Abstract
Marine yeasts have tremendous potential in industrial applications but have received less attention than terrestrial yeasts and marine filamentous fungi. In this study, we have screened marine yeasts for amylolytic activity and identified an amylase-producing strain PH-Gra1 isolated from sea algae. PH-Gra1 formed as a coral-red colony on yeast–peptone–dextrose (YPD) agar; the maximum radial growth was observed at 22 °C, pH 6.5 without addition of NaCl to the media. Based on the morphology and phylogenetic analyses derived from sequences of internal transcribed spacer (ITS) and a D1/D2 domain of large subunit of ribosomal DNA, PH-Gra1 was designated Sporidiobolus pararoseus. S. pararoseus is frequently isolated from marine environments and known to produce lipids, carotenoids, and several enzymes. However, its amylolytic activity, particularly the optimum conditions for enzyme activity and stability, has not been previously characterized in detail. The extracellular crude enzyme of PH-Gra1 displayed its maximum amylolytic activity at 55 °C, pH 6.5, and 0%–3.0% (w/v) NaCl under the tested conditions, and the activity increased with time over the 180-min incubation period. In addition, the crude enzyme hydrolyzed potato starch more actively than corn and wheat starch, and was stable at temperatures ranging from 15 °C to 45 °C for 2 h. This report provides a basis for additional studies of marine yeasts that will facilitate industrial applications.
1. Introduction
Yeasts are ascomycetous or basidiomycetous fungi, and have been widely used in food, agriculture, medicine, and biofuels [Citation1]. In addition to terrestrial environments, yeasts inhabit marine environments including seawater, sediment, seaweeds, marine plants and animals. These marine yeasts play a critical role in biodegradation and nutrient recycling in the ocean [Citation2]. Moreover, several yeasts are pathogenic to sea animals [Citation3].
Marine yeasts have gained attention from scientists and industry because of their numerous and diverse bioactive properties. In the pharmaceutical industry, marine yeasts such as Rhodotorula glutinis, Aureobasidium pullulans, and Candida membranifaciens can be used to produce astaxanthin, siderophore, and riboflavin [Citation4,Citation5]. In addition, marine-derived Pichia salicaria and Yarrowia lipolytica produce ethanol and antimicrobial silver nanoparticles, respectively [Citation6,Citation7]. Marine yeasts also serve as bioresources for industrial enzymes including lipase, cellulase, inulinase, protease, and xylanase [Citation7–9].
Amylase is a group of enzymes that hydrolyze the α-glucosidic linkages of starch to form sugars. It consists of α-amylase, β-amylase, glucoamylase, isoamylase and pullulanase, and is used in the food, brewing, detergent, and textile industries. Animals, plants, and microorganisms produce amylolytic enzymes. In fungi, it is well known that filamentous species such as Aspergillus oryzae and A. niger secrete extracellular amylase to digest starch [Citation10,Citation11]. Furthermore, α-amylase and glucoamylase in some yeast species, including Candida antarctica, Cryptococcus flavus, Filobasidium capsuligenum, Lipomyces kononenkoae, Pichia burtonii, and Schwanniomyces castellii have been characterized [Citation12–17]. Of the marine yeasts, glucoamylase from A. pullulans N13d has been studied in detail [Citation18]. Overall, however, amylase-producing marine yeasts remain poorly understood relative to terrestrial yeasts.
In this study, we screened marine yeast isolates for amylolytic activity. An amylase producing strain, PH-Gra1, isolated from sea algae was identified as a basidiomycetous yeast Sporidiobolus pararoseus based on morphological and molecular analyses. The effects of temperature, pH, NaCl concentration, and incubation period on the amylolytic activity of PH-Gra1 were determined. Moreover, substrate specificity and stability have been investigated.
2. Materials and methods
2.1. Fungal isolation and culture conditions
Sea algae Grateloupia sp. was collected from Pohang, Republic of Korea (36.13′35.5″N, 129.35′23.23″E), washed with sterile distilled water three times, and cut into approximately 1 cm pieces using a surgical blade. The segments were placed on potato dextrose agar (PDA; BD Difco, Sparks, MD) and malt extract agar (MEA; Oxoid, Hampshire, UK) containing ampicillin (0.01%, w/v), streptomycin (0.01%, w/v), and NaCl (3%, w/v), and incubated at 28 °C for 7 days. The following procedures were conducted as previously described [Citation19]. After isolation, the yeast strains were cultured on yeast extract peptone dextrose (YPD) broth or agar at 28 °C unless otherwise described. PH-Gra1 was deposited in the Korean Collection for Type Cultures (strain number KCTC 27936).
2.2. Screening yeast isolates for amylolytic activity
To obtain amylase-producing marine yeast strains, amylolytic activity was assessed by culturing yeasts on nutrient agar containing 0.2% starch (GeorgiaChem, USA) as previously described [Citation20]. Commercial amylase from A. oryzae (Sigma-Aldrich, St-Louis, MO, USA) and sterile H2O were inoculated on the same media as positive and negative controls, respectively. After incubation at 28 °C for 7 days, Lugol solution (Sigma) was poured onto the plates and positive strains were determined by the presence of a light purple zone around the colony.
2.3. DNA extraction, PCR, and sequence analysis
To isolate genomic DNA (gDNA), PH-Gra1 was incubated in YPD broth at 28 °C and 200 rpm for 3 days. Yeast cells were collected by centrifugation at 3,000 rpm for 10 min, frozen and ground in a mortar using liquid nitrogen, and suspended in lysis buffer, following established methods [Citation19].
Polymerase chain reaction (PCR) was conducted using two primer sets: ITS1 and ITS4 were used to amplify the internal transcribed spacer (ITS) region and NL1 and NL4 were used to amplify the D1/D2 domain of a large subunit (26S) of rDNA [Citation21]. The resultant ∼0.6-kb DNA segments for each primer set were purified using a Gel Extraction Kit (Qiagen, Hilden, Germany). The primer sets above were used to perform sequencing in the forward and reverse direction by Macrogen Inc. (http://dna.macrogen.com/kor). The ITS and D1/D2 sequences of PH-Gra1 were used as query sequences for BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) search to find sequence similarity. Sequence alignment and phylogenetic analyses were performed using established methods [Citation19].
2.4. Microscopy
For the morphological analysis, PH-Gra1 was cultured in potato dextrose broth (PDB) and YPD broth at 28 °C and 200 rpm for 2 days. Microscopic observations were conducted using a Leica CTR6000 microscope (Leica, Wetzlar, Germany).
2.5. Preparation of the extracellular crude enzyme
PH-Gra1 was inoculated in 10 mL of basal media containing 0.5% (w/v) starch at 28 °C and 200 rpm for 5 days. Collection, concentration, and buffer exchange of the supernatant were conducted as previously described [Citation19]. The extracellular protein in the resultant crude enzyme was quantified [Citation22], and a 100 μL solution containing 3 μg protein was used to assess the amylolytic activity of PH-Gra1 under various conditions.
2.6. Effect of temperature, pH, concentration of NaCl, and incubation time on amylolytic activity
A solution of 100 μL of PH-Gra1 crude enzyme containing 3 μg of protein was incubated with 1 mL of 0.5% starch dissolved in 20 mM sodium phosphate buffer (pH 6.0) at 15 °C, 22 °C, 30 °C, 37 °C, 45 °C, and 55 °C for 1 h. For the optimum pH test, the crude enzyme was incubated with 1 mL of 0.5% starch solution at various pH (20 mM acetate buffer for pH 4.0 and pH 5.0, 20 mM sodium phosphate buffer for pH 6.5 and pH 7.5, and 20 mM glycine-NaOH buffer for pH 9.0 and pH 10.5) at 37 °C for 1 h. For the optimum NaCl level test, the crude enzyme was incubated with 1 mL of 0.5% starch with a 20 mM sodium phosphate buffer (pH 6.0) supplemented with 0%, 0.1%, 0.5%, 1.5%, 3.0%, and 5.0% (w/v) NaCl at 37 °C for 1 h. To examine the effect of incubation time on the amylolytic activity of PH-Gra1, the crude enzyme was incubated with 1 mL of 0.5% starch at 37 °C for 0 min, 15 min, 30 min, 60 min, 120 min and 180 min. One milliliter of the reaction mixture was used to evaluate the amylolytic activity by DNS assay.
2.7. Hydrolysis of different starch sources by PH-Gra1 amylase
The crude enzyme was incubated with 1 mL of 20 mM sodium phosphate buffer (pH 6.0) containing 0.5% starch from potato (GeorgiaChem), corn (Sigma-Aldrich), and wheat (Sigma-Aldrich) at 37 °C and 55 °C for 1 h. One milliliter of the reaction mixture was used to evaluate the amylolytic activity by DNS assay.
2.8. 3,5-Dinitrosalicylic acid (DNS) assay
The amount of reducing sugar produced by amylase associated with PH-Gra1 was measured by 3,5-dinitrosalicylic acid (DNS) assay as previously described [Citation19] with a few modifications. Starch (0.5% w/v) in a 20 mM sodium phosphate buffer (pH 6.0) was used as a substrate. Blanks of the substrate (starch without the crude enzyme) and enzyme (crude enzyme without starch) were included as controls. A standard curve was constructed using maltose monohydrate dissolved in 20 mM sodium phosphate buffer (pH 6.0). The amylolytic activity unit was defined as the amount of a reducing sugar “maltose” (μmol/mL) produced per hour under tested conditions.
2.9. Thermal stability of PH-Gra1 amylase
A solution of 100 μL of PH-Gra1 crude enzyme containing 3 μg of protein was pre-incubated at 15 °C, 30 °C, 45 °C, 50 °C, 55 °C, and 60 °C for 1 h or 2 h, and promptly chilled in ice. Thereafter, the crude enzyme was incubated with 1 mL of 0.5% starch solution in 20 mM sodium phosphate buffer (pH 6.0) at 37 °C for 1 h. The residual amylolytic activity was evaluated by DNS assay as described above, and the enzymatic activity without pre-incubation was considered as control and taken as 100%.
2.10. Statistical analysis
The experimental data were analyzed using the GraphPad Prism software (version 5.0). All experiments were performed in biological triplicates unless otherwise indicated. Data were analyzed using one-way ANOVA followed by a Tukey’s multiple comparison test.
2.11. Accession numbers
The ITS and D1/D2 sequences of PH-Gra1 were deposited into GenBank under accession numbers MK994014 and MK994015, respectively.
3. Results
3.1. Screening of amylase-producing marine yeasts
Hydrolysis of starch by commercial amylase (positive control) produced a transparent area with a light purple periphery on nutrient agar containing 0.2% starch, when treated with Lugol solution (). In contrast, inoculation of sterile H2O and non-amylase-producing isolates (negative controls) did not give the light purple region under the same conditions. Based on this result, amylolytic activity of yeast isolates was determined by the presence of a light purple halo around the fungal colony against a navy background. An algae-derived isolate, named PH-Gra1, exhibited amylolytic activity and grew readily on nutrient agar, establishing a flattened coral-red colony with smooth surface ().
Figure 1. Yeast isolates tested for amylolytic activity. PH-Gra1 was inoculated onto nutrient agar with 0.2% starch. Lugol solution was used to visualize the region of starch that had been hydrolyzed by amylolytic activity (“before” = prior to Lugol solution treatment; “after” = after Lugol solution treatment). Amylolytic activity was determined by the presence of a light purple region (indicated as white arrows) around the colony against a navy background. Commercial amylase was inoculated on the same medium as a positive control (+). Sterile H2O and a non-amylase-producing strain were included as negative controls (−).

3.2. Growth of PH-Gra1 in various culture conditions
Radial growth of PH-Gra1 was examined at various temperatures, pH, and NaCl concentrations. When cultured on YPD media, PH-Gra1 grew at 15 °C, 22 °C, and 30 °C whereas growth was not observed at 37 °C, 45 °C, and 55 °C after 5 days of incubation (). The largest colony was found at 22 °C, and the colony morphology varied with temperature. At 15 °C, PH-Gra1 formed light pink colonies and growth was limited compared to the growth that formed the coral-red colonies at 22 °C and 30 °C. The colonies were wrinkled with an irregular periphery at 15 °C and 22 °C, whereas they were circular in shape with a smooth surface at 30 °C.
Figure 2. Growth and morphological characteristics of PH-Gra1. (A) PH-Gra1 spores were sequentially diluted (106, 105, 104, and 103 in 5 μl H2O), inoculated on YPD media, and cultured at different temperatures, pH, and NaCl concentrations. PH-Gra1 exhibited optimum growth at 22 °C, pH 6.5 without addition of NaCl to the media; (B) Microscopic images of budding yeast cells of PH-Gra1 cultured at 28 °C on YPD. Scale bar = 10 μm.
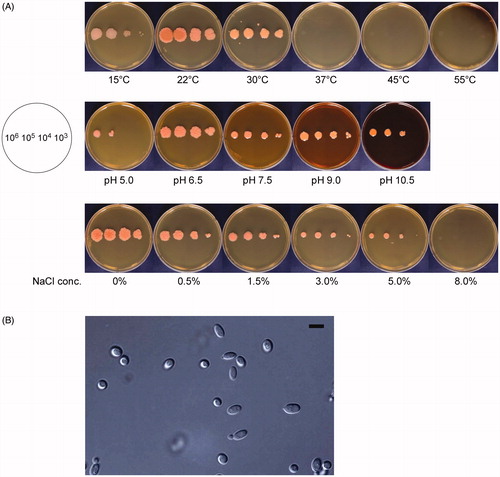
The pH sensitivity of PH-Gra1 growth was tested on YPD media at pH 5.0, 6.5, 7.5, 9.0, and 10.5. The largest colonies were observed at pH 6.5; these colonies had a wrinkled surface and an irregular periphery (). Growth was drastically and slightly inhibited at pH 5.0 and pH 10.5, respectively. Round colonies with smooth surfaces were produced at pH 7.5, 9.0, and 10.5, in contrast to the wrinkled irregular colonies at pH 6.5.
As PH-Gra1 was derived from marine environments, the effect of NaCl concentrations on its growth was investigated. Addition of NaCl to the medium inhibited growth of PH-Gra1, and growth did not occur at 8.0% (w/v) NaCl (). At the lowest NaCl concentrations (0 and 0.5% NaCl), PH-Gra1 formed wrinkled colonies. With increasing NaCl concentration, the colonies became smaller with smoother surfaces.
Cells of PH-Gra1 viewed under the microscope were subglobose or elliptical in shape on PDA and YPD media (). The size was approximately 2.5–4.0 μm in width × 4.0–7.0 μm in length (N = 10), and bud formation was observed. There was no detectable difference in cell morphology between the wrinkled and smooth colonies described above (data not shown).
3.3. Phylogenetic analysis
Yeast identification by phylogenetic analysis is generally conducted using both ITS and D1/D2 domain sequences [Citation23]. The sequences of a 572-bp ITS and a 594-bp D1/D2 domain segment were analyzed for molecular identification of PH-Gra1 (GenBank accession number MK994014 and MK994015, respectively). A BLAST search using the ITS sequence as a query revealed that PH-Gra1 showed a high degree of similarity to Sporidiobolus pararoseus CBS 491 (100% identity), S. pararoseus SF1-2 (99.48%), S. pararoseus CBS 5331 (99.30%), and Sporobolomyces patagonicus CBS 9657 (99.13%). In a neighbor-joining phylogenetic tree, PH-Gra1 was placed on the same clade as S. pararoseus CBS 491 and S. patagonicus CBS 9657 (88% degree of confidence) ().
Figure 3. Phylogenetic analysis of Sporidiobolus pararoseus strain PH-Gra1, based on ITS and a D1/D2 domain of large subunit of rDNA. Neighbor-joining phylogenetic trees generated using sequences of: (A) ITS and (B) a D1/D2 domain of large subunit of rDNA (LSU). The numbers at nodes indicate the percentage bootstrap values based on 1000 replications (values <50% are not shown). Sporidiobolus salmonicolor CBS 490 was included as an outgroup taxon. The scale bar indicates the number of nucleotide substitutions per site.
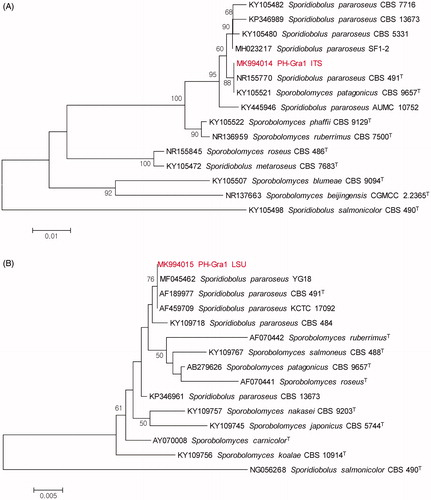
A BLAST search conducted using the D1/D2 domain sequence as a query revealed that PH-Gra1 showed a high degree of similarity to S. pararoseus YG18 (100% identity), S. pararoseus CBS 491 (100%), S. pararoseus KCTC 17092 (100%), and S. pararoseus CBS 484 (99.66%). The neighbor-joining phylogenetic analysis of a D1/D2 domain segment showed that PH-Gra1 belongs to the same clade as these four species by 76% degree of confidence (). The sequence identity of the PH-Gra1 D1/D2 segment to the corresponding sequence from S. patagonicus CBS 9657 was 98.82%. Taken together, based on the morphological and molecular analyses, PH-Gra1 was identified as a basidiomycetous yeast, S. pararoseus. PH-Gra1 was deposited in the Korean Collection for Type Cultures (strain number KCTC 27936).
3.4. Effect of temperature, pH, NaCl concentration, and incubation time on amylolytic activity of PH-Gra1
The optimum temperature, pH, and NaCl concentration for amylolytic activity were investigated using 0.5% starch as a substrate. When the substrate was incubated with crude enzymes from PH-Gra1 at 15 °C, 22 °C, 30 °C, 37 °C, 45 °C, and 55 °C (pH 6.0), the maximum activity was observed at 55 °C (). Amylolytic activities increased gradually with increasing temperature; the amylolytic activity at 55 °C was approximately six times higher than the activity at 15 °C.
Figure 4. Effects of temperature, pH, NaCl concentration, and incubation time on the amylolytic activity of PH-Gra1. A crude enzyme solution containing equal amounts of protein was incubated with 0.5% starch at the selected (A) temperature, (B) pH, (C) NaCl concentration, and (D) incubation time. Amylolytic activity was calculated from the mean and standard deviation of three biological replicates. Alphabetical letters on the graph indicate differences that are statistically significant (p < .05) by ANOVA analysis followed by Tuckey’s test.
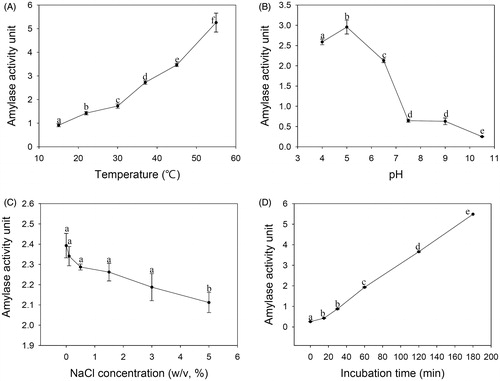
The effect of pH on amylolytic activity was examined at pH 4.0, 5.0, 6.5, 7.5, 9.0, and 10.5 (37 °C, 1 h). The maximum and the minimum activities were observed at pH 5.0 and pH 10.5, respectively (). Amylolytic activity at pH 5.0 was about twelve times higher than at pH 10.5, and the activity dropped drastically between pH 6.5 and pH 7.5.
Amylolytic activity was measured at 0%, 0.1%, 0.5%, 1.5%, 3.0%, and 5.0% (w/v) NaCl concentrations (37 °C, pH 6.0). There were no differences in the activities from 0% to 3.0% NaCl (p > 0.05 by one-way ANOVA). The minimum amylolytic activity was observed at 5% NaCl ().
The effect of incubation time on amylolytic activity was examined at 0, 15, 30, 60, 120, and 180 min (37 °C, pH 6.0). The maximum activity was observed after 180 min incubation, and there was no significant difference in activities between 15 min and 30 min (p > 0.05 by one-way ANOVA). Amylolytic activity increased in proportion to the incubation time from 30–180 min ().
3.5. Hydrolysis of different starch sources by PH-Gra1 amylase
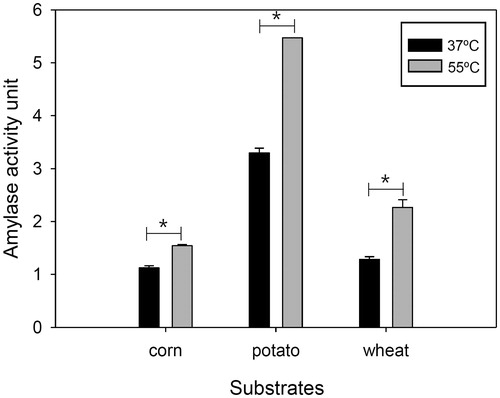
The extracellular crude enzyme of PH-Gra1 digested potato starch more actively than corn and wheat starch (). The amylase activity to corn and wheat starch was 34% and 39% as potato starch, respectively, at 37 °C and pH 6.0. At 55 °C, the amylase activity increased in all starch sources compared to at 37 °C, and the activity to corn and wheat starch was 28% and 41% as potato starch, respectively.
Figure 5. Hydrolysis of different starch sources by PH-Gra1 amylase. A crude enzyme of PH-Gra1 was incubated with 0.5% starch from potato, corn, and wheat at 37 °C and 55 °C for 1 h. Amylolytic activity was presented by the mean and standard deviation of three biological replicates. “*” indicates differences that are statistically significant (p < .05) by t-test between 37 °C and 55 °C.
3.6. Thermal stability of the extracellular amylase of PH-Gra1
To examine thermal stability of PH-Gra1 amylase, the crude enzyme was pre-incubated at 15 °C, 30 °C, 45 °C, 50 °C, 55 °C, and 60 °C for 1 h or 2 h. When pre-incubated at 15 °C, 30 °C, and 45 °C, the crude enzyme showed about 94–98% residual activity relative to the control (no pre-incubation of the enzyme). In contrast, the activity significantly decreased by pre-incubation at 50 °C and 55 °C exhibiting approximately 43–87% residual activity relative to the control. The activity dramatically decreased at 60 °C, retaining approximately 8% of the control activity ().
Table 1. Thermal stability of extracellular crude amylase of PH-Gra1.
4. Discussion
Compared to terrestrial yeasts, which are widely used in industrial process, little is known of the marine-derived yeasts. In the present study, we screened yeast strains isolated from marine environments for amylolytic activity to facilitate industrial applications of marine yeasts.
Based on the phenotypic and phylogenetic analyses, an amylase-producing strain, designated PH-Gra1, was identified as a basidiomyceteous yeast, Sporidiobolus pararoseus. As previously described [Citation24] and demonstrated in this study (), S. pararoseus is closely related to Sporobolomyces patagonicus. The ITS sequences of these two yeasts are similar (≥99% identity), but analysis of the D1/D2 domain sequences showed that these strains are as distinct species, confirming that PH-Gra1 is S. pararoseus. Previously, S. pararoseus has been isolated from fruits, aquatic plants, and green algae [Citation25]. It is also oleaginous and produces carotenoids and enzymes including lipase and amylase [Citation26–28]. However, the amylolytic activity of S. pararoseus has not been characterized in detail, particularly in terms of optimum conditions and stability of the enzyme.
Amylase accounts for about 30% of world enzyme production, and is extensively used in the textile, food, detergent, and fermentation industries [Citation29]. Amylolytic enzymes include 3 families of glycoside hydrolases (GHs): GH 13 (α-amylase [EC 2.1.1.1]); GH 14 (β-amylase [EC3.2.1.2]); and GH 15 (glucoamylase [EC3.2.1.3]). The enzymes α-amylase, β-amylase, and glucoamylase associated with S. pararoseus have not been listed within the Carbohydrate Active enZymes (CAZy) classification system (http://www.cazy.org). While α-amylase acts at random locations at the starch chain, resulting in production of various oligosaccharides including glucose, glucoamylase acts at the non-reducing end of starch, resulting in production of glucose [Citation30]. Maltose is one of the main end products generated by the activity of α-amylase and β-amylase on starch [Citation31].
Amylolytic activity was maximized at 55 °C, pH 5.0, 0%–3.0% (w/v) NaCl, and 180 mins of incubation time in the tested conditions (). The optimum temperatures and pH for amylolytic activity by PH-Gra1 are similar to those previously reported for amylolytic yeasts and filamentous fungi [Citation29,Citation32]. For example, optimum amylolytic activity by Candida antarctica, Cryptococcus flavus, Lipomyces kononenkoae, Saccharomycopsis fibuligera, and Talaromyces pinophilus occurs at 50 °C–70 °C and pH 4.0–6.0. In addition, several filamentous fungi including Aspergillus awamori, Aspergillus foetidus, Paecilomyces variotii, Trichoderma viride, and Fusarium vasinfectum produce amylase of which maximum activities are observed at 45 °C–70 °C and pH 4.0–5.0.
Thermal stability provides processing advantages in enzyme applications, and is a critical factor for commercial exploitation. Amylolytic activity of PH-Gra1 was stable up to 45 °C for 2 h, retaining more than 95% activity relative to the control (no pre-incubation). After 1 h pre-incubation at 55 °C, which is the optimum temperature for amylolytic activity of PH-Gra1, residual activity was approximately 67% relative to the control. A few species such as L. kononenkoae and A. niger produce amylase stable at 65 °C for 20 min and 30 min, respectively [Citation32]. On the other hand, many known fungal amylases of A. awamori, A. foetidus, C. flavus, and Tetracladium sp. are stable over the temperature range of 40 °C–50 °C, consistent with thermal stability of PH-Gra1 amylase [Citation32]. Given the similar optimum conditions and stability, comparative analyses of the enzyme efficiency in amylolytic fungi, yeasts in particular, would be required to identify the most favorable fungal strains for amylase application.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hatoum R, Labrie S, Fliss I. Antimicrobial and probiotic properties of yeasts: from fundamental to novel applications. Front Microbiol. 2012;3:421.
- Hyde KD, Jones EBG, Leano EM, et al. Role of fungi in marine ecosystems. Biodivers Conserv. 1998;7(9):1147–1161.
- Higgins R. Bacteria and fungi of marine mammals: a review. Can Vet J. 2000;41(2):105–116.
- Wang WL, Chi ZM, Chi Z, et al. Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresour Technol. 2009;100(9):2639–2641.
- Wang L, Chi Z, Wang X, et al. Isolation and characterization of Candida membranifaciens subsp. flavinogenie W14-3, a novel riboflavin-producing marine yeast. Microbiol Res. 2008;163(3):255–266.
- Apte M, Sambre D, Gaikawad S, et al. Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express. 2013;3(1):32.
- Kathiresan K, Saravanakumar , Senthilraja KP. Bio-ethanol production by marine yeasts isolated from coastal mangrove sediment. Int Multidiscip Res J. 2011;1:19–24.
- Chi Z, Chi Z, Zhang T, et al. Production, characterization and gene cloning of the extracellular enzymes from the marine-derived yeasts and their potential applications. Biotechnol Adv. 2009;27(3):236–255.
- Duarte AWF, Dayo-Owoyemi I, Nobre F, et al. Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles. 2013;17(6):1023–1035.
- Pedersen H, Nielsen J. The influence of nitrogen sources on the alpha-amylase productivity of Aspergillus oryzae in continuous cultures. Appl Microbiol Biotechnol. 2000;53(3):278–281.
- Uguru GC, Akinyanju JA, Sani A. The use of yam peel for growth of locally isolated Aspergillus niger and amylase production. Enzyme Microb Technol. 1997;21(1):48–51.
- Clementi F, Rossi J. Alpha-amylase and glucoamylase production by Schwanniomyces castellii. Antonie Van Leeuwenhoek. 1986;52(4):343–352.
- Mot R, Verachtert H. Purification and characterization of extracellular alpha-amylase and glucoamylase from the yeast Candida antarctica CBS 6678. Eur J Biochem. 1987;164(3):643–654.
- De Mot R, Verachtert H. Purification and characterization of extracellular amylolytic enzymes from the yeast Filobasidium capsuligenum. Appl Environ Microbiol. 1985;50(6):1474–1482.
- Moranelli F, Yaguchi M, Calleja GB, et al. Purification and characterization of the extracellular alpha-amylase activity of the yeast Schwanniomyces alluvius. Biochem Cell Biol. 1987;65(10):899–908.
- Spencer-Martins I, Uden N. Extracellular amylolytic system of the yeast Lipomyces kononenkoae. Eur J Appl Microbiol Biotechnol. 1979;6(3):241–250.
- Takeuchi A, Shimizu-Ibuka A, Nishiyama Y, et al. Purification and characterization of an alpha-amylase of Pichia burtonii isolated from the traditional starter “murcha” in Nepal. Biosci Biotechnol Biochem. 2006;70(12):3019–3024.
- Li H, Chi Z, Wang X, et al. Purification and characterization of extracellular amylase from the marine yeast Aureobasidium pullulans N13d and its raw potato starch digestion. Enzyme Microb Technol. 2007;40(5):1006–1012.
- Chung D, Baek K, Bae SS, et al. Identification and characterization of a marine-derived chitinolytic fungus, Acremonium sp. YS2-2. J Microbiol. 2019;57(5):372–380.
- Hankin L, Anagnostakis SL. The use of solid media for detection of enzyme production by fungi. Mycologia. 1975;67(3):597–607.
- Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998;73(4):331–371.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254.
- Bai FY, Zhao JH, Takashima M, et al. Reclassification of the Sporobolomyces roseus and Sporidiobolus pararoseus complexes, with the description of Sporobolomyces phaffii sp. nov. Int J Syst Evol Microbiol. 2002;52(6):2309–2314.
- Libkind D, Gadanho M, van Broock M, Sampaio JP. Sporidiobolus longiusculus sp. nov. and Sporobolomyces patagonicus sp. nov., novel yeasts of the Sporidiobolales isolated from aquatic environments in Patagonia, Argentina. Int J Syst Evol Microbiol. 2005;55(1):503–509.
- Hong SG, Lee KH, Bae KS. Diversity of yeasts associated with natural environments in Korea. J Microbiol. 2002;40:55–62.
- Chaiyaso T, Manowattana A. Enhancement of carotenoids and lipids production by oleaginous red yeast Sporidiobolus pararoseus KM281507. Prep Biochem Biotechnol. 2018;48(1):13–23.
- Chaiyaso T, Srisuwan W, Techapun C, et al. Direct bioconversion of rice residue from canteen waste into lipids by new amylolytic oleaginous yeast Sporidiobolus pararoseus KX709872. Prep Biochem Biotechnol. 2018;48(4):361–371.
- Mase T, Sonoda M, Morita M, et al. Characterization of a lipase from Sporidiobolus pararoseus 25-A which produces cheese flavor. Food Res Technol Res. 2010;17(1):17–20.
- Djekrif DS, Gillmann L, Bennamoun L, et al. Amylolytic yeasts: producers of alpha-amylase and pullulanase. Int J Life Sci Scienti Res. 2016;2:339–354.
- Pavezzi FC, Gomes E, Silva R. d. Production and characterization of glucoamylase from fungus Aspergillus awamori expressed in yeast Saccharomyces cerevisiae using different carbon sources. Braz J Microbiol. 2008;39(1):108–114.
- Bilderback DE. A Simple Method to Differentiate between alpha- and beta-Amylase. Plant Physiol. 1973;51(3):594–595.
- Sharma A, Satyanarayana T. Microbial acid-stable alpha-amylase: characteristics, genetic engineering and applications. Process Biochem. 2013;48(2):201–211.
