tested tumor cell lines. These results suggest that phleichrome has the potential for further development as a PDT agent related to its bactericidal and antitumor activities.
Acknowledgments
The authors thank the Institute of Molecular Biology and Genetics at Chonbuk National University for kindly providing the facilities for this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Prażmo EJ, Kwaśny M, Łapiński M, et al. Photodynamic therapy as a promising method used in the treatment of oral diseases. Adv Clin Exp Med. 2016;25:799–807.
- Ackroyd R, Kelty C, Brown N, et al. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–669.
- Masiera N, Bojarska A, Gawryszewska I, et al. Antimicrobial photodynamic therapy by means of porphycene photosensitizers. J Photochem Photobiol B Biol. 2017;174:84–89.
- Sperandio FF, Huang YY, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 2013;8:108–120.
- Baltazar LM, Ray A, Santos DA, et al. Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Front Microbiol. 2015;6:202.
- Yoshihara T, Shimanuki T, Araki T, et al. Phleichrome: a new phytotoxic compound produced by Cladosporium phlei. Arg Biol Chem. 1975;39:1683–1684.
- So KK, Jo IS, Chae MS, et al. Improved production of phleichrome from the phytopathogenic fungus Cladosporium phlei using synthetic inducers and photodynamic ROS production by phleichrome. J Biosci Bioeng. 2015;119:289–296.
- Gnanamanickam SS, Smith DA. Selective toxicity of isoflavonoid phytoalexins to Gram-positive bacteria. Phytopathology. 1980;70:894–896.
- So Y, Lee SY, Han AR, et al. Rosmarinic acid methyl ester inhibits LPS-induced NO production via suppression of MyD88- dependent and -independent pathways and induction of HO-1 in RAW 264.7 cells. Molecules. 2016;21:1083.
- Zhu TC, Finlay JC. The role of photodynamic therapy (PDT) physics. Med Phys. 2008;35:3127–3136.
- Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32:415–425.
- Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–364.
- O'Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–1074.
- Alves E, Costa L, Carvalho CM, et al . Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009;9:70.
- Merchat M, Spikes JD, Bertoloni G, et al. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B Biol. 1996;35:149–157.
- Caminos DA, Spesia MB, Durantini EN. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem Photobiol Sci. 2006;5:56–65.
- Banfi S, Caruso E, Buccafurni L, et al. Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2006;85:28–38.
