Abstract
Citric acid is a commercially valuable organic acid widely used in food, pharmaceutical, and beverage industries. In this study, 260 yeast strains were isolated from soil, bread, juices, and fruits wastes and preliminarily screened using bromocresol green agar plates for their ability to produce organic acids. Overall, 251 yeast isolates showed positive results, with yellow halos surrounding the colonies. Citric acid production by 20 promising isolates was evaluated using both free and immobilized cell techniques. Results showed that citric acid production by immobilized cells (30–40 g/L) was greater than that of freely suspended cells (8–19 g/L). Of the 20 isolates, two (KKU-L42 and KKU-L53) were selected for further analysis based on their citric acid production levels. Immobilized KKU-L42 cells had a higher citric acid production rate (62.5%), while immobilized KKU-L53 cells showed an ∼52.2% increase in citric acid production compared with free cells. The two isolates were accurately identified by amplification and sequence analysis of the 26S rRNA gene D1/D2 domain, with GenBank-based sequence comparison confirming that isolates KKU-L42 and KKU-L53 were Candida tropicalis and Pichia kluyveri, respectively. Several factors, including fermentation period, pH, temperature, and carbon and nitrogen source, were optimized for enhanced production of citric acid by both isolates. Maximum production was achieved at fermentation period of 5 days at pH 5.0 with glucose as a carbon source by both isolates. The optimum incubation temperature for citric acid production by C. tropicalis was 32 °C, with NH4Cl the best nitrogen source, while maximum citric acid by P. kluyveri was observed at 27 °C with (NH4)2 SO4 as the nitrogen source. Citric acid production was maintained for about four repeated batches over a period of 20 days. Our results suggest that apple and banana wastes are potential sources of novel yeast strains; C. tropicalis and P. kluyveri which could be used for commercial citric acid production.
1. Introduction
Microbe-based systems are important and versatile biotechnological processes for the production of various chemical substrates because of the limited space required for their cultivation, their rapid growth, and diverse physiological and biochemical properties [Citation1]. Citric acid is one of the most common microbial fermentation-based commercial products for which there is a never ending demand. In 2008, global production of citric acid exceeded 1.6 million tons, and is only expected to increase in the future [Citation2,Citation3]. Because of its low toxicity and high solubility and palatability, citric acid plays an essential role in the beverage, food, pharmaceutical, cosmetic, and household cleaning product industries, amongst others [Citation4,Citation5]. Citric acid is generally produced on a commercial scale either by fermentation or chemical synthesis, although the vast majority (>90%) is produced via fermentation [Citation6]. A large number of microorganisms, including both bacteria and fungi, are used to produce citric acid [Citation7,Citation8]. Although fungal species Aspergillus niger is traditionally used for commercial citric acid production, systems using various yeast species have recently attracted some attention [Citation9,Citation10]. Bioprocesses for citric acid production using yeasts have several advantages compared with Aspergillus processes, including a larger spectrum of substrates, decreased sensitivity to low dissolved oxygen concentrations and heavy metals, genetic and mechanical stability, and fewer health hazards [Citation11].
Three fermentation methods, including surface fermentation, submerged fermentation, and solid-state fermentation, can be used for the industrial production of citric acid. However, these conventional fermentation mechanisms suffer from various drawbacks such as nutritional limitations, low cell density, and batch-mode operations with significant down times [Citation12]. In addition to environmental conditions, strain isolation and selection are important factors for the regulation of product formation during fermentation [Citation13]. There is significant interest in improving the production of citric acid using immobilized cell techniques [Citation9,Citation14].
The aims of this study were: (i) to obtain new yeast isolates that could produce high yields of citric acid; (ii) to identify citric acid-producing yeasts at the species level using polymerase chain reaction (PCR)-based amplification and sequence analysis of the D1/D2 region of the 26S rRNA gene; (iii) to optimize the nutritional and environmental factors affecting citric acid production; and (iv) to improve citric acid production using an immobilization technique and repeated-batch culture.
2. Materials and methods
2.1. Samples collection
Yeast strains were isolated from fruits, juices, dates, tomatoes, bread wastes, and soil samples collected from different locations in the Asir region of Saudi Arabia. The samples were maintained in a clean plastic container at room temperature and stored until rot to be ready to use.
2.2. Isolation and purification of yeast isolates
The yeast strains were isolated and purified according to the method described by Kurtzman and Fell [Citation15].
Dilution plate method and direct touch method were used for yeast isolation. For dilution plate method, one gram of each sample was placed in a conical flask containing 50 mL sterile distilled water. The suspension was shaken on mechanical shaker for 30 min. A 0.1 mL of the suspension was added to the yeast extract peptone dextrose (YEPD) agar medium and incubated at 27 °C for 48–72 h. The YEPD agar plates contained (g/L): 10 g yeast extract; 20 g peptone; 20 g glucose, and 20 g agar (pH 5.5). The 100 μg/mL penicillin–streptomycin solution was added to the autoclaved agar medium to avoid bacterial growth. For direct touch method, one gram of each sample was directly touched (4–5 times) on the YEPD agar medium. Five sterile Petri dishes were used for each sample. The dishes were incubated at 27 °C for 48–72 h. After incubation, individual colonies were selected randomly according to their different morphological characteristics, and were purified by single colony isolation after triple re-streaking and kept in slants of the same medium at 4 °C for further study.
2.3. Screening of acid producing yeasts
All yeast isolates were primarily screened for organic acid production by determining the acid unitage (AU) values using the fermentation medium containing (g/L): glucose 100, NH4Cl 0.6, KH2PO4 1, MgSO4·7H2O 1.0, yeast extract 1.0, CaCO3 4.0, bromocresol green 0.2, Triton X-10 1.5 mL (pH 5.5). A loopful of yeast colony was inoculated on agar plates and incubated at 27 °C for 48 h. Formation of a yellow halo around the colonies indicated the ability of isolates to produce organic acid. The AU values of the colonies were determined by dividing the diameter (mm) of the yellow zone by the diameter (mm) of the colonies [Citation16]. The promising colonies having notable AU values were selected for further study and kept as stock cultures at 4 °C on YEPD agar medium. The isolates showing the highest levels of acid production were secondary screened for citric acid production using submerged cultures by free and immobilized cell techniques [Citation17]. The inoculum was prepared by incubation of the isolates at 27 °C, 150 rpm for 48 h in a YEPD agar medium. The production of citric acid was carried out in 250 mL flasks containing 50 mL of fermentation medium (without bromocresol green) in an incubator shaker at 27 °C and 150 rpm for five days. After incubation, the medium was centrifugation at 6000 rpm for 20 min and the supernatant was used for the determination of citric acid production.
2.4. Molecular identification of the selected yeasts
Genomic DNA was extracted from the isolated yeast strains according to our previously described method [Citation18]. The D1/D2 domain of the 26S rRNA gene was amplified using universal primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) and the PCR conditions and parameters described by Kurtzman and Robnett [Citation19]. PCR was performed in 50 µL as a final volume containing GoTaq (Promega, Madison, WI, USA) green master mix, 1 µL DNA sample and each primer 1 µL (at a concentration of 0.5 mM). PCR was under the following conditions: initial denaturation at 95 °C for 5 min, followed by 36 cycles at 94 °C for 2.0 min, 52 °C for 1.0 min, 72 °C for 2.0 min; final extension at 72 °C for 7 min, holding at 4 °C. Briefly, 5 μL of PCR products was then analyzed using 1.5% 0.59 TBE agarose gel electrophoresis. The gel was stained with ethidium bromide, visualized under UV light, and photographed. Product of the correct size was purified with a TaKaRa Agarose Gel DNA Purification Kit version 2.0 and sequenced in both directions using an ABI 3730 automated sequencer (Macrogen, Seoul, Korea). The 26S rRNA gene sequences from the isolates were aligned and compared with known 26S rRNA gene sequences in the GenBank database using BLASTn analysis (http://www.ncbi.nlm.nih.gov/BLAST/). To determine the exact taxonomic positions of the isolates, phylogenetic trees were constructed using MEGA version 4.0, as described previously [Citation18]. The partial 26S rRNA gene sequences obtained in this study for strains KKU-L42 and KKU-L53 have been deposited in the DNA Data Bank of Japan (www.ddbj.nig.ac.jp/), the European Molecular Biology Laboratory (www.embl.de/), and GenBank (http://www.ncbi.nlm.nih.gov) nucleotide sequence databases under accession numbers KU728119 and KU728120, respectively.
2.5. Optimization of culture conditions for citric acid production
Citric acid production by the selected strains was evaluated using fermentation medium [Citation17,Citation20]. The inoculum was prepared by growing the yeast culture in the YEPD medium at 27 °C, 150 rpm for 48 h. After immobilization technique, the calcium alginate pellets (4 g) were washed with distilled sterile water and suspended in a 250-mL Erlenmeyer flask containing 50 mL of fermentation medium and incubated at 27 °C in an incubator shaker at 150 rpm for 8 days. At regular (1 day) intervals, the triplicate samples were harvested. The pellets were removed by centrifugation (6000 rpm for 20 min) and used for growth determination and the supernatant was used for glucose and citric acid assay. Factors affecting citric acid production, including pH (3–7), temperature (18–42 °C), carbon source (sucrose, mannitol, glucose, fructose, maltose, starch, ethanol, glycerol), and nitrogen source (urea and ammonium salts; ammonium sulfate monobasic, ammonium sulfate, ammonium ferrous sulfate, diammonium hydrogen-phosphate, ammonium dihydrogen phosphate, ammonium chloride), were then investigated. All experiments were conducted in triplicate.
2.6. Immobilization of yeast cells
Sodium alginate was used to prepare the immobilized cells as described previously [Citation21]. It was autoclaved for 5 min at 121 °C and stored overnight at 4 °C to facilitate de-aeration. To form an alginate–cell mixture, 5 mL of cell suspension was added to 50 mL of 3% (wt/vol) alginate solution and then dropped into 1.5% (wt/vol) CaCl2 by using 2 mL pipettes to prepare the immobilized cells of approximately 3 mm in diameter. Pellets were hardening in the same solution with stirring for 1 h at 20 °C, washed three times with 0.9% (wt/vol) NaCl solution and stored in CaCl2 solution at 4 °C.
2.7. Repeated-batch fermentation
Repeated-batch cultures were conducted by running the citric acid fermentation for the optimum period, at the end of each cycle the immobilized cells were washed (2% (wt/vol) CaCl2 for 30 min) for curing the gel capsules by maintaining their mechanical structure and increasing their productive life, fresh production medium was added and the fermentation was continued. Curing the gel capsules with 2% CaCl2 after each fermentation batch not only prevented the disruption of the capsules and maintained their mechanical structure but it also increased significantly the productive life of the biocatalysts as described by Konsoula and Liakopoulou-Kyriakides [Citation22].
2.8. Analytical methods
Microbial growth: After fermentation process, the culture was centrifuged and both cell growth in freely suspended cultures and immobilized cells in alginate matrix were determined as cell dry weight (drying in an oven at 70 °C). Biomass were determined from the difference between the total weight of the alginate gel capsules containing cells and that of alginate capsules without cells (blank) prepared under the same conditions. Glucose assay: The culture supernatant was used for the calorimetric determination of consumed glucose (%) by Sigma-Aldrich kit according to the manufacturer’s recommendations using a double beam UV/vis scanning spectrophotometer at 500 nm. Citric acid assay: Anhydrous citric acid was estimated by pyridine-acetic anhydride method [Citation23] using a double beam UV/vis scanning spectrophotometer (Model 1601PC: Shimadzu, Kyoto, Japan). The citric acid content in supernatant was determined by adding 1 mL of sample (containing from 25 to 200 mg of citric acid), 1.3 mL of pyridine, and 5.7 mL of acetic anhydride to a test tube. After incubation for 30 min at 32 °C, the color intensity was estimated at 420 nm. Citric acid standard was prepared from anhydrous citric acid at 0.1–0.3 g/L. It was also performed using K-CITR enzymatic test kit (Megazyme, Wicklow, Ireland).
2.9. Statistical analysis
The results were analyzed by comparing the means of the obtained data with the values for individual controls. Results are expressed as mean ± standard deviation. Significant differences among values were analyzed using analysis of variance (one-way ANOVA). Results were considered significant at p < 0.05. Statistical analysis was performed using SPSS version 15.0 software [Citation24].
3. Results and discussion
3.1. Isolation and screening of citric acid-producing yeast isolates
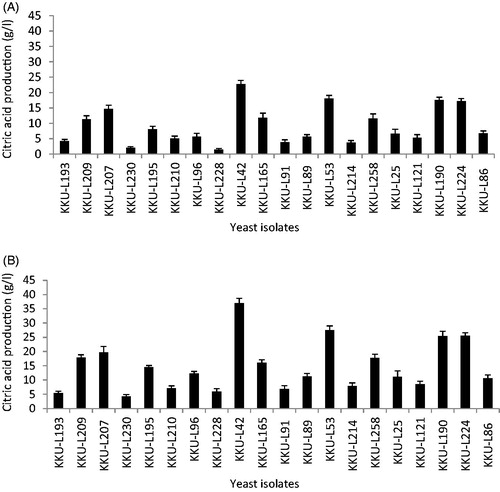
In this study, 260 yeast isolates were successfully isolated from various samples collected from the southern region of Saudi Arabia. The isolates were screened for their ability to produce organic acids using bromocresol green agar plates test, with 251 isolates showing a positive result (yellow halo formation around the colonies). The halo zone diameters gradually increased from 2 mm at 24-h post-inoculation to about 16 mm at 48-h post-inoculation, which was similar to observations made using the same screening method for organic acid-producing yeasts [Citation16,Citation25]. The highest organic acids producing ability was detected by two yeast isolates: KKU-L42 isolated from banana wastes (16.2 mm), and KKU-L53 isolated from apple wastes (15.4 mm). Twenty promising yeast isolates were subjected to further screening for their ability to produce citric acid using free and immobilized cell technique. Results showed that citric acid production by immobilized cells (30–40 g/L) was significantly greater than that of the freely suspended cells (8–19 g/L) within the same period (). The use of immobilized cells as a novel fermentation technology has been investigated for many microbial products, and several advantages of this method over free cells have been identified [Citation14,Citation26]. Of the 20 isolates examined, isolates KKU-L42 and KKU-L53 showed the highest levels of citric acid production (36.9 g/L and 27.5 g/L, respectively). Using the immobilized cell technique, isolate KKU-L42 showed a 62.5% increase in production compared with free cells, while immobilized KKU-L53 cells showed a 52.2% increase in citric acid production. These isolates were therefore selected for further studies. The increased citric acid production by the immobilized cells than by the free cells may be due to the formation of strong gel capsules possessing high substrate mass transfer rates, higher cell density, the low viscosity of the broth substantially, facilitating oxygen transfer with less agitation [Citation27]. Despite several carriers such as k-carrageenan, polyurethane gel, nylon web, and polyurethane foam used for immobilization of the yeast cells, entrapped cells in alginate exhibited highest citric acid productivity [Citation28]. The highest citric acid productivity by Candida guilliermondii was recorded with alginate-bead-immobilized cells because of its large surface area for cell attachment [Citation26].
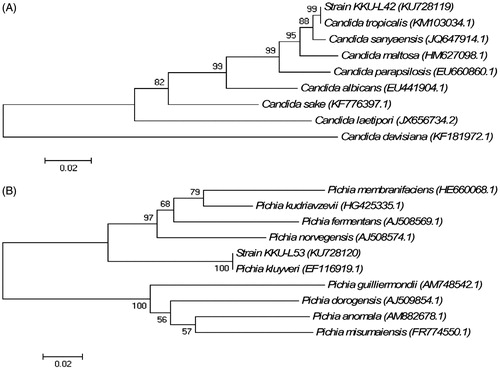
3.2. 26S rRNA gene D1/D2 domain sequence-based identification and phylogenetic analysis
Genomic DNA was obtained from isolates KKU-L42 and KKU-L53 and used as a template for amplification and sequencing of the D1/D2 region of the 26S rRNA gene which has gained recognition in yeast taxonomy as important identification tool [Citation19,Citation29–31]. Alignment of the resulting sequences against published 26S rRNA gene sequences in the GenBank database confirmed that the KKU-L42 and KKU-L53 sequences showed 99% nucleotide identity to the corresponding regions in Candida tropicalis and Pichia kluyveri, respectively. To confirm the identification of each strain, a number of representative Candida and Pichia 26S rRNA gene sequences were selected from the GenBank database for construction of phylogenetic trees. The trees showed that the strain KKU-L42 sequence clustered with the C. tropicalis sequences (), while the sequence from strain KKU-L53 clustered with the P. kluyveri sequences (), confirming the original identification of the two strains. The citric acid-producing yeast isolates were obtained from pineapple, plantain, and sugar cane waste and molecular identified as Saccharomyces cerevisiae, Schizosaccharomyces pombe, C. tropicalis, Pichia guilliiermondii, Debaromyces sp., Candida parapsilosis, Candida rugosa, and Candida krusei [Citation1].
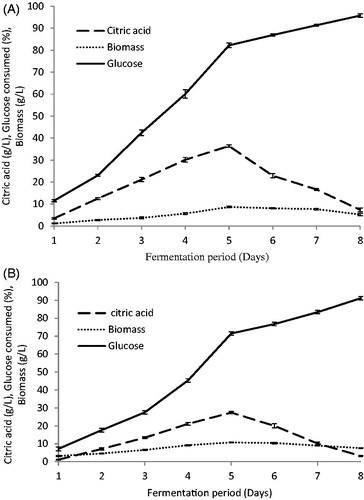
3.3. Effects of time course on citric acid production
Yeast growth and citric acid production were influenced by the fermentation time. As shown in , citric acid was secreted early on in the active growth phase, reaching a maximum near the end of the logarithmic phase. Maximum production was obtained after 5 days of cultivation for both C. tropicalis (36.3 g/L) and P. kluyveri (27.4 g/L). At the end of the optimum fermentation period, biomass and glucose consumed were 8.66 g/L and 82.2% for C. tropicalis and 10.76 g/L and 71.46% for P. kluyveri. The citric acid production was observed to be dependent on microbial growth suggesting that these organisms may be sensitive to metabolite repression [Citation32,Citation33]. The decrease in productivity at the later phase of growth probably resulted from the following factors: (i) the age of the microbes used, (ii) the inhibitory effects of high concentrations of citric acid, (iii) decay in the enzyme system responsible for biosynthesis of citric acid, and (iv) depletion of sugar in the culture broth [Citation32]. As has been shown previously, the optimum incubation time for maximum citric acid production varies with the microbes and fermentation conditions. The optimum citric acid production by S. cerevisiae and C. guilliermondii was attained after 3–5 days of incubation [Citation34], 40 h for Candida lipolytica [Citation35], 12 days for Yarrowia lipolytica [Citation8,Citation10], while using of P. kluyveri to enhance the flavor of fish sauce, it produced citric acid, malic acid, and succinic acid within the first 3 days of fermentation [Citation36].
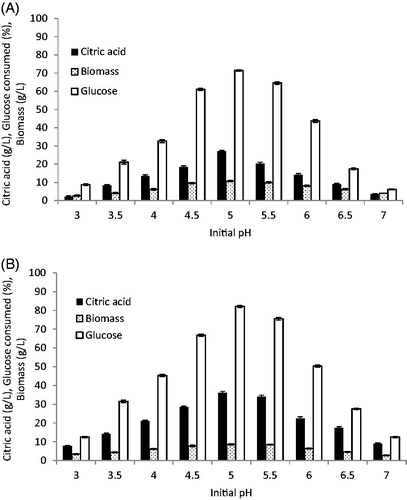
3.4. Effect of initial pH on citric acid production
The pH of the fermentation medium is known to be a critical parameter for citric acid production by yeasts. In the current study, the influence of initial pH on citric acid production was examined over a pH range of 3.0–7.0. The results presented in showed that a pH of 5.0 was the most favorable for citric acid production and growth of the two strains. Our results were in agreement with those of Papanikolaou et al. [Citation37] and Ferreira et al. [Citation38] and Y. lipolytica, using glucose as carbon source and finding that the highest citric acid concentration and biomass productivity were obtained at pH 5. Above optimum pH, citric acid production was markedly decreased, at pH 5.5, C. tropicalis showed a 5.9% decrease in citric acid production compared with that at pH 5.0, with only 75.6% of glucose being consumed, while P. kluyveri showed a 25.1% reduction in citric acid production and only 64.5% of the available glucose was consumed. Furthermore, below pH 5.0, the citric acid concentration decreases due to the accumulation of some polyalcohols like erythritol and arabitol instead of citric acid [Citation39] and inhibition of transport of citrate from cell membrane [Citation40]. The initial pH of the fermentation medium must be very well defined and optimized depending on the microorganism, substrate, and production technique [Citation41]. Several studies mentioned that the optimum pH for citric acid production was varied even for the same strain at a broad value between pH 3.5 and pH 7.0. The maximum citric acid production by Y. lipolytica was achieved at pH 5.5–6.0 [Citation8,Citation10,Citation25,Citation42], Y. lipolytica 57 and Y. lipolytica NBRC 1658 at pH ranges of 5.2–7.0 [Citation17], S. cerevisiae at pH of 4.5 [Citation6], whereas for S. cerevisiae and C. guilliermondii at pH 3.5 [Citation34], while for S. cerevisiae and C. tropicalis citric acid was produced at pH 6 and pH 4.0 for P. gulliermondii [Citation1].
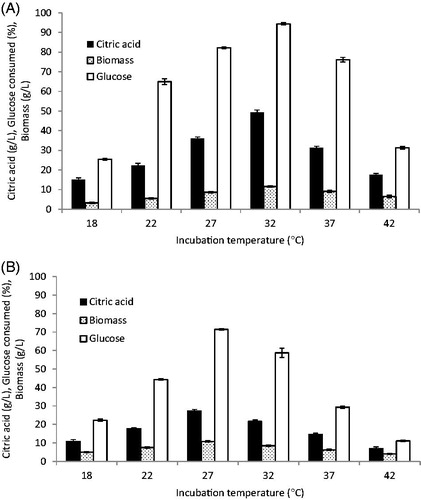
3.5. Effect of incubation temperature on citric acid production
Incubation temperature plays an important role in citric acid production by affecting all the physiological activities of microbes [Citation8,Citation10]. To determine the optimal temperature for citric acid production, fermentations were carried out at temperatures ranging from 22 to 42 °C (). The results revealed a significant relationship between citric acid production and incubation temperature up to 32 °C for C. tropicalis and up to 27 °C for P. kluyveri, followed by a gradual decrease. Increasing the incubation temperature of C. tropicalis from 27 °C to 32 °C resulted in a 36.6% increase in citric acid production and glucose consumption of 94.4%. These results were in harmony with the citric acid productions of Y. lipolytica which decreased when temperature was increased from 30 to 35 °C [Citation17]. At high temperature than optimal value, the fermentation process is very rapid with excessive growth causing large amounts of sugar to be oxidized to CO2, resulting in decreased yields of citric acid and increased levels of oxalic acid and gluconic acid in addition to denaturation of citrate synthase [Citation43]. Various studies have reported different temperatures for maximum citric acid production, suggesting that the optimal temperature depends on the strain [Citation10]. The citric acid was produced between 24 and 31 °C for Candida oleophila [Citation44], 30 °C for S. cerevisiae and C. guilliermondii [Citation34,Citation41], 27 °C for Y. lipolytica [Citation25,Citation42], and 30 °C for S. cerevisiae and C. tropicalis [Citation1].
3.6. Effects of carbon source on citric acid production
The ability of the two novel yeast strains to utilize different carbon sources (ethanol, glycerol, starch, glucose, sucrose, fructose, maltose, and mannitol) for the production of citric acid was conducted. The results revealed that citric acid production is strongly affected by the carbon source, with glucose being the preferred carbon source for both strains (). Other than fructose, sucrose, and maltose, all of the carbon sources examined supported growth and citric acid production by the two yeast strains. Glycerol was the second most favorable carbon source, resulting in citric acid production levels of about 80.89% and 82.88% than those obtained using glucose for C. tropicalis and P. kluyveri, respectively. When fructose and sucrose were used as carbon sources, the citric acid yield of C. tropicalis decreased to only 13.69% and 34.56%, respectively, of the yield obtained using glucose. Similarly, citric acid production by P. kluyveri decreased to 8.24% and 33.6% of levels obtained using glucose when fructose and maltose were used as the carbon source, respectively. The presence of easily metabolized carbohydrates is essential for optimum production of citric acid [Citation45]. Candida strains can produce citric acid from various carbohydrates, although glucose has generated increasing interest [Citation39]. The basic principle of utilizing glucose as a carbon source for citric acid production extensively investigated where the high activity of citrate synthase is pronounced for yeast strains [Citation46]. The highest citric acid production was achieved at optimum sugar concentration, because such high concentration of the carbon source lead to suppression of α-ketoglutarate dehydrogenase while, at low glucose levels, the yeast size and its shape is affected [Citation47]. The highest amounts of citric acid produced by Y. lipolytica were observed in the production medium containing sunflower oil (50–66.2 g/L) as a carbon source [Citation25], sucrose (127–140 g/L) [Citation41], and crude glycerol (8–10 g/L) [Citation38].
Table 1. Effect of carbon sources on citric acid production by Candida tropicalis (F value = 279.651) and Pichia kluyveri (F value = 85.656).
3.7. Effects of nitrogen source on citric acid production
The effects of seven different nitrogen sources (diammonium hydrogen phosphate, ammonium dihydrogen phosphate, ammonium chloride, ammonium ferrous sulfate, urea, ammonium phosphate monobasic, and ammonium sulfate) on citric acid production was also investigated. As illustrated in , the results revealed that the ammonium salts were the best sources of nitrogen for the synthesis of citric acid by both strains. Citric acid production and sugar consumption by C. tropicalis were highest when ammonium chloride was used as the nitrogen source, reaching 50.53 g/L and 94.53%, respectively. Furthermore, while ammonium sulfate and diammonium hydrogen phosphate did not result in any significant change in the production of citric acid, urea and ammonium ferrous sulfate significantly decreased the production of citric acid. In comparison, optimum citric acid production (35.4 g/L) and glucose consumption (81.56%) by P. kluyveri were achieved using ammonium sulfate as the nitrogen source, corresponding to 33.28% and 10.06% increases, respectively, over the control medium. Again, diammonium hydrogen phosphate and ammonium dihydrogen phosphate did not result in any significant change in the production of citric acid, while urea and ammonium ferrous sulfate significantly reduced citric acid production. Interestingly, Çelik et al. [Citation25] reported that optimum citric acid production (66.2 g/L) by Y. lipolytica was achieved using ammonium sulfate as the nitrogen source, while Yalcin [Citation48] showed that ammonium chloride was the optimum nitrogen source for citric acid production by Y. lipolytica (50 g/L). On the other hand, C. tropicalis produced 132.2 mg/L using sodium nitrate as an optimum nitrogen source [Citation1]. The biochemical role of intracellular ammonium ions in the regulation of the production of citric acid was clarified; the increase of intracellular ammonium ions could lead to the prevention of citrate’s inhibition of phosphofructokinase activity, which would result in an overproduction of citric acid [Citation46,Citation47].
Table 2. Effect of nitrogen sources on citric acid production by Candida tropicalis (F value= 107.536.) and Pichia kluyveri (F value = 44.401).
3.8. Repeated-batch fermentation
One of the most important benefits of immobilized cells is their ability to stably produce citric acid under repeated-batch cultivation. Separately, immobilized C. tropicalis and P. kluyveri cells were collected from the fermentation medium after 5 days and transferred into fresh sterile fermentation medium. This process was repeated six times. The citric acid and glucose concentrations over this period are shown in . The results indicated that immobilized cells of both strains showed high citric acid productivity upon re-use. The culture activity of C. tropicalis remained stable up until the third cycle, and cells retained about 93.93% of their initial efficiency during the fourth batch. However, citric acid production and glucose consumption decreased from the fourth batch and a dramatic reduction was observed during the fifth batch. In comparison, culture activity of P. kluyveri remained stable up until the fourth cycle before reducing to about 60.73% of the initial activity during the fifth batch. Therefore, repeated-batch fermentation with calcium alginate beads was successfully run for four batches for both strains. The repeated-batch culture technique is superior to batch cultivation for reducing the costs of fermentation by eliminating the need for multiple sterilization and inoculum preparation steps [Citation49]. The activity of the repeat batch culture of Y. lipolytica remained stable after cultivation for more than 58 days which reduces the costs of fermented sterilization and inoculum preparation compared to batch fermentation [Citation32]. Also, repeated batch production of citric acid by C. oleophila ATCC 20177 [Citation46] and C. guilliermondii [Citation26] seems to have many advantages compared with the traditional industrial processes. The decline of the cultural activity after the fourth cycle may be occurring because of clogging of pores of the alginate immobilized cells. Thus, citric acid was accumulated inside the gel and could not penetrate the outside of the gel which causes the inhibition of the citrate synthase [Citation2].
Table 3. Repeat batch culture for citric acid production by Candida tropicalis (F value =279.651) and Pichia kluyveri (F value = 374.689).
In conclusion, 260 yeast isolates were successfully isolated from Saudi Arabia. A total of 251 yeast isolates were able to produce organic acids. The productivity of citric acid by immobilized cells was significantly greater than that of the freely suspended cells. The sequences of domains D1 and D2 of the 26S rRNA gene and phylogenetic analysis confirmed the identification of the two promising citric acid-producing isolates as C. tropicalis and P. kluyveri. Maximum citric acid production was achieved after 5 days of fermentation with optimum pH 5.0 and glucose as a carbon source for the two isolates. Optimum incubation temperature for citric acid production by C. tropicalis was 32 °C with ammonium chloride as the best nitrogen source, while it was 27 °C with the ammonium sulfate as a good nitrogen source for P. kluyveri. The activity of the cultures remained stable after a repeat batch culture for four cycles of 20 days. The results demonstrated that the two yeast strains C. tropicalis and P. kluyveri could be recommended for citric acid production using a repeat batch culture technique by immobilized cells.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Afolabi FT, Adeyemo SM, Balogun HO. Fermentation conditions and process optimization of citric acid production by yeasts. Int J Biotechnol. 2018;7:51–63.
- Ates S, Dingil N, Bayraktar E, et al. Enhancement of citric acid production by immobilized and freely suspended Aspergillus niger using silicone oil. Process Biochem. 2002;38:433–436.
- Auta HS, Abidoye KT, Tahir H, et al. Citric acid production by Aspergillus niger cultivated on Parkia biglobosa fruit pulp. Int Schol Res Notic. 2014;2014. Article ID 762021.
- Yadegary M, Hamidi A, Alavi SA, et al. Citric acid production from sugarcane bagasse through solid state fermentation method using Aspergillus niger mold and optimization of citric acid production by Taguchi method. Jundishapur J Microbiol. 2013;6:e7625.
- Khan R, Gupta AK. Screening and optimization of organic acid producers from mine areas of Chhattisgarh region, India. Int J Curr Microbiol Appl Sci. 2015;4:103–111.
- Soccol CR, Vandenberghe LP, Rodrigues C, et al. New perspectives for citric acid production and application. Food Technol Biotechnol. 2006;44:141–149.
- Angumeenal AR, Venkappayya D. An overview of citric acid production. LWT – Food Sci. Technol. 2013;50:367–370.
- Wang LF, Wang ZP, Liu XY, et al. Citric acid production from extract of Jerusalem artichoke tubers by the genetically engineered yeast Yarrowia lipolytica strain 30 and purification of citric acid. Bioprocess Biosyst Eng. 2013;36:1759–1766.
- Ashour A, El-Sharkawy S, Amer M, et al. Production of citric acid from corncobs with its biological evaluation. J Cosmet Dermatol Sci Appl. 2014;4:141–149.
- Liu X, Wang X, Xu J, et al. Citric acid production by Yarrowia lipolytica SWJ-1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind Crop Prod. 2015;78:154–160.
- Moeller L, Zehnsdorf A, Aurich A, et al. Substrate utilization by recombinant Yarrowia lipolytica growing on sucrose. Appl Microbiol Biotechnol. 2012;93:1695–1702.
- Khosravi Darani K, Zoghi A, Alavi SA, et al. Application of Plackett Burman design for citric acid production from pretreated and untreated wheat straw. Iran J Chem Chem Eng. 2008;27:91–104.
- Radwan H, Alanazi FK, Taha EI, et al. Development of a new medium containing date syrup for production of bleomycin by Streptomyces mobaraensis ATCC 15003 using response surface methodology. Afr J Biotechnol. 2010;9:5450–5459.
- Mostafa YS, Alamri SA. Optimization of date syrup for enhancement of the production of citric acid using immobilized cells of Aspergillus niger. Saudi J Biol Sci. 2012;19:241–246.
- Kurtzman CP, Fell JW. Definition, classification and nomenclature of the yeasts. In: Kurtzman CP, Fell JW, editors. The yeasts, a taxonomic study. Amsterdam: Elsevier Science BV; 1998. p. 3–5.
- Shaikh Z, Qureshi P. Screening and isolation of organic acid producers from samples of diverse habitats. Int J Curr Microbiol Appl Sci. 2013;2:39–44.
- Karasu-Yalcin S, Tijen Bozdemir M., Yesim Ozbas Z. Effects of different fermentation conditions on growth and citric acid production kinetics of two Yarrowia lipolytica strains. Chem Biochem Eng Q. 2010;24:347–360.
- Hesham A. New safety and rapid method for extraction of genomic DNA from bacteria and yeast strains suitable for PCR amplifications. J Pure Appl Microbiol. 2014;8:383–388.
- Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998;73:331–371.
- Rodarte M, Dias D, Vilela D, et al. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Sci Agron. 2011;33:457–464.
- Bayraktar E, Mehmetoglu U. Production of citric acid using immobilized conidia of Aspergillus niger. Appl Biochem Biotechnol. 2000;87:117–125.
- Konsoula Z, Liakopoulou-Kyriakides M. Starch hydrolysis by the action of an entrapped in alginate capsules α-amylase from Bacillus subtilis. Process Biochem. 2006;41:343–349.
- Marrier JR, Boulet M. Direct determination of citric acid in milk with an improved pyridine-acetic anhydride method. J Dairy Sci. 1958;41:1683–1692.
- SPSS. SPSS base 15.0 user’s guide. Chicago (IL): SPSS.; 2006.
- Çelik G, Bahriye Uçar F, Akpınar O, et al. Production of citric and isocitric acid by Yarrowia lipolytica strains grown on different carbon sources. Turk J Biochem. 2014;39:285–290.
- Tisnadjaja D, Gutierrez NA, Maddox IS. Citric acid production in a bubble-column reactor using cells of the yeast Candida guilliermondii immobilized by adsorption onto sawdust. Enzyme Microb Technol. 1996;19:343–347.
- Verbelen PJ, De Schutter DP, Delvaux F, et al. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol Lett. 2006;28:1515–1525.
- Mansfeld J, Förster M, Hoffmann T, et al. Coimmobilization of Yarrowia lipolytica cells and invertase in polyelectrolyte complex microcapsules. Enzyme Microb Technol. 1995;17:11–17.
- Kurtzman CP. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res. 2003;4:233–245.
- Guffogg SP, Thomas-Hall S, Holloway P, et al. Novel psychrotolerant member of the hymenomycetous yeasts from Antarctica: Cryptococcus watticus sp. nov. Int J Syst Evol Microbiol. 2004;54:275–277.
- Hesham A, Wang Z, Zhang Y, et al. Isolation and identification of a yeast strain capable of degrading four and five ring aromatic hydrocarbons. Ann Microbiol. 2006;56:109–112.
- Arzumanov TE, Shishkanova NV, Finogenova TV. Biosynthesis of citric acid by Yarrowia lipolytica repeat-batch culture on ethanol. Appl Microbiol Biotechnol. 2000;53:525–529.
- Ishaq A, Ali S, Ikram-Ul-Haq QM. Time course profile of citric acid fermentation by Aspergillus niger and its kinetic relation. J Biol Sci. 2002;2:760–761.
- Acourene S, Ammouche A. Optimization of ethanol, citric acid, and α-amylase production from date wastes by strains of Saccharomyces cerevisiae, Aspergillus niger, and Candida guilliermondii. J Ind Microbiol Biotechnol. 2012;39:759–766.
- Pazouki M, Felse PA, Sinha J, et al. Comparative studies on citric acid production by Aspergillus niger and Candida lipolytica using molasses and glucose. Bioprocess Eng. 2000;22:353–361.
- Gao P, Xia W, Liu S. Use of wine and dairy yeasts as single starter cultures for flavor compound modification in fish sauce fermentation. Front Microbiol. 2019;10.
- Papanikolaou S, Muniglia L, Chevalot I, et al. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol. 2002;92:737–744.
- Ferreira P, Lopes M, Mota M, et al. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem Eng J. 2016;110:35–42.
- Yalcin SK, Bozdemir MT, Ozbas ZY. Citric acid production by yeasts: fermentation conditions, process optimization and strain improvement. Appl Microbiol Microbial Biotechnol. 2010;9:1374–1382.
- Mattey M. The production of organic acids. Crit Rev Biotechnol. 1992;12:87–93.
- Förster A, Aurich A, Mauersberger S, et al. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol. 2007;75:1409–1417.
- Urak S, Yeniay O, Karasu-Yalcin S. Optimization of citric acid production from a carrot juice-based medium by Yarrowia lipolytica using response surface methodology. Ann Microbiol. 2015;65:639–649.
- Ambati P, Ayyanna C. Optimizing medium constituents and fermentation conditions for citric acid production from Palmyra jaggery using response surface method. World J Microbiol Biotechnol. 2001;17:331–335.
- Crolla A, Kennedy KJ. Optimization of citric acid production from Candida lipolytica Y-1095 using n-paraffin. J Biotechnol. 2001;89:27–40.
- Finogenova TV, Puntus IF, Kamzolova SV, et al. Mutant Yarrowia lipolytica strains producing citric acid from glucose. Appl Biochem Microbiol. 2008;44:197–202.
- Anastassiadis S, Rehm HJ. Continuous citric acid secretion by a high specific pH dependent active transport system in yeast Candida oleophila ATCC 20177. Electron J Biotechnol. 2005;8:26–42.
- Max B, Salgado JM, Rodríguez N, et al. Biotechnological production of citric acid. Braz J Microbiol. 2010;41:862–875.
- Yalcin SK. Enhancing citric acid production of Yarrowia lipolytica by mutagenesis and using natural media containing carrot juice and celery byproducts. Food Sci Biotechnol. 2012;21:867–874.
- Ji H, Yu J, Zhang X, et al. Characteristics of an immobilized yeast cell system using very high gravity for the fermentation of ethanol. Appl Biochem Biotechnol. 2012;168:21–28.