Abstract
Candelariella is a widespread lineage of lichenized ascomycetes with ambiguous relationships among species that have not solved completely. In this study, several specimens belonging to Candelariella were collected from China and South Korea, and the internal transcribed spacer region was generated to confirm the system position of the newly collected specimens. Combined with a morphological examination and phylogenetic analysis, two new areolate species, Candelariella rubrisoli and C. subsquamulosa, are new to science. Detail descriptions of each new species are presented. In addition, C. canadensis is firstly reported from China mainland.
Keywords:
1. Introduction
The family Candelariaceae Hakul., primarily described by Hakulinen [Citation1], is comprised of four genera around the world: Candelariella Müll. Arg. (ca. 50 spp.) with the areolate to squamulose thallus, Candelaria A. Massal. (9 spp.) with foliose to subfruticose thallus, Candelina Poelt (3 spp.) with placoid to subfoliose thallus, and Placomaronea Räsänen (6 spp.) with squamulose to umbilicate thallus [Citation2–4]. Although molecular analyses have been conducted, many taxonomic questions remain to be answered. Neither Candelariella nor Candelaria was monophyletic indicated by phylogenetic analysis [Citation4,Citation5]. Polyspored or 8-spored asci is a very important characteristic to distinguish the species in genus Candelariella, but the fertile organism is frequently absent in the sorediate group, which makes species identification much more difficult. Hence, molecular data are expected to solve this issue [Citation6].
Candelariella are estimated to be particularly diverse in Asia and America [Citation6–12], but delimitation of the species needs to be assessed critically. The patterns in morphological diversity suggest that this genus is represented by five species in South Korea [Citation13–20] and fourteen species in China mainland and Taiwan [Citation7,Citation21]. The morphological, chemical and DNA traits were integrated to elucidate the diversity of species of Candelariella in South Korea and Southwest of China and estimate their relationships.
2. Material and methods
2.1. Material and morphological studies
All newly collected specimens included in this study were deposited in Sunchon National University, Korean Lichen Research Institute (KoLRI). The morphology examination and anatomical characteristics were recorded under a dissecting microscope (Model SMZ 745 T; Nikon, Tokyo, Japan) and Olympus BX 50 microscope (Olympus, Tokyo, Japan), and photographs were taken using a HD-Measure LTHS-300 (Leetech Co., Seoul, South Korea) microscope and Carl Zeiss MicroImaging with Axio Cam ERc 5 s imaging system (Carl Zeiss, Gottingen, Germany). The minimum (min.) and maximum values (max.) of the thallus width, soredia, apothecia, and ascospore size are recorded. The number (N) and mean values (in italics) were measured. Secondary metabolites were studied by a spot test and thin layer chromatography in solvent C, as described in [Citation22] and [Citation23]. UV test was performed with a UV Chamber (VL-6.LC; Viber Lourmat, Collégien, France) under a long (366 nm) wavelength.
2.2. Molecular studies
The total genomic DNA was extracted from newly collected specimens using the NucleoSpin Plant II Kit (Clontech Laboratories, Mountain View, CA) according to the manufacturer’s instructions. The internal transcribed spacer (ITS) region, including the 5.8S ribosomal RNA gene, and the 5’portion of the large subunit of the ribosomal RNA (28S) were targeted by PCR using the primers pairs, ITS4 and ITS1F [Citation24], LR0R [Citation25], and LR5 [Citation26], respectively. The protocols of PCR amplification are described in the previous report [Citation27]. Sequencing was accomplished by the genomic companies GenoTech (Daejeon, Korea) and Macrogen (Daejeon, Korea). Newly obtained sequences for the ITS region of the Candelariella species () were assembled and edited using SeqMan (DNASTAR, Madison, WI) and Mega 7.0 [Citation28], and complemented with publically available sequences () into a matrix and aligned with Mafft v7.273 [Citation29]. The ambiguous regions were identified and excluded using Gblocks 0.91 b [Citation30]. Pycnora xanthococca (Sommerf.) Hafellner was used as the outgroup since Pycnora has been recognized as a possible sister clade to Candelariaceae [Citation31].
Table 1. Voucher information of the ITS and 28S sequences newly generated in this study.
2.3. Phylogenetic analysis
The matrix was analyzed under the criterion of maximum likelihood (ML) using RAxML v7.2.6 [Citation32] with the GTR + I + G model. The nodes supported by ML bootstrap values >70% were estimated from the consensus trees obtained from 1000 nonparametric bootstrapping pseudoreplicates. Bayesian inference (BI) was performed with MrBayes v3.1.2 [Citation33], applying the best-fitted substitution models (GTR + T + G) based on the Akaike information criterion using jModelTest 3.7 [Citation34]. The BIs were conducted using four chains and run for 2 million generations. The trees were sampled every 1000 generations with the first 20% of the tree discarded. The remaining trees were used to generate a majority-rule consensus tree with posterior probabilities (PP); clades with a PP value ≥ 0.95 were considered to be significantly supported.
3. Results
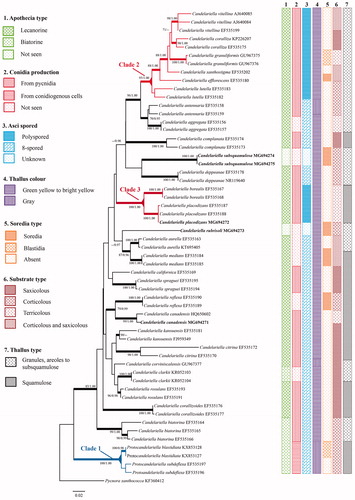
The matrix was comprised of 57 sequences representing 30 taxa. After excluding the ambiguous sites, 471 sites were retained for the Candelariella matrix. The phylogenetic analyses resolved all species of Candelariella that were represented by two or more accessions as robust clades (). Newly sequenced accessions from East Asia, were either mostly closely related to known taxa (e.g., Candelariella placodizans, C. canadensis; ), resolved as singletons (i.e., Candelariella rubrisoli; ), or as novel clades (i.e., C. subsquamulosa; ) that were proposed as new species. Seven characters are listed (apothecia type, conidia production, asci spored type, thallus color and type, soredia and substrate type) were combined with a phylogenetic tree to examine the lineages of Candelariella (), but neither 8-spored nor polyspored species of Candelariella were monophyletic.
Figure 1. Most likely phylogenetic relationships among species of Candelariella inferred from ITS sequences with distribution of selected characters. Nodes supported by ML bootstrap values >70% and Bayesian posterior probabilities >0.95 are identified by thickened branches; the clades with polyspored asci and conidiogenous cell were marked with color.
3.1. Taxonomy of Candelariella rubrisoli D. Liu & J.-S hur, sp. nov
MycoBank No.: MB824741
Similar to C. efflorescens, but differs in an irregular subsquamulose thallus, usually slightly ascending from one side.
Type: China, Yunnan Province, Kunming City, Dongchuan District, Huagou Village, 25°55′23″N, 103°05′03″E, 2403 m, on Pinus armandi, 3 August 2017, J.-S. Hur & D. Liu CH170039 (holotype, KoLRI!). Accession number: ITS = MG694273, LSU = MH101758 ().
Figure 2. Candelariella rubrisoli. (A, B) Habit; (C) thallus breaking into soredia; (D) soredia; (E) transverse section of thallus [Scale bar: (B–D) 0.5 mm, (E) 20 µm].
![Figure 2. Candelariella rubrisoli. (A, B) Habit; (C) thallus breaking into soredia; (D) soredia; (E) transverse section of thallus [Scale bar: (B–D) 0.5 mm, (E) 20 µm].](/cms/asset/b4a042f5-78b1-4a95-ba63-31c520c87df4/tmyb_a_1583785_f0002_c.jpg)
Thallus corticolous, crustose, areolate to subsquamulose, irregular, scattered or several aggregation to imbricate sometimes, green yellow, (50)60–140–220(280) µm (N = 56). Subsquamulose thalli often dissolved, and margin broke into fine, granular soredia and dispersed inward or to the lower side, covering the entire upper surface of the squamules and forming a ± continuous leprose or pulverulent crust, which is greenish under the soredia layer due to the exposure of photobiont. Soredia granular, spherical, (30)40–54–70(80) µm (N = 85), bright yellow to orange. Thallus layers not well developed, upper cortex (pseudocortex) indistinct, 7–10 µm thick, of 1–2 layer non-gelatinized hyphae. Photobiont chlorococcoid, ca. 6.9–11.3 µm in diam., dispersed in the thallus. Hypothallus and prothallus absent. Apothecia and pycnidia not seen.
Chemistry: K–, KC–, C–, PD–; Calycin and pulvinic acid as major substances.
Etymology: The epithet “rubrisoli” refers to the type location, where is famous for its red soil lands.
Ecology: Candelariella rubrisoli was found growing on Pinus armandi, together with Flavopunctelia flaventior, Heterodermia, Lepraria, Ochrolechia, Parmotrema, Rinodina and Usnea rubescens.
Remarks: Candelariella rubrisoli is characterized by the areolate to subsquamulose thallus, which usually breaks and ultimately dissolves into the soredia. Candelariella rubrisoli resembles C. efflorescens R. C. Harris & W. R. Buck, but differs in having larger soredia ((30)40–54–70(80) vs. 15–40(–50) µm [Citation6]). It is also similar to C. reflexa (Nyl.) Lettau, but differs in having smaller squamules, and to C. xanthostigmoides (Müll. Arg.) R. W. Rogers, which has smaller soredia ((20)18.6–32.4–46.2(60) µm [Citation35]) and frequently grows on the deciduous trees in North America.
Comparison with C. sorediosa Poelt & Reddi, the similar corticolous species was primarily described from Nepal [Citation36], and from Taiwan [Citation10]. However, Lendemer and Westberg [Citation35] treated C. sorediosa as a distinct species based on the examination of holotype (Poelt L434, M), which is “very meager, a few minute areoles are slightly raised from substrate, and sorediate from the margin,” this species seems even not frequently recorded in the Asia. Yakovchenko et al. [Citation7] treated the growth direction of soredia as a significant character to distinguish the sorediate species of Candelariella in their key part, with a short description that the soredia dispersed to the lower side of thallus, in contrast, soredia of C. rubrisoli dispersed inward and lacking district direction.
Candelariella rubrisoli is similar to C. granuliformis, however, C. granuliformis usually not forming distinct soralia but breaking into blastidia and frequently occurs in terricolous habit [Citation37]. Candelariella rubrisoli resembles C. biatorina, C. subdeflexa, C. lutella, and C. deppeanae, but differs in having soredia; Candelariella rubrisoli can be distinguished from C. antennaria, C. blastidiata, C. oleaginescens, and C. aurella in having bright yellowish areolate to subsquamulose thallus. There are no sexual propagules present, and it is hard to make clear the polyspored or 8-spored asci, however, the phylogenetic tree also suggests that C. rubrisoli separated from other species.
3.2. Taxonomy of Candelariellia subsquamulosa D. Liu & J.-S hur, sp. nov
MycoBank No.: MB824742
Similar to C. reflexa, but differs by the green to dark green thallus with the margins remaining intact (i.e., not breaking into soredia).
Type: South Korea, Jeollanam-do, Suncheon-si, Jungang-ro 225, Sunchon National University, 34°58′01″N, 127°28′48″E, 47 m, on Cerasus sp., 26 September 2017, D. Liu 171419 (holotype, KoLRI!). Accession number: ITS = MG694274, LSU = MH101759 ().
Figure 3. Candelariella subsquamulosa. (A, B) Habitat; (C) thallus; (D, F) soredia; (E) transverse section of thallus [Scale bar: (A, B) 2 cm, (C, D) 0.5 mm, (E, F) 20 µm].
![Figure 3. Candelariella subsquamulosa. (A, B) Habitat; (C) thallus; (D, F) soredia; (E) transverse section of thallus [Scale bar: (A, B) 2 cm, (C, D) 0.5 mm, (E, F) 20 µm].](/cms/asset/81b79a25-09e5-4065-a510-f28bd7c13129/tmyb_a_1583785_f0003_c.jpg)
Thallus corticolous, crustose, granular to subsquamulose, irregular, ± round, flattened to somewhat convex, scattered or aggregation to imbricate sometimes, not dissolved, tightly attached to the substrate, (60)90–170–370(440) µm (N = 38). Upper surface green to dark green, slightly shiny under optical microscopy, margin irregular, usually not breaking into soredia. Soredia granular, spherical, (30) 40–60–70(80) µm (N = 40), bright yellow to orange, discrete or aggregation into a sorediate crust, with a thallus areole-like appearance, covering the thallus or directly widespread on the substrate. Thallus layers not well developed, upper cortex (pseudocortex) indistinct, 7–8 µm thick, of 1–2 layers of non-gelatinized hyphae. Photobiont chlorococcoid, ca. 5–10 µm in diameter, dispersed in the thallus, but usually close to the upper cortex. Hypothallus indistinct, with silvery hyphae interwoven. Apothecia and pycnidia not seen.
Chemistry: K–, KC–, C–, PD–; Calycin and pulvinic acid as major substances.
Ecology: Candelariellia subsquamulosa was found growing on conifer and cherry flower trees, together with buelliod lichens, Lepraria, and Phaeophyscia spp.
Etymology: The epithet “subsquamulosa” refers to the green to dark green subsquamulose thallus.
Additional examined specimens: South Korea. Jeollanam-do, Suncheon-si, Jungang-ro 225, Sunchon National University, 34°58′01″N, 127°28′48″E, 47 m, on Cerasus sp., 26 September 2017, D. Liu 171418; 1 December 2017, D. Liu 171451, 171452; Jeollabuk-do, Namwon-si, Geumji-myeon, 35°19′11″N, 127°16′45″E, 260 m, on pine tree, 5 June 2015, D. Liu 152676.
Remarks. Candelariellia subsquamulosa is characterized by its green to dark green granular to subsquamulose thallus, which never breaks into the soredia and is covered with numerous bright yellow to orange soredia. This species usually grows on the bark of cherry and conifers. The color of soredia is variable, always orange on conifers or under an open area, but is yellow to yellow greenish when on a cherry tree or under low light or in the shade.
Differences among species of Candelariella are usually based on the following characteristics: substrate, thallus color, and type, asci 8-spored or polyspored and soredia present or absent. The genus holds ca. 50 species around the world, and five species produce soredia: C. efflorescens, C. medians (Nyl.) A. L. Sm., C. reflexa, C. sorediosa, and C. xanthostigmoides.
Candelariellia subsquamulosa is most similar to C. reflexa in having soredia, and often growing on bark, but differs in having green granular to subsquamulose thalli, and an intact thallus margin. The soredia of C. subsquamulosa usually adhere to or are scattered over the surface and can be separated easily from the thallus or substrate. Furthermore, C. reflexa has much larger and indistinctly effigurate thalli, with lobes up to 0.6 mm long [Citation6], and when well developed, it can have an almost rosette-like thallus, and be sorediate from the center of the areoles.
Candelariellia subsquamulosa is also similar to C. efflorescens, but can be distinguished by its thallus not dissolved and obscured by soredia. In addition, the soredia of C. efflorescens usually form along the margin of thallus. Candelariellia efflorescens is recorded from Europe [Citation38], North America [Citation6], and South Korea [Citation18]. The species always grows on bark (mainly broadleaved trees) or bryophytes [Citation6], but one specimen was found on siliceous rock in South Korea [Citation18]. In contrast, C. subsquamulosa was only found in colonizing bark.
Candelariellia subsquamulosa also resembles also the sorediate species C. medians, which occurs in North Africa, Europe, Southwest Asia, and China [Citation9,Citation21,Citation39], but C. medians forms placoid thalli, and is usually found on nitrogen-enriched rocks, particularly where birds often rest and leave their droppings [Citation39].
Candelariellia subsquamulosa differs from the other soradiate species, C. xanthostigmoides, in thallus not dissolved into soredia, and in soredia without obvious spreading direction, comparison with C. xanthostigmoides, soredia of latter appears to begin at the margin, spreading inward [Citation35].
3.3. Taxonomy of Candelariella canadensis H. Magn.
Ark. Bot. ser. 2, 2: 216 (1952)
= Candelariella hudsonica Hakul., Ann. Bot. Soc. Zool.-Bot. Fenn. “Vanamo” 27: 49 (1954), synonymized in [Citation40].
= Candelariella nepalensis Poelt & Reddi, Khumbu Himal [Universitätsverlag Wagner, Innsbruck–München] 6: 8 (1969), synonymized in [Citation40].
Thallus composed of large granules 100–400 µm (N = 30), aggregation or becoming large then forming lobes, up to 1–3 mm wide, ± rosettes adnate to the substrate; surface yellow to dark yellow, smooth in the lobe center and pulverulent near the lobe margin and granules. Thallus 200–330 µm thick, pseudocortex uneven, 9.6–12 µm. Photobiont chlorococcoid, ca. 5–10 µm diam. Apothecia lecanorine, 0.33–0.59–0.86 mm (N = 17) diam., thalline margin to 160 µm thick, entire when young, becoming incised and ultimately with few granules remaining. Proper margin 25–106 µm thick, composed of radiating hyphae with elongated cells. Disc yellow to brown, flat at first, or turning convex or even globose sometimes. Hymenium 60–82 µm thick. Paraphyses simple, sometimes branch on the tip, swollen at the tip but not blacken, up to 5.5 µm wide. Asci clavate, 8-spored, 58.7(58.8)–62.6–66.5(69) × (10.1)10.5–12.7–14.6(15.2) µm (N = 16). Ascopores colorless, narrowly ellipsoid, simple, or with thin septa, or with oil droplets sometimes, the end usually attenuated, 13.1(13.3)–15.2–17.3(17.5) × (5.1)5.9–6.6–7.4(8) µm (N = 37). Pycnidia not seen ().
Figure 4. Species of Candelariella in China. (A, C–F) Candelariella canadensis. (A) Habit; (C) apothecia; (D) transverse section of thallus; (E) transverse section of apothecia; (F) ascospores; (B) Candelariella placodizans [Scale bar: (A–C) 0.5 mm, (D) 20 µm, (E) 50 µm, (F) 10 µm].
![Figure 4. Species of Candelariella in China. (A, C–F) Candelariella canadensis. (A) Habit; (C) apothecia; (D) transverse section of thallus; (E) transverse section of apothecia; (F) ascospores; (B) Candelariella placodizans [Scale bar: (A–C) 0.5 mm, (D) 20 µm, (E) 50 µm, (F) 10 µm].](/cms/asset/452ac605-67c8-49b4-b4ed-a5b9658ef57a/tmyb_a_1583785_f0004_c.jpg)
Chemistry: Thallus K–, KC–, C–, PD–, apothecia disc K slightly red; Calycin and pulvinic acid as major substances.
Ecology: Candelariella canadensis was found growing on the moss over rock or directly grow on the rock.
Examined specimen: CHINA, Yunnan Province, Kunming City, Luquan Co., near the peak of Jiaozi Snow Mt., 26°5′6″N, 102°51′9″E, 3970 m, on moss, 3 August 2017, J.-S. Hur & D. Liu CH170062 (KoLRI). Accession number: ITS = MG694271, LSU = MH101756.
Remarks: Candelariella canadensis is characterized by the well-developed pulverulent thallus and often convex apothecia with the granules scattered around the margin. [Citation41] treated this species was as a synonym of C. terrigena, whereas Westberg treated as a synonym of C. citrina, based on the examination of holotypes of C. terrigena and C. terrigena var. placodimorpha collected from Colorado (C. C. Plitt, H) and New Mexico (S. Shushan & W. A. Weber S6893, TUR), and considered the type specimen of C. terrigena to be a mixture of two species, including C. rosulans (dark yellow thallus and narrowly ellipsoid spores) and C. citrina (greenish thallus and shorter, ovoid to citriform spores) [Citation12]. Then Westberg investigated the initial cited specimens and holotype of C. canadensis (Freucher 1469, lectotype, C), C. hudsonica (CANL No. 12961, holotype) and C. nepalensis (Poelt L441, holotype, M), and concluded that the latter two species were conspecific [Citation40]. Our phylogenetic tree shows that our specimen collected from Jiaozi Snow Mountains forms a clade with C. terrigena (HQ650602), the specimen used in [Citation42] may be a misidentification of C. canadensis, this species was reported as C. nepalensis from Taiwan growing on moss and soil over 3000 m altitude [Citation10], and is here recorded as new from mainland of China. Morphology, anatomy, and chemistry of Chinese material match the description [Citation40], but have longer asci (58.7(58.8)–62.6–66.5(69) × (10.1)10.5–12.7–14.6(15.2) vs. 52–57 × 13–17 µm). As a conclusion, GenBank sequence of C. terrigena (HQ650602) may be a misidentification of C. canadensis.
3.4. Taxonomy of Candelariella placodizans (Nyl.) H. Magn.
In Lynge, Rep. Fifth Thule Exped. 1921–24, 2: 23 (1935)
= Candelariella himalayana Poelt & Reddi, Khumbu Himal [Universitätsverlag Wagner, InnsbruckMünchen] 6: 8 (1969), synonymized in [Citation40].
Candelariella placodizans is characterized by squamulose thallus, upper surface of squamules yellow to bright pulverulent, and the lecanorine apothecia with polyspored asci. This species usually appear in the alpine and subalpine habitats on moss or plant debris [Citation6]. Our specimen lacks apothecia but the thallus morphology, chemistry, and ecology are consistent with the description in [Citation6]. It is also robustly resolved based on ITS data in the C. placodizans clade ().
Chemistry: Thallus K–, KC–, C–, PD–; Calycin and pulvinic acid as major substances.
Ecology: Candelariella placodizans is an arctic alpine lichen that grows on terricolous bryophytes. In China, it was found in the rocky areas of mountains in Taiwan over 3240 m [Citation7] and in Yunnan over 3900 m, where it occurred with C. canadensis.
Examined specimens: China, Yunnan Province, Kunming City, Luquan Co., near the peak of Jiaozi Snow Mt., 26°5′6″N, 102°51′9″E, 3970 m, on moss, 3 August 2017, J.-S. Hur & D. Liu CH170084 (KoLRI). Accession number: ITS = MG694272, LSU = MH101757.
4. Discussion
Poelt recognized Candelariella subdeflexa was a unique and suggested it a “protocandelariella” in family Candelariaceae [Citation43], and the morphology and molecular phylogeny strongly supported the close relationship between Candelariella blastidia and C. subdeflexa, which formed a single clade and was separated from the other species in Candelariaceae, Clade 1 [Citation4,Citation44]. These two species produce conidia from conidiogenous cells on the lower surface, and differ from the other species of Candelariella in producing conidia from the pycinidia on the upper surface. In addition, soredia or blastidia, apothecia type, and thallus color have a positive role in distinguishing species, but was not significant for distinguishing the potential different groups of 8-spored species of Candelariella prior to this study [Citation4].
Candelariella in a broad sense is characterized by granules to squamulose thalli, 8-spored or polyspored asci, given lower support of the main clade. The relationship among 8-spored species remains ambiguous but species with polyspored asci are separated into two single clades with strong support. Clade 2, Candelariella s. str., with the type species C. vitellina, is characterized by granulose, areolate to subsquamulose thalli. Clade 3 is characterized by squamulose thalli and usually occurs on the bryophytes in the rocky mountainous areas above the tree line (i.e., >3000 m).
The 8-spored or polyspored asci is a significant character to distinguish the species of Candelariella and Candelaria, however, the apothecia usually rarely present in sorediate species, which makes the delimitation incredible only from the size of the squamules or soredia. For this case, molecular data is a potential method for the discovery of species and their relationship within the genus Candelariella.
Supplemental Material
Download MS Word (22.1 KB)Acknowledgements
The authors are grateful to Prof. Bernard Goffinet (University of Connecticut) for reviewing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hakulinen R. Die flechtengattung Candelariella Müll. Arg., mit besonderer berücksichtigung ihres auftretens und ihrer verbreitung in Fennoskandien. Ann Bot Soc Zool Bot Fenn ‘Vanamo'. 1954;27:1–127.
- Westberg M. The lichen genus Candelariella in Western North America [dissertation]. Department of Biology, Lund University; 2005.
- Westberg M, Clerc P. Five species of Candelaria and Candelariella (Ascomycota, Candelariales) new to Switzerland. MycoKeys. 2012;3:1–12.
- Westberg M, Arup U, Kärnefelt I. Phylogenetic studies in the Candelariaceae (lichenized Ascomycota) based on nuclear ITS DNA sequence data. Mycol Res. 2007;111:1277–1284.
- Westberg M, Arup U. Candelaria pacifica sp. nova (Ascomycota, Candelariales) and the identity of Candelaria vulgaris. Bibioth Lichenol. 2011;106:353–364.
- Westberg M. Candelariella (Candelariaceae) in western United States and northern Mexico: the polysporous species. Bryologist. 2007;110:375–390.
- Yakovchenko L, Davydov EA, Ohmura Y. Candelariella placodizans (Candelariaceae) reported new to mainland China and Taiwan based on morphological, chemical and molecular phylogenetic analyses. Tawania. 2016;6:159–164.
- Yakovchenko L, Ahti T, Westberg M. Candelariella biatorina new to Asia from the Russian Far East. Herzogia. 2013;26:207–212.
- Westberg M, Sohrabi M. A conspectus of the lichen genus Candelariella (Candelariaceae, Ascomycota) in Southwest Asia with emphasis on Iran. Nova Hedw. 2012;95:531–546.
- Aptroot A, Sparrius LB. New microlichens from Taiwan. Fungal Divers. 2003;14:1–50.
- Westberg M. Candelariella (Candelariaceae) in western United States and northern Mexico: the species with biatorine apothecia. Bryologist. 2007;110:365–374.
- Westberg M. Candelariella (Candelariaceae) in western United States and northern Mexico: the 8-spored, lecanorine species. Bryologist. 2007;110:391–419.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 6. Acta Bot Hung. 2017;59:137–260.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 5. Acta Bot Hung. 2016;58:319–396.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 4*. Acta Bot Hung. 2016;58:75–136.
- Kondratyuk SY, Lőkös L, Farkas E, et al. New and noteworthy lichen-forming and lichenicolous fungi 2. Acta Bot Hung. 2015;57:77–141.
- Kondratyuk SY, Lőkös L, Farkas E, et al. New and noteworthy lichen-forming and lichenicolous fungi 3*. Acta Bot Hung. 2015;57:345–382.
- Aptroot A, Moon KH. 114 New reports of microlichens from Korea, including the description of five new species, show that the microlichen flora is predominantly Eurasian. Herzogia. 2014;27:347–365.
- Kondratyuk SY, Lőkös L, Tschabanenko S, et al. New and noteworthy lichen-forming and lichenicolous fungi. Acta Bot Hung. 2013;55:275–349.
- Hur JS, Koh YJ, Harada H. A checklist of Korean lichens. Lichenology. 2005;4:66–95.
- Wei JC. An enumeration of lichens in China. Beijing: International Academic Publisher; 1991.
- Elix JA. A Catalogue of Standardized Chromatographic Data and Biosynthetic Relationships for Lichen Substances. 3rd ed. Canberra: The Author; 2014.
- Orange A, James P, White F. Microchemical methods for the identification of lichens. 2nd ed. London: British Lichen Society; 2010.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innnis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315–322.
- Rehner SA, Samuels GJ. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res. 1994;98:625–634.
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246.
- Liu D, Wang XY, Li JW, et al. Contributions to the lichen flora of the Hengduan Mountains, China (6): revisional study of the genus Canoparmelia (lichenized Ascomycota, Parmeliaceae). Plant Divers Resour. 2014;36:781–787.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Katoh K, Standley DM. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics. 2016;32:1933–1942.
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552.
- Bendiksby M, Timdal E. Molecular phylogenetics and taxonomy of Hypocenomyce sensu lato (Ascomycota: Lecanoromycetes): extreme polyphyly and morphological/ecological convergence. Taxon. 2013;62:940–956.
- Stamatakis A. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313.
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755.
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256.
- Lendemer JC, Westberg M. Candelariella xanthostigmoides in North America. Opuscula Philolichenum. 2010;8:75–81.
- Poelt J, Reddi BV. Candelaria und Candelariella. Lichenes, Candelariaceae (Flechten des Himalaya 4). Khumbu Himal. 1969;6:1–16.
- Westberg M, Morse CA, Wedin M. Two new species of Candelariella and a key to the Candelariales (lichenized Ascomycetes) in North America. Bryologist. 2011;114:325–334.
- Kubiak D, Westberg M. First records of Candelariella efflorescens (lichenized Ascomycota) in Poland. Pol Bot J. 2011;56:315–319.
- Arup U, Westberg M. Candelariella medians new to Sweden. Graphis Scripta. 2005;17:1–2.
- Westberg M. The identity of Candelariella canadensis. Lichenologist. 2010;42:119–122.
- Thomson JW. Notes on American Arctic species of Candelariella. Revista da Faculdade de Cienas de Lisboa II. 1973;17:747–759.
- Schmull M, Miadlikowska J, Pelzer M, et al. Phylogenetic affiliations of members of the heterogeneous lichen-forming fungi of the genus Lecidea sensu Zahlbruckner (Lecanoromycetes, Ascomycota). Mycologia. 2011;103:983–1003.
- Poelt J. Zur Kenntnis der Flechtengattung Candelariaceae. Ein Beitrag Mit Besonderer Berücksichtigung Einiger Südamerikanischer Arten – Phyton (Austria). 1974;16:189–210.
- Yakovchenko LS, VondráK J, Ohmura Y, et al. Candelariella blastidiata sp. nov. (Ascomycota, Candelariaceae) from Eurasia and North America, and a key for grey thalli Candelariella. Lichenologist. 2017;49:117–126.
