Abstract
Peach (Prunus persica L.) is one of the major fruit crops in South Korea, along with apple, persimmon, and Asian pears. Peach anthracnose is a continuing threat to growers and is accountable for enormous economic loss. In July 2018, anthracnose of peach appeared at different peach orchards in Gyeongsangbuk-do region, Korea. The typical anthracnose symptoms (brown, circular, and necrotic lesions) were observed on the fruits. Anthracnose of peach was surveyed in different peach orchards of Gyeongsangbuk-do, and 20 fungal isolates from 19 diseased fruits were collected. Multigene phylogenetic analyses coupled with morphological characteristic analysis approaches were used for identifying the fungal species isolated from diseased fruits. This study confirmed three Colletotrichum species. Based on the results, Colletotrichum siamense are reported for the first time as causal agents of peach anthracnose alongside C. fructicola and C. fioriniae, which has been reported previously. Pathogenicity assays were performed for the three isolates representing all the species identified, and Koch’s postulates on detached healthy peach fruits were verified. All the identified species were pathogenic on peach fruits as the typical anthracnose symptoms were reproduced. Significant variations in the virulence were observed among fungal species on peach fruit.
1. Introduction
Peach (Prunus persica L. Batsch) is economically a very important fruit crop in Korea. Generally, people eat it as a table fruit. In Korea, it is used in different food industries for making jam (Boksoongah jam), jelly (jelly in jelly), tea (Boksoongah hong Chaa), and juice (Kolpeach). Addition of peach fruit could increase the total phenolic content of beer [Citation1]. Peach has medicinal value as it contains polyamines, γ-aminobutyric acid (GABA), proline, and caffeoylquinic acid, a bioactive polyphenol with high antioxidant activity [Citation1,Citation2]. They are considered as the fruit of happiness, riches, honor, and longevity. Since peach is a delicious fruit containing different nutrients and phytochemicals, some studies investigated the quality characteristics and antioxidant potential of beer prepared by the addition of peach. Peach has various health benefits [Citation3,Citation4] and also contains caffeoylquinic acid, a bioactive polyphenol with high antioxidant activity and beneficial effect on human health [Citation5]. Consumption of peach also potentially protects humans from against various chronic diseases by scavenging the reactive oxygen species.
Anthracnose caused by the fungal genus Colletotrichum is a devastating threat for the cultivation of peach worldwide, including South Korea and USA [Citation6,Citation7]. Anthracnose produces sunken lesions with a circular ring on the fruit and results in the loss of yield and fruit quality [Citation6]. Various fungal species belong to the genus Colletotrichum [Citation6,Citation8]. Colletotrichum gloeosporioides and C. acutatum are species complexes and comprise more than 22 and 30 species, respectively [Citation8,Citation9]. Species within a complex share common morphological characteristic, such as conidial shape and size, colony color, and growth rate. Based on morphological characteristics, identification of species within the complex is very difficult. Furthermore, morphological characteristics of fungal species vary with growth media, temperature, light, and host dependence [Citation8]. Some morphological characteristics, particularly colony color, may change or be lost with repeated subculturing [Citation8]. Molecular tools using multiple sequences have become more popular to delineate the Colletotrichum species. The study of morphological characteristics using molecular analysis is a reliable approach for species-level identification of the pathogen [Citation10].
To date, C. gloeosporioides and C. fioriniae have been identified and reported as causal agents of peach anthracnose in South Korea [Citation7,Citation11,Citation12]. Hu et al. [Citation6] identified C. acutatum, C. truncatum, C. siamense, and C. fructicola as causal agents of peach anthracnose using calmodulin (CAL), glyceraldehyde-3- phosphate dehydrogenase (GAPDH), and b-tubulin (TUB2) sequence data. Conversely, Kim and Hong [Citation11] identified Colletotrichum species in peach based on morphological characteristics, which is not enough [Citation8,Citation9]. In our previous study, we identified C. foriniae and C. fructicola using molecular and morphological analysis. There is also a chance of peach infection with diversified Colletotrichum species. Many studies have reported that diverse Colletotrichum species are responsible for anthracnose of a single host, for example persimmon, Japanese plum, and pear [Citation13–15]. Novel Colletotrichum species are continuously being identified by applying advanced identification methods [Citation10,Citation15]. To improve biosecurity and develop peach anthracnose management strategies, accurate diagnosis of responsible Colletotrichum species is essential.
The objective of this study was morphological and molecular characterization of Colletotrichum species associated with peach anthracnose in Korea and investigation of the virulence of identified Colletotrichum species.
2. Materials and methods
2.1. Fungal isolates and sampling
In 2018, 20 Colletotrichum isolates were collected from infected peach fruits in commercial orchards of Sangju, Gimcheon, Yechon, Yeongcheon, and Cheongdo, South Korea. Infected fruits (three fruits per orchard) showing typical anthracnose were collected and brought to the laboratory. The fruits were cleaned by washing with tap water. Disease lesions (5 mm × 5 mm size) were disinfested in 1% NaOCl for 1 min, washed twice with sterilized water, and dried with sterilized tissue paper. Disinfested tissue was plated on water agar (WA) and incubated at 25 °C in the dark for two days. Newly emerging hyphae from diseased tissue on WA were transferred to a Petri plate containing potato dextrose agar (PDA). Colonies showing typical morphological characteristics of Colletotrichum species were purified by hyphal tipping twice one fresh PDA. Fungal colonies (20) were grouped into two based on morphology. Colonies with pink color were placed in first group, and those with whitish gray color were placed in the second group. There were 14 isolates in the former and six in the latter. A sub sample of seven isolates (three from the first group and four from the second group) was selected to confirm species as morphological characteristic of isolates with in group were identical. All collected isolates were preserved in the laboratory on PDA slant at 4 °C in the dark.
2.2. Genomic DNA extraction
For extraction of genomic DNA, around 10 g mycelia were collected from an actively growing 4-day-old culture. DNA from each isolate was extracted using a HiGeneTM Genomic column type DNA Prep Kit (BIOFACT, Yuseong-Gu, Daejeon, Korea) following the manufacturer’s instructions. The crude DNA (1 µL) was used for PCR amplification of the target region.
2.3. Multigene amplification and sequencing
The following loci were amplified and sequenced: internal transcribed spacers (ITS) and a partial sequence of beta-tubulin (TUB2), calmodulin (CAL), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), actin (ACT), chitin synthase 1 (CHS-1), and the Apn2bulin (TUB2), calmodulin, and the partial mating type (ApMat). Identification of isolates into C. gloeosporioides sensu lato (s.l.) was done using six loci (ITS, TUB2, GAPDH, CHS-1, CAL, and ApMat), whereas identification of isolates into C. acutatum s.l. was done using four loci (ITS, TUB2, GAPDH, and CHS-1). To amplify ITS, TUB2, GAPDH, CHS-1, CAL, and ApMat gene, primer pairs ITS1 + ITS4 [Citation16], Bt2a + Bt2b [Citation17], GDF + GDR [Citation18], CHS-79F + CHS-345R [Citation19], CL1C + CL2C [Citation8] and AM-F + AM-R [Citation20] were used, respectively. PCR reaction for each gene was run as described by Hassan et al. [Citation13]. The resulting PCR amplicons were purified and then sequenced commercially at Macrogen, Inc. (Seoul, Korea). The consensus sequence of each gene was obtained by assembling forward and reverse sequences with SeqMan (v. 7.1.0.44; DNASTAR, Inc.) and was then deposited in GenBank ().
Table 1. Genebank accession number of present Colletotrichum isolates.
2.4. Sequence alignment, concatenation and phylogenetic analysis
For confirming the phylogenetic relationship of current isolates, sequences (respective genes) of ex-type Colletotrichum species in C. gloeosporioides s.l. and C. acutatum s.l. were retrieved from the GenBank database; these were deposited by Weir et al. [Citation8] and Damm et al. [Citation9], respectively. Individual gene sequence data from current isolates and data retrieved from GenBank were aligned with each other using the MUSCLE tool in MEGA 6 [Citation21] and edited manually. Gaps in aligned sequences were considered as missing data. Each of the resulting multiple gene sequence alignments were concatenated in Mesquite v. 2.75 [Citation22]. Maximum likelihood (ML) and maximum parsimony (MP) phylogenetic analyses were conducted with MEGA v. 6.0 [Citation21] and Bayesian inference (BI) with MrBayes v. 3.2 [Citation23]. For sampling trees and estimating posterior probabilities of the model parameter in BI phylogenetic analysis, Markov chain Monte Carlo (MCMC) sampler was employed as described by Hassan et al. [Citation14]. The resulting 50% majority rule consensus tree was visualized using FigTree v 1.3.1 [Citation24].
2.5. Morphological characterization
Three isolates (ICKb18, ICKb21 and ICKb36) representing one Colletotrichum species each were selected for morphological characterization. Mycelium plugs (5 mm) were transferred to a fresh PDA plate from the actively growing culture. Morphological characteristics of each colony, including color, mycelial growth rate, and conidia and appressorial characteristics, were evaluated after eight days of incubation at 25 °C in the dark. Conidial mass from the culture was mounted in sterilized distilled water or lactic acid. The size (lengths and widths) of 30 randomly selected conidia were measured using an Olympus BX43 microscope at 400× magnification. The slide with conidia mounted in water was put in a Petri plate containing moist tissue and incubated at 25 °C in the dark for enhancing appressoria formation. After three days of incubation, the sizes of 30 randomly chosen appressoria were measured as the size of conidia was measured.
2.6. Pathogenicity assay
Representative isolates (ICKb18, ICKb21, and ICKb36) of each species were tested for pathogenicity on peach fruits. The conidial suspension (1 × 106) of each isolate was used for inoculation on detached peach fruits. Stock conidial suspension was prepared from 20-day-old PDA culture, and then, 1 × 106 conidial suspension was prepared from it. Ripe peach fruits were disinfected with 70% ethanol for 1 min, rinsed twice with sterilized distilled water, and dried by blotting. Unwounded and wounded peach fruits were inoculated by dropping a 10-µL droplet of the conidial suspension at three equidistant points. Fruits were wounded to a depth of 1 mm using a sterile pin. The same amount of water was used to inoculate control unwounded and wounded fruits. The inoculated fruits were incubated in a humid plastic box (containing moist tissue) at 25 °C with a photoperiod of 12–12-h light/dark cycle for seven days. After seven days of incubation, lesion diameter (LD) was measured using the scale ruler. Disease incidence (DI) was estimated in the term percentage of lesion development compared with the total number of points inoculated. Five fruits were tested for each isolate and control.
2.7. Statistical analysis
The data were averaged, and standard deviation was calculated using MS excel. All data related to conidial and appressoria size and lesion diameter are presented as the average. Mean values of lesion diameter were compared through least significant different test in the SAS (SAS Inc., Cary, NC, USA).
3. Results
3.1. Multigene phylogenetic analysis
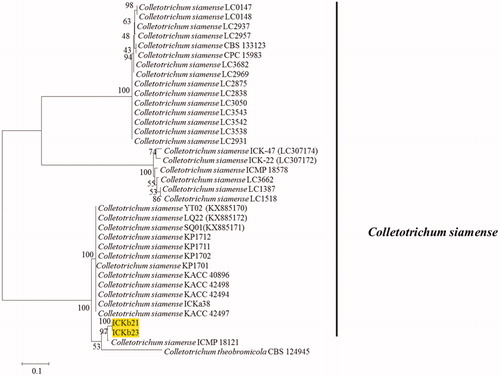
Seven representative isolates and reference isolates [Citation8,Citation9] were subjected to multilocus phylogenetic analysis. Phylogenetic analysis for four fungal isolates belonging to C. gloeosporioides s.l. were conducted using the combined sequences of ITS, TUB2, GAPDH, CHS-1, and CAL (). The final dataset consisted of 37 taxa and 2391 characters (nucleotide positions), including alignment gap (considered as missing data). The fungal species C. boninense was included as the outgroup in this phylogenetic analysis. In Bayesian analysis, 5650 trees were sampled to construct a 50% majority rule consensus tree and calculate the posterior probabilities. The topologies of BI, ML, and MP trees were congruent. The isolates recovered from infected peach fruits were clustered in two clades in the C. gloeosporioides s.l. phylogenetic tree: two isolates formed a clade with the ex-type isolates of C. fructicola (ICMP 18581), whereas another two isolates were clustered with the ex-type isolates of C. siamense (ICMP 18642). The isolates belonging to C. siamense clade were further confirmed by phylogenetic analysis using ApMat gene sequence data ().
Figure 1. A 50% majority rule consensus tree obtained by Bayesian inference analysis based on ITS, TUB2, GAPDH, CHS-1, and CAL sequence alignment. Bayesian posterior probability values ≥ 0.5 and bootstrap support values ≥ 50%; these are shown at the nodes (PP/ML/PM). C. boninense was used as the outgroup. The used reference Colletotrichum spp. within the C. gloeosporioides species complex obtained from Weir et al. [Citation8]. Isolates in this study are shown different color blocks.
![Figure 1. A 50% majority rule consensus tree obtained by Bayesian inference analysis based on ITS, TUB2, GAPDH, CHS-1, and CAL sequence alignment. Bayesian posterior probability values ≥ 0.5 and bootstrap support values ≥ 50%; these are shown at the nodes (PP/ML/PM). C. boninense was used as the outgroup. The used reference Colletotrichum spp. within the C. gloeosporioides species complex obtained from Weir et al. [Citation8]. Isolates in this study are shown different color blocks.](/cms/asset/efaeb927-97df-4b31-acf3-448f076f2e73/tmyb_a_1763116_f0001_c.jpg)
Figure 2. A maximum likelihood tree based on AptMat sequence alignment. Present isolates are indicated by the yellow block. Colletotrichum theobromicola CBS 124945 is used as the outgroup.
For phylogenetic analysis of the C. acutatum complex, the concatenated sequences of ITS, TUB2, GAPDH, and CHS-1 were used, which comprised 1576 nucleotide positions including alignment gap and 44 taxa. Colletotrichum pseudoacutatum (CBS 43677) was included as the outgroup in this phylogenetic analysis. A total of 4991 tree samples were subjected to BI analysis to construct 50% majority rule consensus tree and estimate posterior probability. There was no basic difference in BI, ML, and MP tree topological structures.
Phylogenetic tree (constructed using BI, ML, and MP statistical approach) of the C. acutatum complex showed that present isolates were clustered with ex-type isolates of C. fioriniae CBS 125396 ().
Figure 3. A 50% majority rule consensus tree obtained by Bayesian inference analysis based on ITS, TUB2, GAPDH, and CHS-1 sequence alignment. Bayesian posterior probability values ≥ 0.5 and bootstrap support values ≥ 50% are shown at the nodes (PP/ML/PM). The used reference was a Colletotrichum sp. from within the Colletotrichum acutatum species complex obtained from Damm et al. [Citation9]. Species isolated in this study are shown in different color blocks.
![Figure 3. A 50% majority rule consensus tree obtained by Bayesian inference analysis based on ITS, TUB2, GAPDH, and CHS-1 sequence alignment. Bayesian posterior probability values ≥ 0.5 and bootstrap support values ≥ 50% are shown at the nodes (PP/ML/PM). The used reference was a Colletotrichum sp. from within the Colletotrichum acutatum species complex obtained from Damm et al. [Citation9]. Species isolated in this study are shown in different color blocks.](/cms/asset/724a0a33-c8a5-42d2-96cb-bbcaaa6b916e/tmyb_a_1763116_f0003_c.jpg)
3.2. Morphological characterization
Distinct morphological characteristics, including colony color and conidial shape, were observed in the isolates of C. gloeosporioides s.l. (C. fructicola and C. siamense) and C. acutatum s.l. (C. fioriniae). Pinkish colony and fusiform conidia of C. fioriniae were easily disguisable from those of C. fructicola and C. siamense (). The conidia of both C. fructicola and C. siamense isolates were almost similar in terms of conidial shape, including cylindrical and obtuse end; however, they can be distinguished from each other by colony color (). The colony color of C. siamense isolates was whitish to gray, whereas that of C. fructicola was olivaceous with a white margin. The size of the conidia of C. siamense isolate was 13.12–20.15 µm × 5.0–7.8 µm (mean ± SD = 17.3 ± 1.5 × 6.2 ± 0. 8 µm), C. fructicola isolate was 12.8–20. 2 × 4.4–7.00 µm (mean ± SD = 16.3 ± 1.1 × 5.1 ± 1.0 µm), and C. fioriniae isolate was 8.1–15.1 × 4.3–7.0 µm (mean ± SD = 12.1 ± 1.3 × 5.4 ± 0.6 µm) (). There was little difference among the present isolates (C. fructicola, C. siamense, and C. fioriniae) in terms of appressoria shape and size (; ). The size of appressoria of C. siamense isolate was 7.1 to 13.2 × 7.1 to 8.9 µm (mean ± SD = 11.0 ± 1.9 × 7.9 ± 1.0 µm), C. fructicola isolate was 8.5–12.0 × 6.4–8.3 µm (mean ± SD = 9.2 ± 1.5 × 7.1 ± 0.5 (µm), and C. fioriniae isolate was 8.5–15.2 × 4.9–9.4 µm (mean ± SD = 12.5 ± 1.5 × 8.2 ± 1.1 µm) ().
Figure 4. Morphological characteristics of present isolates belonging to different Colletotrichum species. (A–C) A 7-day-old colony on PDA; (D–F) Conidia. G-I. Appressoria.
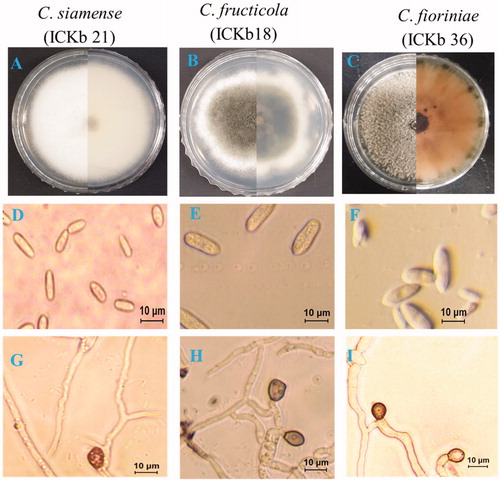
Table 2. Morphological characterization of representative Colletotrichum spp. isolates identified in this study.
3.3. Pathogenicity assay
Three isolates (one from each Colletotrichum species) were selected for confirming Koch’s postulates with a spore suspension on detached peach fruits. All Colletotrichum species were pathogenic irrespective of the lesion size and % disease incidence under both wounded and unwounded conditions (). C. fructicola, C. siamense, and C. fioriniae were more virulent under wounded conditions than under unwounded conditions. The average DI of each isolate was 100% under wounded condition and 30%–50% under unwounded condition. The average LD of each isolate ranged from 9 mm to 22 mm under wounded condition and 2 to 3 mm under unwounded condition. LD produced by all isolate in unwounded condition were statistically insignificant. The average LD value of C. fioriniae was 9.5 mm, which was significantly smaller than that of C. siamense or C. fructicola. The LD value of C. siamense was 18.7 mm and C. fructicola was 21.5 mm ().
Table 3. Disease incidence and lesion diameter produced by different Colletotrichum on apple fruit.
4. Discussion
The genus Colletotrichum was first introduced to the family Glomerellaceae (Glomerellales, Sordariomycetes) by Corda in 1831 [Citation25]. Since then, numerous species have been introduced to this genus. Many species have been introduced, typified, and synonymized through several revisions of the genus Colletotrichum in the past decade, and this process is still going on. Species belonging to Colletotrichum can be endophytic, parasitic, or saprobic [Citation8,Citation9,Citation26–29]. Colletotrichum species are currently identified based on morphological and molecular analyses. Identification of Colletotrichum species solely based on morphological characteristics is not reliable and may lead to misidentification [Citation8,Citation26]. Therefore, mycologist recommend using a polyphasic approach, including the analysis of geographical, ecological, morphological, and genetic data to identify the pathogen at the species level [Citation26,Citation27]. DNA sequence data are very crucial to identify species diversity within a genus. Phylogenetic analysis using concatenated sequences of multiple genes is a proven powerful tool for delimitation of species within a genus [Citation26–28].
The main objective of this study was to identify Colletotrichum species associated with peach anthracnose using morphological and multi-locus phylogenetic analyses and pathogenicity tests to confirm Koch’s postulates. This study revealed three species belonging to the C. gloeosporioides complex (C. siamense and C. fructicola) and the C. acutatum complex (C. fioriniae). Among these, C. siamense was confirmed to be associated with peach anthracnose for the first time in South Korea. The isolates of C. siamense and C. fructicola were morphologically typical of C. gloeosporioides s.s. (mean species) responsible for peach anthracnose according to Kim and Hong [Citation11]. However, Kim and Hong [Citation11] identified C. gloeosporioides s.s. based on morphological characteristics alone, which cannot unequivocally identify an isolate at the species level. Reportedly, 22 species and one subspecies within C. gloeosporioides s.l. (species complex) were micromorphologically almost similar [Citation8]. Eight gene sequences were used to separate the species within C. gloeosporioides s.l. by Weir et al. [Citation8]. In this study, we combined ITS, TUB2, GAPDH, CHS-1, and CAL sequence data for solid identification of isolates from peach to be either C. siamense or C. fructicola. Many scholars suggested that not all eight gene sequences are required to reliably separate Colletotrichum species within C. gloeosporioides s.l. [Citation6]. Weir et al. [Citation8] suggested that ITS, TUB2, GAPDH, CHS-1, and CAL gene sequence data are effectively distinguished between C. siamense and C. fructicola. Hu et al. [Citation6] used concatenated sequences of TUB2, GAPDH, and CAL for clearly identifying C. siamense and C. fructicola isolates by comparing them against sequences available in GenBank. Another important fact of this study is the use of ApMat gene sequence to confirm C. siamense isolates (). The sequences of ApMat gene are a powerful tool for the identification of C. siamense [Citation8,Citation20]. C. siamense was isolated from diseased fruits for the first time. In a previous study, we have described C. fructicola as the causal agent of peach anthracnose [Citation30].
Some isolates from peach were also identified as C. fioriniae, which belongs to the C. acutatum complex (clade 3). The morphological characteristics, including pink colony and fusiform conidia, of present isolates are very typical and similar to those of C. fioriniae, as reported by Damm et al. [Citation9]. Thirty-one species have been excepted within the C. acutatum complex by Damm et al. [Citation9]. They used concatenated sequences of ITS, ACT, TUB2, CHS-1, GAPDH, and HIS3 for delineating species within the C. acutatum complex, whereas, in this study, we used combined sequences of ITS, TUB2, GAPDH, and CHS-1 for identifying C. fioriniae isolates. The only representative species of clade 3 which is distinct within the C. acutatum complex is C. fioriniae, and it can be separated using any sequence of ITS, ACT, TUB2, CHS-1, GAPDH, and HIS3 [Citation9]. The isolates of C. fioriniae from peach were easily distinguished from those of the C. gloeosporioides complex (C. siamense and C. fructicola) because of their pinkish colony ().
C. siamense, C. fructicola, and C. fioriniae are believed to have a wide range of hosts [Citation8,Citation9]). In Korea, these three species have been frequently reported as causal agents of anthracnose affecting different fruit crops, such as apple, Japanese plum, and persimmon [Citation13,Citation14,Citation31,Citation32]. Pathogenic C. siamense has previously been isolated from Diospyros kaki, Malus domestica, and Prunus salicina [Citation13,Citation14,Citation31], C. fructicola from Malus domestica and Prunus salicina [Citation13,Citation14,Citation31,Citation32], and C. fioriniae from Lycium chinense, Prunus salicina, Prunus persica, and Solanum melongena in South Korea [Citation13,Citation14,Citation31,Citation33,Citation34].
The result of the pathogenicity test suggests that all the Colletotrichum species described in this study are virulent on detached peach fruits irrespective of DI and DL (). All the three Colletotrichum species were re-isolated from artificially inoculated peach fruits and identified as the same species based on morphological characteristics, thus confirming Koch’s postulates.
Figure 5. Anthracnose symptoms on naturally and artificially inoculated peach fruits. (A) Symptomatic fruits in peach orchard; (B) Colletotrichum siamense (Isolate ICKb21); (C) Colletotrichum fructicola (ICKb18); (D) Colletotrichum fioriniae (ICKb 36).

In conclusion, this study provides the first morphological, molecular, and pathological characterization of Colletotrichum species in association with peach anthracnose in South Korea. More specifically, this study is also the first report on peach anthracnose caused by C. siamense in South Korea.
Acknowledgements
We would like to thank all of our lab colleagues for their precious help during the study period.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jung KM, Kim SY, Seo EC, et al. Influence of peach (Prunus persica L. Batsch) fruit addition on quality characteristics and antioxidant activities of beer. ijSciences. 2017;3(08):186–191.
- Cao S, Song C, Shao J, et al. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J Agric Food Chem. 2016;64(25):5215–5222.
- Manzoor M, Anwar F, Mahmood Z, et al. Variation in minerals, phenolics and antioxidant activity of peel and pulp of different varieties of peach (Prunus persica l.) fruit from Pakistan. Molecules. 2012;17(6):6491–6506.
- Yang Z, Ma Y, Chen L, et al. Differential transcript abundance and genotypic variation of four putative allergen-encoding gene families in melting peach. Tree Genet Genom. 2011;7(5):903–916.
- Luo J, Butelli E, Hill L, et al. Atmyb12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008;56(2):316–326.
- Hu MJ, Grabke A, Schnabel G. Investigation of the Colletotrichum gloeosporioides species complex causing anthracnose fruit rot of peach in South Carolina. Plant Dis. 2015;99(6):797–805.
- Lee D, Hassan O, Kim C, et al. First report of peach (Prunus persica) anthracnose caused by Colletotrichum fioriniae in Korea. Plant Dis. 2018;102(12):2650.
- Weir BS, Johnston PR, Damm U. The Colletotrichum gloeosporioides species complex. Stud Mycol. 2012;73:115–180.
- Damm U, Cannon PF, Woudenberg JHC, et al. The Colletotrichum acutatum species complex. Stud. Mycol. 2012;73:37–113.
- De Silva DD, Ades PK, Crous PW, et al. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017;66(2):254–267.
- Kim WG, Hong SK. Occurrence of anthracnose on peach tree caused by Colletotrichum species. Plant Pathol J. 2008;24(1):80–83.
- KSPP (Korean Society of Plant Pathology). List of plant diseases in Korea. 5th ed. Seoul: Korean Society of Plant Pathology; 2009.
- Hassan O, Jeon JY, Chang T, et al. Molecular and morphological characterization of Colletotrichum species in the Colletotrichum gloeosporioides complex associated with persimmon anthracnose in South Korea. Plant Dis. 2018;102(5):1015–1024.
- Hassan O, Lee YS, Chang T. Colletotrichum olletotrichume, Y. S. and rsimmon anthrPrunus salicina) Anthracnose in South Korea. Sci Rep. 2019;9(1):12089.
- Fu M, Crous PW, Bai Q, et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia. 2019;42(1):1–19.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315–322.
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330.
- Templeton MD, Rikkerink EHA, Solon SL, et al. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene. 1992;122(1):225–230.
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–556.
- Silva DN, Talhinhas P, Várzea V, et al. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycologia. 2012;104(2):396–409.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729.
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.75. 2011. Available from: http://mesquiteproject.org.
- Ronquist F, Teslenko M, van der Mark P, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542.
- Rambaut A, Drummond A. FigTree v1. 3.1: tree figure drawing tool. Edinburgh, UK: Institute of Evolutionary Biology; 2009. Available from: http://tree.bio.ed.uk/software/figtree.
- Corda A. Die Pilze Deutschlands. In: Sturm J, editor. Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen. Vol. 3. Nürnberg: Sturm; 1831.p. 21–32.
- Jayawardena RS, Hyde KD, Damm U, et al. Notes on currently accepted species of Colletotrichum. Mycosphere. 2016;7(8):1192–1260.
- Cannon PF, Damm U, Johnston PR, et al. Colletotrichum current status and future directions. Stud Mycol. 2012;73:181–213.
- Hyde KD, Cai L, McKenzie EHC, et al. Colletotrichum: a catalogue of confusion. Fungal Diver. 2009;39:1–17.
- Liu F, Wang M, Damm U, et al. Species boundaries in plant pathogenic fungi: a Colletotrichum case study. BMC Evol Biol. 2016;16(1):81.
- Lee D, Hassan O, Kim C, et al. First report of peach (Prunus persica) anthracnose caused by Colletotrichum fructicola in Korea. Plant Dis. 2020;104(5):1556–1556.
- Oo MM, Yoon HY, Jang HA, et al. Identification and characterization of Colletotrichum species associated with bitter rot disease of apple in South Korea. Plant Pathol J. 2018;34(6):480–489.
- Kim C, Hassan O, Lee D, et al. First report of anthracnose of apple caused by Colletotrichum fructicola in Korea. Plant Dis. 2018;102(12):2653–2653.
- Oo MM, Oh SK. Identification and characterization of new record of grape ripe rot disease caused by Colletotrichum viniferum in Korea. Mycobiology. 2017;45(4):421–425.
- Xu SJ, Aktaruzzaman M, Kim BS, et al. First report of anthracnose caused by Colletotrichum fioriniae on eggplant fruits in Korea. Plant Dis. 2018;102(12):2642–2642.
