Abstract
Root-feeding Scarabaeidae, particularly white grubs are considered among the most harmful coleopteran insect pests in turfgrass. In this work, sixteen entomopathogenic fungal species were assayed against flower chafer beetle, Protaetia brevitarsis (Coleoptera: Scarabaeidae) and Metarhizium anisopliae JEF-314 showed high virulence. The control ability of the isolate JEF-314 has been in detail tested for a model insect flower chafer beetle. Further analyses showed insect stage-dependent virulence where the fungal virulence was the highest against smaller instar larvae. Additionally, we confirmed that millet-based solid cultured granule was effective against the soil-dwelling larval stage. The isolate also showed a similar ability for a representative pest (Popillia spp.) in laboratory conditions. Our results clearly suggest a high potential of M. anisopliae JEF-314 to control the flower chafer beetle, possibly resulting in controlling of root-feeding white grubs in turfgrass. Based on the insect life cycle and susceptibility to the fungus, late spring and summer time would be the optimum time to apply JEF-314 granules for an effective control. Further characterization of the efficacy of the fungus under field conditions against the Scarabaeidae beetles might provide an efficient tool to control this beetle in an environment-friendly way.
1. Introduction
Most of Scarabaeidae species (Coleoptera: Scarabaeidae) represent important pests in agriculture, forestry, and horticulture when they occur in high density [Citation1]. Among them, the root-feeding white grubs are considered the most destructive species in turfgrass. Major turfgrass-damaging white grubs are brown chafer (Adoretus tenuimaculatus), oriental beetle (Blitopertha orientalis) and Japanese beetle (Popillia japonica). In general, and similarly to most white grubs, they have one generation per year where the larval stage is the most harmful, feeding on plant roots between spring and fall before pupation [Citation2]. Infestations of the larvae can damage a variety of agricultural and horticultural crops and warm and cool season grasses [Citation3]. The subterranean habitats of these white grubs make them especially difficult to control because insecticides applied to the surface must move into the soil [Citation4].
Currently, chemical insecticides are the primary pesticides used to control these beetles. Even though they provide effective preventive and early curative control against larval stages, their application negatively affects hymenopteran parasitoids of the beetles. Moreover, neonicotinoids are in general mixed with pyrethroids that have an extremely broad active spectrum resulting in negative effects on the insects’ natural enemies [Citation3]. Furthermore, those chemical pesticides persist in the environment contaminating soil, wetlands, ground water, non-target plants and vertebrate prey in addition to human diets [Citation5,Citation6]. Due to the lack of efficient safe management strategies for these beetles and the harmful effects of the available chemical pesticides, alternative control strategies are needed. Natural insect pathogens could represent an effective and environmentally safe alternative, particularly soil-borne entomopathogenic fungi. Such fungi naturally colonize in soils, plant roots as rhizosphere colonizers, other plant parts as endophytes and insect pests [Citation7]. In particular, Metarhizium anisopliae is distributed worldwide and is a natural pathogen to coleopteran insects [Citation8]. Since the isolation and identification of this fungal species around 140 years ago, it has become one of the important microbial agents used for control of many pests all over the world. Indeed, most of the commercial formulations of fungi-based pesticides belong to 5 species, with two-thirds of them based on M. anisopliae and Beauveria bassiana [Citation9,Citation10].
In addition to the availability of commercial strategies to produce M. anisopliae-based pesticides and the high infectivity of this fungi to coleopteran species, it represents an environmentally friendly alternative since it has no effect on plants and only minor effects on soil organisms [Citation8]. These factors together render the fungus an interesting alternative for control of white grubs. In this context, we aimed to characterize a candidate M. anisopliae isolate as an alternative to control these insect pests. Nevertheless, due to difficulties in rearing and maintaining the target Scarabaeidae beetles in laboratory conditions, we used the white spotted flower chafer, Protaetia brevitarsis as an insect model in the present study. This species is widely distributed in Korea, Japan, China, Taiwan and some parts of Europe [Citation11,Citation12]. Its life cycle comprises the embryo, larva, pupa, and imago stages, of which the larval stage is the longest and is the stage when the insect feeds and stores energy for the rest of the life cycle [Citation13]. Depending on habitat conditions, adults can be observed in Korea from May to October with greatest abundance in July. They are gregarious and diurnal insects that overwinter as third instar larvae in the soil [Citation11]. Herein, we described an isolate of M. anisopliae and characterized its insecticidal potential against the white-spotted flower chafer in laboratory conditions. Lastly, we assessed the millet-based solid cultured granules to investigate its ability to reach the soil-dwelling larvae and built a theoretical model of its application in the fields.
2. Materials and methods
2.1. Fungal strains
The studied fungal species, belong to the Jeonbuk National University Collection of Entomopathogenic Fungi (JEF), Korea, except for the isolates ERL1578 and ERL836 of Beauveria bassiana that were provided by the Entomology Research Laboratory, University of Vermont, Burlington, VT, USA. The fungal species and isolates were cultured and maintained on one-quarter strength Sabouraud dextrose agar (1/4 SDA; Difco, Lawrence, KS, USA) in the dark at 25 °C for colony growth. To identify the Metarhizium anisopliae JEF-314 (Public number in National Agricultural Biotechnology Information Center: NU-1005-00001 and Patent number: 10-2017-0018944), internal transcribed spacer (ITS) region and beta-tubulin (β-tubulin) gene were amplified using primers ITS1 (forward: 5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (reverse: 5′-TCC TCC GCT TAT TGA TAT GC-3′) and Bt1F (forward: 5′- GGT CCC TTC GGT CAG CTC TTC C-3′) and Bt1R (reverse: 5′-CAG CCA TCA TGT TCT TAG GGT C-3′). The PCR products were sent to the sequencing service company (Macrogen, Seoul, Korea) for direct sequencing and submitted to NCBI data base (ITS, MN704643; β-tubulin, under registration). The isolated fungus was further characterized under a stereomicroscope (Nikon SMI745T; Nikon Corp., Tokyo, Japan) and conidiogenesis and spores were observed under an optical microscope (Primo Star; Carl Zeiss, Göttingen, Germany).
2.2. Insects
Flower chafer beetles were provided by National Institute of Agricultural Sciences, Rural Development Administration, Korea (www.rda.go.kr). They were reared in fine wood chips at 25 ± 2 °C and 50-60% relative humidity (RH) in a chamber (30 × 20 × 25 cm) with a 16:8 (L/D) photoperiod. Insect jelly (Chungam beetle jelly, Korea) was used as feed for the adults. Turfgrass-damaging white coleopteran larvae (TWC; Popillia spp. (Scarabaeidae)) were collected under the grass of a golf course in Naju-si, Jeollanam-do, Korea (34°52′01.7″N 126°47′20.8″E) in October 2019 and immediately used for the experiment.
2.3. Determination of culture time for conidial attachment on test larvae
The JEF-314 isolate was cultured in 1/4 SDA medium for either 7 or 14 days at 25 ± 1 °C. Afterward, five larvae were added to each culture plate. Seven days after treatment, individual infected larvae were placed into Eppendorf tubes containing 1 ml of 0.03% siloxane solution as a standard surfactant, then vortexed to liberate larva-attached conidia into the surfactant solution. The conidia concentrations were counted using a hemocytometer under a microscope. Three biological replicates were performed.
2.4. Phylogenetic analysis
The sequences (ITS and β-tubulin) of the M. anisopliae isolate JEF-314 and 30 homologous sequences of various Metarhizium species, obtained following NCBI blast (http://www.ncbi.nlm.nhi.gov) of the sequenced JEF-314 (Supplementary Table S1), were aligned using ClustalX software [Citation14] and visualized with the GeneDoc program [Citation15]. Evolutionary distances were calculated for aligned sequences by the Maximum-Likelihood method [Citation16] and the phylogenetic tree was constructed using the MEGA6 program [Citation17]. One thousand replicates were analyzed by bootstrap analysis.
2.5. In vitro fungal virulence assay
Sixteen fungal species, Beauveria bassiana (JEF-006, JEF-007, ERL836 and ERL1578), Beauveria brongniartii, Clonostachys rogersoniana, Cordyceps militaris, Isaria fumosorosea, Lecanicillium attenuatum, Metacordyceps brittlebankisoides, Metacordyceps taii, Metarhizium anisopliae (JEF-314), Metarhizium brunneum, Metarhizium figidum, Metarhizium lepidiotae, Paecilomyces javanicus, Paecilomyces lilacinus, Pochonia bubillosa and Pochonia suchlasporia, were used to screen for virulence against the chafer beetle. Five third instar larvae were placed in direct contact with 4 g of each fungal granule in insect breeding dishes. The breeding dishes were humidified adding 1 ml distilled water each day and larvae mortality was recorded daily for 12 days.
For further characterization of M. anisopliae JEF-314 virulence, the fungus was cultured on 1/4 SDA at 25 ± 1 °C for 14 days. Eggs, larvae and adults of the flower chafer beetle, received from the National Academy of Agricultural Science, RDA (Rural Development Administration), Korea, were collected from rearing cages. Five flower chafer beetles were exposed to the fungal-cultured plates for 1 h at room temperature, then transferred to a 90 × 15 mm Petri dish with filter paper (Whatman) previously humidified with 1 ml of sterilized water. Finally, Petri dishes, containing infected insects or non-treated insects, were kept at 25 ± 1 °C and 40 ± 10% relative humidity. The bioassays were replicated three times for all stages. The hatching eggs or survival of the different developmental stages were recorded daily for 7 days. Three biological replicates were performed.
Injection bioassay of JEF-314 was conducted to justify the use of flower chafer beetles as model insect for root-feeding coleopteran larvae. Conidial suspension of the isolate JEF-314 was obtained by scraping with 0.03% siloxane solution from a two-week-old 1/4 SDA plate and adjusted to 1 × 107 conidia/ml using a hemocytometer. Each flower chafer beetle and TWC larvae were injected with a 20 µl of the conidial suspension using a 1 ml syringe (REF301321; BD, Singapore), then transferred to a 90 × 15 mm Petri dish as mentioned above. Fermented sawdust was supplied as a food source. The 0.03% siloxane solution was used as control and the insects were checked daily for mortality. The bioassay was repeated three times and 10 insects were used per replicate.
2.6. Solid culture for preparation of fungal granules
The efficacy of M. anisopliae JEF-314 fungal granules was tested in different types of granules to finally produce the fungus on Italian millet (Setaria italica), as previously described [Citation18]. Briefly, 200 g of Italian millet was placed in a polyethylene bag (22 cm width × 32 cm length). Then 100 ml of sterilized water supplemented with citric acid (320 µl of 50% stock solution) was added. The Italian millet was placed in a microwave for 5 min, followed by autoclaving at 121 °C for 15 min. After cooling, 1 ml of fungal liquid culture of the isolate was inoculated into Italian millet. The polyethylene bags were held at 25 ± 1 °C with a 16:8 (Light: Dark) photoperiod for two weeks. After cultivation, the cultured millet was dried at room temperature for 2 days to less than 10% moisture.
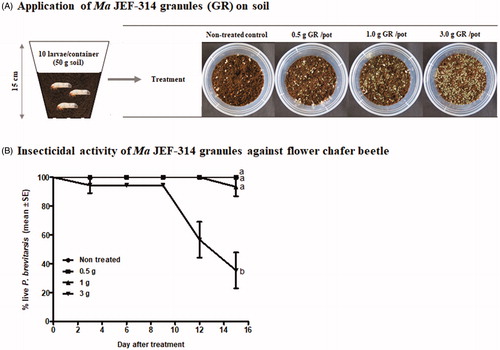
2.7. Virulence assay of JEF-314 granules
M. anisopliae JEF-314 granule formulation was tested for efficacy against the flower chafer beetle soil-dwelling larvae. About 50 g of nursery bed soil was placed in a plastic cup (11.5 cm diameter and 8.0 cm height), onto which 10 flower chafer beetle larvae were let to dwell in the soil. After 1 h, 0.5 g, 1 g or 3 g of the fungal granules were applied to the surface of soil. The plastic cups were covered with plastic cover with meshes to keep the humidity. This assay was conducted at 25 ± 2 °C. Each treatment was replicated three times, and larvae were monitored for 16 days after treatment. Mortality was recorded each day, and three biological replicates were performed.
2.8. Data analyses
In all the experiments including the conidia production assay and the percentages of live insect (No. of live insects/No. of infested insects) were arc-sine transformed to produce normally distributed data. These data were analyzed using a generalized linear model (GLM). The overall analyses were followed by Tukey’s honestly significant difference (HSD) test for multiple comparisons. All the analyses were conducted using the SPSS program version 12.0 (SPSS, Inc., Chicago, IL, USA) at the 0.05 (α) level of significance.
3. Results
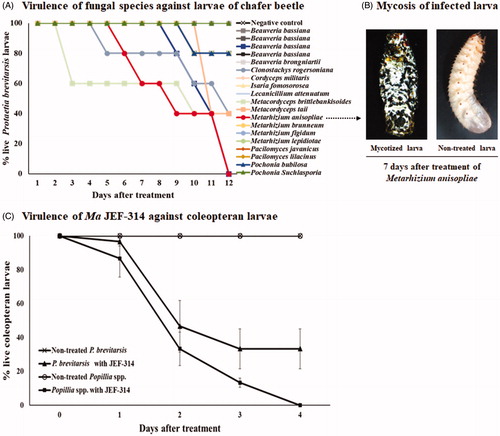
3.1. Virulence of fungal species against P. brevitarsis larvae
Sixteen entomopathogenic fungi species were screened for pathogenicity against the flower chafer beetle. Only four of them showed certain virulence: M. taii killed 60% of the treated larvae, C. rogersoniania killed 50%, M. brittlebankisoides killed 40% and M. anisopliae killed all the treated larvae, suggesting that the latter species has great potential for chafer beetle control (). According to the obtained results, we chose M. anisopliae JEF-314 to better characterize its insecticidal potential against P. brevitarsis. In addition, the M. anisopliae JEF-314 was confirmed to have similar virulence to the chafer beetle and TWC larvae (F15,48 = 5.498; p > 0.001) (). The isolate showed 100% and 66% insect mortality in the chafer beetle and TWC larvae after 4 days of injection. It is worth noting that the screening was performed as a unique replication to obtain preliminary data on the infectivity of the largest possible number of fungi species to finally select the most effective species for detailed virulence characterization against the chafer beetle.
Figure 1. Screening of entomopathogenic fungi against P. brevitarsis. (A) Virulence of different entomopathogenic fungal species and isolates against larvae; (B) Photographs showing P. brevitarsis larva mycosis 7 days after treatment with the highly infective species M. anisopliae JEF-314 compared to non-treated larva; (C) Virulence of the JEF-314 against P. brevitarsis and turfgrass-damaging white coleopteran larvae.
To perform further in vitro virulence assays, we studied the optimum fungal culture time for conidia production. No or very limited conidia production was obtained in the 7-day fungi culture compared to conidia production ranging from 2.08 ± 2.72 × 107 to 3.1 ± 4.17 × 107 conidia per ml for all analyzed species cultured for 14 days (Supplementary Figure S1). Therefore, we decided to use 14-day cultured fungi for further virulence assays.
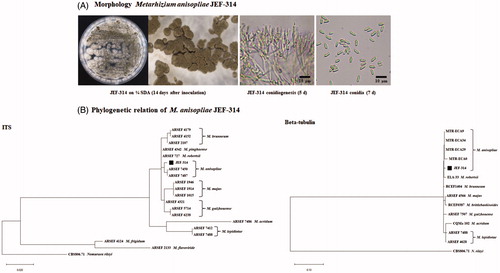
3.2. Identification of M. anisopliae JEF-314
The ITS and β-tubulin of the identified isolate were sequenced and subjected to blast analysis. The blast analyses and morphological characteristics unambiguously showed that the studied fungus is M. anisopliae isolate. Conidia of the fungus were whitish but later turned to green in color and cylindrical in shape. Its phialides were cylindrical and had short narrow necks (). Further phylogenetic analyses clearly revealed the isolate JEF-314 grouped with the reference sequences of M. anisopliae (). Based on morphological and molecular sequence analyses, the isolate JEF-314 was identified as M. anisopliae.
Figure 2. Characterization of the isolate JEF-314 of M. anisopliae. (A) Morphological characterization of the JEF-314 isolate: M. anisopliae cultured 14 days at 25 °C on a one-quarter strength SDA plate and Microscopic observation of the 14 days cultured fungus under a stereoscopic microscope (×40); 5 days conidiogenesis and 7 days conidia microphotographs under the optical microscope, respectively (×400); (B) Phylogenetic trees of the isolate JEF-314 and other Metarhizium isolates based on ITS and beta-tubulin sequences. Evolutionary distance was calculated for aligned sequences by Maximum Likelihood analyses.
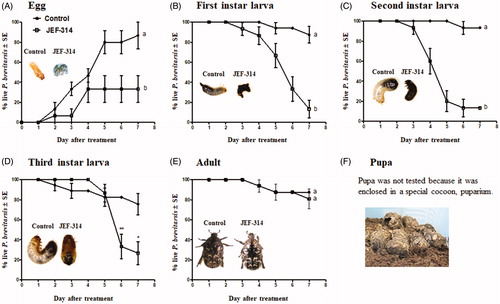
3.3. Stage-dependent virulence of M. anisopliae JEF-314 against flower chafer beetle
With a goal of characterizing the virulence of the isolate JEF-314 of M. anisopliae, we analyzed its infectivity against all developmental stages of the flower chafer beetle P. brevitarsis seulensis. The fungus drastically decreased the percentage of hatching eggs (F1,44=6.405; p = 0.015) from 86.67 ± 23.09% to 33.33 ± 23.09% (). In the case of larvae, the fungus was highly virulent to first, second and third instar larvae, with 7-day killing percentages of 86.67 ± 8.78% (F1,46=18.37; p < 0.0001), 86.67 ± 8.78% (F1,46=9.404; p = 0.004) and 73.33 ± 11.42% (F1,5=16.236; p = 0.01; statistics for 7 days after treatment), respectively (). Meanwhile, 80% of the adults used in the bioassay survived until the end of the toxicity assay being highly tolerant to the isolate ().
Figure 3. Stage-dependent virulence of M. anisopliae JEF-314 against Protaetia brevitarsis seulensis. (A) Percentage of hatching eggs following infection with JEF-314. a and b denote significant differences; (B–D) Survival curves of first, second and third instar larvae infected with JEF-314, respectively; a and b denote statistically significant differences and * and ** refers to p < 0.05 and p < 0.001 respectively; (E) Survival curve of infected adults. The photographs in the various graphs show the infection symptoms of the different development stages of the insect caused by M. anisopliae JEF-314 compared to non-infected eggs or larvae, 7 days after treatment, as well as the puparium formed by pupae (F). In the case of treated eggs, we show the mycosis of the freshly hatched larva from the infected egg.
3.4. Granular application of JEF-314 against soil-dwelling larvae
Second instar larvae were used to assess the efficacy of application of the isolate in soil following the application of different amounts of the fungus granules (). The results showed that the highest tested concentration, corresponding to 3 g grains added to 50 g nursery bed soil, was highly toxic to the beetle larvae, killing 66.39 ± 12.22% (F1,34=7.286; p = 0.011) of the total larvae 15 days after treatment. However, the larvae were tolerant to lower doses corresponding to 0.5 g or 1 g grains placed in 50 g soil (). These results suggest the fungus was successfully released from the millet grains and reached the soil-dwelling larvae with killing them in a dose-dependent manner.
4. Discussion
In this work, we characterized the fungus M. anisopliae JEF-314 following screening of various entomopathogenic fungi for virulence against P. brevitasis. We showed that this isolate is highly virulent to the beetle larvae and inhibits the insect egg hatching in laboratory experimental conditions. Larval stages are the longest of the insect life cycle, where the insect feeds on various sources of decaying cellulose, including roots of grass and weed, rice straw and Oakwood [Citation13]. The subterranean habitats of the white grubs, make them difficult to control since applied insecticides have to move into the soil. In addition, repeated application of certain insecticides into the soil results in increased biodegradation by soil microorganisms resulting in reduced residual effectiveness [Citation19,Citation20]. In general, management of white grubs, depends on the ability of the pesticide to reach the soil-dwelling larvae and on a combination of cultural and chemical tools aimed to maintain the beetle populations below economic damaging level. For this purpose, organochlorine pesticides such as DDT, which residues persist long-term in the environment and are harmful to non-target organisms and human beings, have long been used. Following the ban of DDT, carbonate and phosphate-based insecticides have become important candidates to manage the beetles. Even though they are less persistent in the environment, they are as toxic and have been proven difficult to use for scarab larvae [Citation1]. An attractive alternative is the use of entomopathogenic fungi, with no or minimal negative effects to human health and the environment. These insect pathogens often cause epizootic outbreaks in nature and are very effective since they directly penetrate the insect cuticle and can therefore be used as contact insecticides [Citation21,Citation22]. In addition to being environmentally-friendly, the price of commercial Metharizium spp. is similar to that of conventional chemical insecticides [Citation22].
In this work, we used the spotted flower chafer beetle as an insect model because it is easily reared in laboratory conditions. In addition, even though this insect is generally considered for its use in Korean traditional medicine and for nutritional affinities, some reports in China point to the fact that it can be considered a pest as well. For instance, the increase of this insect population in China resulted in extensive damage to sweet corn [Citation23]. Our results indicate that M. anisopliae was the most effective against the flower chafer beetle in comparison to other entomopathogenic fungal species. Various reports have shown that M. anisopliae is a soil-borne fungus abundant in field soils [Citation24,Citation25], which might reflect their possible pathogenicity against white grubs colonizing underground habitats. The differences in susceptibility between M. anisopliae JEF-314 and the other tested species might reflect differences in the infection process and insect-fungi interactions. Recently, significant insights into the molecular mechanisms underlying the fungal infection and specificity have resulted in a description of specificity-related genes [Citation26,Citation27]. Therefore, characterization of the mechanisms underlying the pathogenicity of the isolate JEF-314 against the chafer beetle will help identify the fungus virulence determinants that are specific to this species and absent in other entomopathogenic fungus. Further experiments showed that eggs and larval stages are particularly susceptible to our newly described M. anisopliae isolate JEF-314, whereas the adults were not susceptible. Pupae were excluded from the assays since the grubs form oval earthen cells to pupate, blocking contact with the fungi (). M. anisopliae is known to exhibit varying levels of mortality against Scarabaeidae species [Citation28]; thus, characterization of highly infective isolates is of great importance for development of biological tools to control those insects. M. anisopliae represents an attractive alternative to control soil-dwelling grubs, both because of its high virulence and because it represents an eco-friendly tool to control those insects in agricultural spaces or turf grass. The fungus was shown to not be phytopathogenic, to have no or low virulence against other soil organisms and the level of conidia entering the water is unlikely to pose any hazard to aquatic organisms [Citation8,Citation29].
Our results showed that application of fungal granules is highly effective against the insect larvae. The fungus was successfully released from the grains and could reach the dwelling larvae. Indeed, granule formulations have been shown to be highly effective for beetles control [Citation2,Citation30]. Therefore, we highly recommend applying fungi-formulated granules in late spring and summer seasons, when the beetle lay eggs and the first larval stage start hatching, since this is when the insects are highly susceptible to the fungus (). Similar suggestions have been made for the June beetle Popyphylla fullo infected with either B. bassiana or M. anisopliae [Citation31] suggesting that our model might be used to control a number of economically important turfgrass-infesting beetles that share the same life cycle such as the Japanese beetle and June beetles. An important factor to control the beetle population is the persistence of the applied formulation in the environment, since the fungus has to persist long enough to infect the target insect. Indeed, the fungal application to the soil is more likely to confer protection from UV rays and high temperatures in addition to providing conditions of relative high humidity, favoring fungal survival and germination. In the particular case of M. anisopliae JEF-314, we recommend application of millet-based granule formulation which provides nutrition for the fungus increasing its persistence [Citation21]. In this context, our previous data suggested that millet granules support high conidia production and high thermal tolerance of fungal species [Citation32]. Moreover, several reports showed that M. anisolpiae persist long enough to reach and infect the host and are able to persist up to 3 years in the soil [Citation33–35]. Entomopathogenic fungi-based granule formulations may provide an advantage over other commercial biological pesticides due to low production cost because of increased production quantities compared to fermentation production. The reduced cost is also associated with availability of commercial equipment and shorter process time [Citation30].
Figure 5. Suggested model for application of the isolate M. anisopliae JEF-314 in the field to control the flower chafer beetle.
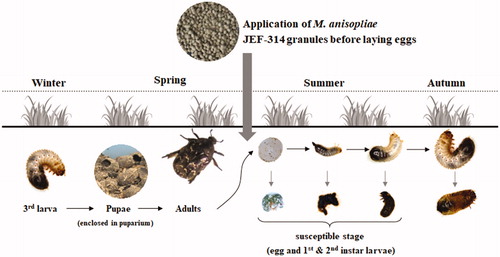
Due to the lack of efficient control strategies of the white grubs, we analyzed the potential of a M. anisopliae JEF-314 to infect the chafer beetle. The results of the performed bioassays showed a clear stage-dependent virulence and suggest that granule formulation of M. anisopliae JEF-314 would be a successful tool to control the beetles when applied at a time corresponding to oviposition and egg hatching. It is likely that the entomopathogenic fungi are highly effective against beetles in general since they can reach soil-dwelling larval stages and act as contact insecticides. However, knowledge of the beetle life cycle and habitat conditions is necessary to assess the optimum application time corresponding to the most susceptible developmental stage. Our data and similar systemic studies are indispensable to solve the control problems associated with soil-dwelling beetles not significantly impacted by other control agents.
Supplemental Material
Download TIFF Image (49.4 KB)Acknowledgements
The authors thank Mr. Sunghyun Kim at Rural Development Administration for providing a colony of flower chafer beetle and information how to rear the insect in an insectary.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jackson TA, Klein MG. Scarabs as pests: a continuing problem. Coleopterists Bull. 2006;60:102–119.
- Behle RW, Goett EJ. Dosage response mortality of Japanese beetle, masked chafer, and June beetle (Coleoptera: Scarabaeidae) adults when exposed to experimental and commercially available granules containing Metarhizium brunneum. J Econ Entomol. 2016;109:1109–1115.
- Gyawaly S, Koppenhöfer A, Wu S, et al. Biology, ecology, and management of masked chafer (Coleoptera: Scarabaeidae) grubs in turfgrass. J Integr Pest Manag. 2016;7:3.
- Potter DA, Braman S. Ecology and management of turfgrass insects. Annu Rev Entomol. 1991;36:383–406.
- Cimino AM, Boyles AL, Thayer KA, et al. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ Health Perspect. 2017;125:155–162.
- Wood TJ, Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res. 2017;24:17285–17325.
- Ramos Y, Portal O, Lysøe E, et al. Diversity and abundance of Beauveria bassiana in soils, stink bugs and plant tissues of common bean from organic and conventional fields. J Invertebr Pathol. 2017;150:114–120.
- Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Technol. 2007;17:879–920.
- Jackson MA, Dunlap CA, Jaronski ST. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl. 2010;55:129–145.
- Vega FE, Goettel MS, Blackwell M, et al. Fungal entomopathogens: new insights on their ecology. Fungal Ecol. 2009;2:149–159.
- Kim HG, Park KH, Lee S, et al. Effects of different diets and temperatures on larval growth of the white-spotted flower chafer, Protaetia brevitarsis (Kolbe)(Coleoptera: Scarabaeidae). Int J Ind Entomol. 2015;31:75–78.
- Kwon O. Effect of different diets on larval growth of Protaetia brevitarsis seulensis (Kolbe) (Coleoptera: Cetoniidae). Entomol Res. 2009;39:152–154.
- Kwak KW, Han MS, Nam SH, et al. Detection of insect pathogen Serratia marcescens in Protaetia brevitarsis seulensis (Kolbe) from Korea. Int J Ind Entomol. 2014;28:25–31.
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680.
- Nicholas KB. GeneDoc: analysis and visualization of genetic variation. Embnew News. 1997;4:14.
- Saitou N. Property and efficiency of the maximum likelihood method for molecular phylogeny. J Mol Evol. 1988;27:261–273.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Song MH, Yu JS, Kim S, et al. Downstream processing of Beauveria bassiana and Metarhizium anisopliae-based fungal biopesticides against Riptortus pedestris: solid culture and delivery of conidia. Biocontrol Sci Technol. 2019;29:514–532.
- Karpouzas DG, Walker A, Froud‐Williams RJ, et al. Evidence for the enhanced biodegradation of ethoprophos and carbofuran in soils from Greece and the UK. Pestic Sci. 1999;55:301–311.
- Niemczyk H, Chapman R. Evidence of enhanced degradation of isofenphos in turfgrass thatch and soil. J Econ Entomol. 1987;80:880–882.
- Lee SJ, Kim S, Kim JC, et al. Entomopathogenic Beauveria bassiana granules to control soil-dwelling stage of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). BioControl. 2017;62:639–648.
- Lovett B, St. Leger RJ. Genetically engineering better fungal biopesticides. Pest Manag Sci. 2018;74:781–789.
- Chen R-z, Li y. A novel plant volatile attractant scheme to protect corn in China from the white-spotted flower chafer (Coleoptera: Scarabaeidae: Cetoniinae). J Pest Sci. 2011;84:327–335.
- Meyling NV, Thorup-Kristensen K, Eilenberg J. Below-and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biol Control. 2011;59:180–186.
- Parker B, Skinner M, Gouli S, et al. Persistence of Beauveria bassiana sensu lato and Metarhizium anisopliae sensu lato in Vermont (USA) forest soil. Biocontrol Sci Technol. 2015;25:768–788.
- Alkhaibari AM, Lord AM, Maffeis T, et al. Highly specific host-pathogen interactions influence Metarhizium brunneum blastospore virulence against Culex quinquefasciatus larvae. Virulence. 2018;9:1449–1467.
- Lee SJ, Lee MR, Kim S, et al. Genomic analysis of the insect-killing fungus Beauveria bassiana JEF-007 as a biopesticide. Sci Rep. 2018;8:12388.
- Ramoutar D, Legrand AI, Alm SR. Field performance of Metarhizium anisopliae against Popillia japonica (Coleoptera: Scarabaeidae) and Listronotus maculicollis (Coleoptera: Curculionidae) larvae in turfgrass. J Entomol Sci. 2010;45:20–26.
- Milner RJ, Lim RP, Hunter DM. Risks to the aquatic ecosystem from the application of Metarhizium anisopliae for locust control in Australia. Pest Manag Sci. 2002;58:718–723.
- Behle RW, Richmond DS, Jackson MA, et al. Evaluation of Metarhizium brunneum F52 (Hypocreales: Clavicipitaceae) for control of Japanese beetle larvae in turfgrass. J Econ Entomol. 2015;108:1587–1595.
- Erler F, Ates AO. Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle. J Insect Sci. 2015;15:44–44.
- Park SE, Kim JC, Lee SJ, et al. Solid cultures of thrips-pathogenic fungi Isaria javanica strains for enhanced conidial productivity and thermotolerance. J Asia-Pac Entomol. 2018;21:1102–1109.
- Milner RJ, Samson P, Morton R. Persistence of conidia of Metarhizium anisopliae in sugarcane fields: Effect of isolate and formulation on persistence over 3.5 years. Biocontrol Sci Technol. 2003;13:507–516.
- Pilz C, Enkerli J, Wegensteiner R, et al. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J Appl Entomol. 2011;135:393–403.
- Samson PR, Milner RJ, McLennan PD. Field trials of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) against Inopus rubriceps (Diptera: Stratiomyidae) in sugarcane. Environ Entomol. 1994;23:749–754.