Abstract
The reexamination of the fungal genus Botryosphaeria on 12 plant species of 10 families was carried out based on molecular phylogenetic analyses using the regions of translation elongation factor 1-α, β-tubulin, DNA-directed RNA polymerase II subunit, and internal transcribed spacer region of rDNA and morphological characteristics. Japanese isolates were divided into five clades and include Botryosphaeria dothidea, B. qingyuanensis, B. sinensis, and Botryosphaeria spp. Two species, B. qingyuanensis and B. sinensis have been newly added to the Japanese mycoflora, but their host plants are not specified. Botryosphaeria tenuispora isolated from Leucothoe fontanesiana and insect galls on fruits of Aucuba japonica has been proposed as a new species.
1. Introduction
Genus Botryosphaeria (Botryosphaeriaceae, Botryosphaeriales) was introduced by Cesati and de Notaris [Citation1]. Botryosphaeria has been known to be a plant pathogenic, endophytic, and saprobic fungus [Citation2–5]. Some species of this genus cause diseases of crops and economic impact on forests and useful trees worldwide [Citation6]. However, some species are known to behave as opportunistic pathogens with weak symptoms or endophytes without symptoms under stressful conditions [Citation6]. Several researchers have discussed these various niches. Marsberg et al. [Citation7] discussed the distinction between the endophyte and the latent pathogen for parts of their life cycle and concluded that it is of little value. Moreover, symbiotic relationships among the host plants, insects inhabiting the gall, and Botryosphaeria spp. have been discovered [Citation8–10].
Botryosphaeria dothidea, a type species of the genus Botryosphaeria, is known for its cosmopolitan distribution and numerous hosts [Citation4,Citation6,Citation7]. Slippers et al. [Citation11] reexamined the B. dothidea based on molecular phylogeny and phenotypic characteristics and proposed several species for those previously identified as B. dothidea. They also emended the species concept with a newly designated epitype of B. dothidea. Thereafter, several species have been described as follows: Botryosphaeria agaves, B. auasmontanum, B. corticis, B. fabicerciana, B. fusispora, B. guttulata, B. kuwatsukai, B. minutispermatia, B. pseudoramosa, B. qingyuanensis, B. ramosa, B. rosaceae, B. scharifii, B. sinensis, and B. wangensis. However, the taxonomical positions of numerous species of Botryosphaeria described without phylogenetic data is still unclear [Citation12,Citation13].
In Japan, according to the database of the common names of plant diseases in Japan [Citation14], 14 species of the genus Botryosphaeria cause diseases of 30 plant species of 21 families. In our previous studies [Citation15], molecular and phylogenetic analyses using the large ribosomal subunit of rDNA (LSU) and DNA-directed RNA polymerase II subunit (RPB2) regions suggested that 9 of 20 isolates identified previously as isolates of the genus Botryosphaeria were that of the genus Neofusicoccum and 9 of 10 isolates of the genus Dothiorella were that of the genus Botryosphaeria. Therefore, in this study, the isolates kept as Botryosphaeriaceae in culture collections were reexamined for their taxonomical position based on multi-locus molecular and phylogenetic analyses using the internal transcribed spacer (ITS) region of rDNA, RPB2, translation elongation factor 1-α (TEF1-α), and β-tubulin (TUB2) and morphological characteristics on host plants and media.
2. Materials and methods
2.1 Sample collection and morphological study
Twenty-four isolates identified as Botryosphaeria and Dothiorella species kept at the Laboratory of Forest Pathology, Forestry and Forest Products Research Institute (Tsukuba, Ibaraki, Japan), the Institute of Fruit Tree and Tea Science, National Agriculture and Food Research Organization (Tsukuba, Ibaraki, Japan), and the Culture Collection of the Laboratory of Phytopathology, Mie University (Tsu, Mie, Japan) were examined. These isolates included those from various host plants and insect galls (). These isolates were cultivated on potato dextrose agar (PDA) medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) or malt agar (Becton Dickinson, Franklin, NJ) at room temperature under room light diffusion. To observe conidiomata and conidia, the isolates were transferred to boiled mulberry agar (BMA [Citation20]). In brief, mulberry leaves were cut into 5 cm squares, boiled for 30–60 s, and dried on a paper towel. These leaves were placed on water agar medium. Mycelial disks containing Botryosphaeria isolates, which had been cultivated for 1 week on PDA, were transferred onto BMA and cultivated for 1 week to 3 months at room temperature under room light diffusion. The specimens were deposited at the Mycological Herbarium at Mie University (MUMH). The examined isolates were maintained at the Culture Collection of Mycological Herbarium, Mie University (MUCC; Tsu, Mie, Japan).
Table 1. List of Japanese Botryosphaeria isolates used in this study.
2.2 Molecular and phylogenetic analyses
Genomic DNA was extracted from mycelial disks after 7 days of culture on PDA plates with DNeasy Ultra Clean Microbial Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Targeted sequences of the ITS region of rDNA and TEF1-α, TUB2, and RPB2 gene-coding regions were amplified using the T100 Thermal Cycler (Bio-Rad, Tokyo, Japan) via polymerase chain reaction (PCR). The total volume of the PCR mixture was 12.5 µL; it consisted of 1–10 ng of genomic DNA, 0.05 µL of 0.25 unit Taq DNA polymerase (Bioline, London, UK; TEF1-α 0.1 µL and RPB2 0.1 µL), 1.25 µL of 10× NH4 reaction buffer (Bioline), 1.9–2.5 mM MgCl2 (Bioline; ITS, RPB2, and TEF1-α 2.5 mM and TUB2 1.9 mM), 2.5–5.0 mM each of deoxyribonucleotide triphosphate mixture (Bioline; ITS 2.5 mM and TEF1-α, TUB2, and RPB2 5.0 mM), 0.2 µM of each primer, and 5.6% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO), which was added only for TEF1-α amplification, and sterilized distilled water up to 12.5 µL.
The PCR conditions were as follows: for ITS: initial denaturation (94 °C, 5 min), 40 cycles of amplification (denaturation 94 °C, 45 s; annealing 48 °C, 30 s; and extension 72 °C, 90 s), and final extension (72 °C, 2 min); for TEF1-α: initial denaturation (94 °C, 5 min), 40 cycles of amplification (denaturation 94 °C, 30 s; annealing 52 °C, 30 s; and extension 72 °C, 45 s), and final extension (72 °C, 2 min); for TUB2: initial denaturation (94 °C, 5 min), 40 cycles of amplification (denaturation 94 °C, 30 s; annealing 52 °C, 30 s; and extension 72 °C, 60 s), and final extension (72 °C, 2 min); and for RPB2: initial denaturation (95 °C, 5 min), touch-down amplification (5 cycles of 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 120 s; 5 cycles of 95 °C for 45 s, 58 °C for 45 s, and 72 °C for 120 s; and 30 cycles of 95 °C for 45 s, 54 °C for 45 s, and 72 °C for 120 s), and final elongation at 72 °C for 8 min. The primer sets are shown in . The amplicon was sequenced in both directions using the respective PCR primers and the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) on an Applied Biosystems 3730xl DNA analyzer installed at the Mie University Advanced Science Research Promotion Center (Tsu, Mie, Japan). The sequences were assembled and aligned with 16 sequences of the Botryosphaeria spp. collected from GenBank using the software MAFFT version 7 [Citation21].
Table 2. PCR primer sets and annealing temperatures.
Maximum likelihood (ML) and Bayesian inference (BI) analyses were used to estimate phylogenetic relationships. ML analyses were performed using raxml HPC-PTHREADS [Citation22]. The strength of the internal branches from the resultant trees was tested by bootstrap analysis [Citation23] using 1000 replications. BI analyses were performed using BEAST version 2.5.1 [Citation24] to estimate the posterior probabilities (PPs) of tree topologies based on the metropolis-coupled Markov chain Monte Carlo (MCMC) searches, which used the MCMC algorithm of four chains in parallel from a random tree topology. The MCMC analysis lasted 10,000,000 generations. Trees were sampled and saved every 1000 generations. The first 25% of the saved trees were discarded, representing the “burn-in” phase, and the PPs were determined from the remaining trees. Representative sequences for all taxa were uploaded to GenBank (). Sequence alignments prepared in this study were deposited in TreeBASE number 26984.
Table 3. List of Botryosphaeria species used for phylogenetic analysis.
3. Results
3.1 Phylogeny
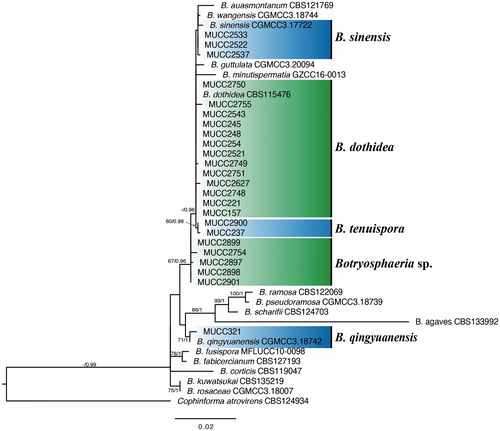
The ITS + TEF1-α + TUB2 + RPB2 combined data matrix of 41 sequences consisted of 1756 characters (ITS: 536, TEF1-α: 280, RPB2: 576, and TUB2: 364) . Cophinforma atrovirens (CBS 124934) was selected as the out taxon. The resultant ML tree is shown in . The topologies of the generated trees from ML and BI analyses were congruent. As a result of the phylogenetic analysis, Japanese isolates formed five groups with the hitherto known species or newly recognized species. These are B. dothidea (MUCC 157, MUCC 221, MUCC 245, MUCC 248, MUCC 254, MUCC 2521, MUCC 2543, MUCC 2627, MUCC 2748–2751, and MUCC 2755), B. tenuispora (MUCC 237 and MUCC 2900), B. qingyuanensis (MUCC 321), B. sinensis (MUCC 2522, MUCC 2533, and MUCC 2537), and Botryosphaeria sp. (MUCC 2754, MUCC 2897–2899, and MUCC 2901).
3.2 Taxonomy
Botryosphaeria dothidea (Moug. ex Fr.) Cesati & De Notaris, Commentario della Società Crittogamologica Italiana 1: 212, 1863.
Teleomorphic state: It has been reported by Slippers et al.[Citation11].
Anamorphic state on the host plants: Conidiomata solitary, globose, dark brown to dark gray, covered with white to dark green hyphae, 419–490 × 355–437 µm; pycnidial wall composed of depressed or irregular cells in five to eight layers, brown to dark brown, blackish around an ostiole, paler toward the conidiogenous region; paraphyzes hyaline, rounded at the apex, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, smooth, phialidic conidiogenesis with periclinal thickening, or holoblastic conidiogenesis after percurrent proliferation at the tip, 1.3–16.3 × 1.5–3.4 µm. Conidia solitary, fusiform to subfusiform, rounded at the apex, convex to truncate at the base, hyaline, aseptate or rarely one-septate, smooth, with granular contents, 15–36 × 3.3–8.4 µm, L/W = 3.45 (min 2.60, max 5.51; n = 101).
Cultural characteristics on PDA: Colonies were gray to dark gray with dense aerial mycelia, reaching 90 mm at 7 days after inoculation.
Host: Prunus sp., Rosa sp. [Citation11], Castanea crenata, Daphniphyllum macropodum, Eucalyptus viminalis, Leucothoe catesbaei, Lindera obtusiloba, Pyrus pyrifolia, Prunus persica, Prunus sp., Saxifraga stolonifera (this study).
Materials examined: on Daphniphyllum macropodum, Japan, Aichi, Nagoya, 14 Nov 2005, by I. Araki & K. Motohashi (MUMH 10467, culture MUCC 157); on Leucothoe catesbaei, ibid, June 19, 2006, by I. Araki & K. Motohashi (MUMH 10395, culture MUCC 221); on Daphniphyllum macropodum, ibid, 18 Jul 2006, by I. Araki & K. Motohashi (MUMH 10425, culture MUCC 245); on Lindera obtusiloba, ibid, 18 Jul 2006, by I. Araki & K. Motohashi (MUMH 10429, culture MUCC 248); on Saxifraga stolonifera, ibid, 18 Jul 2006, by I. Araki & K. Motohashi (MUMH 10437, culture MUCC 254); on Prunus sp., Japan, Ibaraki, Tsukuba, May 1993, by T. Yamada (culture MUCC 2521 = MAFF 410826); on Eucalyptus viminalis, Japan, Tokyo, Koto, 2 Jul 1986, by unknown (culture MUCC 2543 = FFPRI 411204); on Pyrus pyrifolia, Japan, Mie, Tsu, 9 Aug 2018, by Y. Hattori (culture MUCC 2627); on Castanea crenata, Japan, Ibaraki, Tsukuba, 4 Sep 2017, by A. Sasaki (culture MUCC 2748 = NP-004); on C. crenata, Japan, Kumamoto, Uki, 8 Sep 2017, by Y. Fukunaga (culture MUCC 2749 = K-001); on Castanea crenata, ibid, 8 Sep 2017, by Y. Fukunaga (culture MUCC 2750 = K-004); on Prunus persica, Japan, Ibaraki, Tsukuba, 27 Jan 2017, by H. Nakamura (culture MUCC 2751; and on Castanea crenata, Japan, Kumamoto, Uki, 8 Sep 2017, by Y. Fukunaga (culture MUCC 2755 = K-027).
Thirteen Japanese isolates were identified as B. dothidea based on their phylogenetic analyses and morphological characteristics. The morphology of conidia varied, with an L/W ratio of 3.4–5.6 (). All isolates grew well and formed conidiomata and conidia on the BMA medium.
Table 4. Morphological characteristics of the genus Botryosphaeria.
Botryosphaeria qingyuanensis G.Q. Li & S.F. Chen, Persoonia 40: 83, 2008.
Teleomorphic state: It has not been reported.
Anamorphic state on the host plants: Symptoms brown to reddish-brown, small at the edge of the leaf, later enlarged and coalescent, expanded toward the whole leaf. Conidiomata amphigenous, epidermal, merged, solitary, scattered, black to dark brown, ellipsoid, 105–146.5 × 87–132 µm; pycnidial wall composed of depressed or irregular cells in three to five layers, brown to dark brown, blackish around an ostiole, paler toward the conidiogenous region; paraphyzes hyaline, rounded at the apex, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, phialidic conidiogenesis, or holoblastic conidiogenesis after percurrent proliferation at the tip, smooth, 6.3–12.8 × 1.4–2.5 µm (n = 10). Conidia solitary, fusiform to ellipsoid, obtuse at both ends, hyaline, aseptate, smooth, with granular contents, 17–24 × 3.6–6.5 µm, L/W = 4.16 (min 2.96, max 6.12; n = 25).
Cultural characteristics on PDA: Colonies were gray to dark gray with dense aerial mycelia, reaching 90 mm at 7 days after inoculation.
Host: Eucalyptus hybrid [Citation27], Gamblea innovans (this study).
Material examined: on Gamblea innovans, Japan, Aichi, Nagoya, 14 Nov 2005, by I. Araki & K. Motohashi (MUMH 10273, culture MUCC 321).
From a phylogenetic analysis, MUCC 321 formed the same clade as B. qingyuanensis (CGMCC 3.18742). The width of the conidia of MUCC 321 was slightly narrower than that of B. qingyuanensis [MUCC 321: 17.5–24 × 3.6–6.5 vs. CGMCC 3.18742: (15) 19.5–24.5 (28.5) × (5) 6–6.5 (7.5); Li et al. 2018]. Botryosphaeria qingyuanensis was isolated from the twigs of a Eucalyptus tree in China and known only from the type locality. MUCC 321 was isolated from the leaf spots on G. innovans. This study was the first report of the new habitat and host plant from Japan.
Botryosphaeria sinensis Y.P. Zhou & Y. Zhang ter, Phytotaxa 245: 45, 2016.
Teleomorphic state: It has been reported [Citation28].
Anamorphic state formed on BMA: Conidiomata formed within 7 days, solitary or aggregate, globose to subglobose, dark brown to dark gray, covered with white to dark green hyphae, 304–382 × 316–400 µm; pycnidial wall composed of depressed or irregular cells in three to five layers, brown to dark brown, blackish around the ostiole, paler toward the conidiogenous region; paraphyzes hyaline, rounded at the apex, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, smooth, phialidic conidiogenesis with periclinal thickening, or holoblastic conidiogenesis after percurrent proliferation at the tip 1.6–2.6 × 6.8–11.2 µm (n = 3). Conidia solitary, fusiform, or irregularly fusiform, rounded at the apex, convex to truncate at the base, hyaline, aseptate or ralely one-two septate, smooth with granular contents, 16–23 × 4.8–6 µm, L/W = 3.72 (min 3.27, max 4.04; n = 5).
Cultural characteristics on PDA: Colonies were gray to dark gray with dense aerial mycelia, reaching 90 mm at 7 days after inoculation.
Host: Juglans regia, Morus sp., Populus sp. [Citation28], Paulownia tomentosa, Prunus sp. (this study).
Materials examined: on Prunus sp., Japan, Ibaraki, Tsukuba, May 1993, by T. Yamada (culture MAFF 410827 = MUCC 2522); on Aucuba japonica, Japan, Tokyo, Minato, 12 Feb 1980, by T. Konayashi (culture MUCC 2533 = FFPRI 411202); and on Paulownia tomentosa, Japan, Niigata, Uonuma, 10 Jul 1978, by H. Hayashi (culture MUCC 2537 = FFPRI 411203).
Note: From the results of the molecular and phylogenetic analyses, three examined isolates were located in the same clade composed of ex-type isolates of B. auasmontanum (CBS 121769), B. sinensis (CGMCC 3.17722), and B. wangensis (CGMCC 3.18744). This clade was statistically supported only in Bayesian trees. Botryosphaeria sinensis, B. wangensis, MUCC 2522, MUCC 2533, and MUCC 2537 formed an inner clade supported moderately with a PP value of 0.91. The conidia size of MUCC 2522 (16–23 × 4.8–6) was somewhat smaller than B. sinensis [(15) 19–29 × 5–7] and B. wangensis [(20.5) 22–26 (29) × (4.5) 5.5–6.5 (7.5); Zhou et al. and Li et al. [Citation24,Citation27]]. The ITS and TEF1-α sequences of MUCC 2522 were identical to B. sinensis. Also, the conidia of MUCC 2537 (14–27 × 3–5.8) were narrower than that of B. sinensis and B. wangensis (). Only a few mutations were observed in the TEF1-α regions of MUCC 2537 compared to B. sinensis.
Botryosphaeria tenuispora Y. Hattori & C. Nakashima, sp. nov. [MB837514], .
Figure 2. Morphological features of Botryosphaeria tenuispora [A–F: MUMH 10420 (MUCC 237) and G–I: MUCC 2900]. (A) Specimen MUMH 10420. (B) Symptoms with pycnidia forming on the leaf of Leucothoe fontanesiana. (C) Vertical section of pycnidium in the leaf tissue. (D) Conidia and conidiophores. (E, F) Conidia. (G) Conidiomata formation on BMA after 7 days. (H) Conidiomata. (I) Conidium and conidiophores. (J) Conidium. Scale bars, 200 μm (C and H) and 25 μm (D–F and I–J).
![Figure 2. Morphological features of Botryosphaeria tenuispora [A–F: MUMH 10420 (MUCC 237) and G–I: MUCC 2900]. (A) Specimen MUMH 10420. (B) Symptoms with pycnidia forming on the leaf of Leucothoe fontanesiana. (C) Vertical section of pycnidium in the leaf tissue. (D) Conidia and conidiophores. (E, F) Conidia. (G) Conidiomata formation on BMA after 7 days. (H) Conidiomata. (I) Conidium and conidiophores. (J) Conidium. Scale bars, 200 μm (C and H) and 25 μm (D–F and I–J).](/cms/asset/d9effbc6-f09a-4665-b7bb-96de691fffaa/tmyb_a_1895486_f0002_c.jpg)
Etymology: Name derived from the shape of the slender conidia.
Teleomorphic state: Unknown.
Anamorphic state formed on the host: Leaf spots brown to yellowish-brown, small at the edge, later enlarged and coalescent, expanded toward the whole of a leaf. Conidiomata epidermal, merged, solitary, scattered, black to dark brown, ellipsoid, 446.68 × 476.03 µm; pycnidial wall composed of depressed or irregular cells in five to eight layers, brown to dark brown, blackish around the ostiole, paler toward the conidiogenous region; paraphyzes hyaline, rounded at the apex, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, phialidic conidiogenesis with periclinal thickening, or holoblastic conidiogenesis after percurrent proliferation at the tip, smooth, 8.8–26.5 × 1.9–4.4 µm. Conidia fusiform to cylindro-clavate, rounded at the apex, convex to truncate at the base with fine frill, hyaline, aseptate, smooth, with granular contents, 23–32 × 4–6.7 µm, L/W = 5.40 (min 3.51, max 6.85; n = 115).
Cultural characteristics on PDA: Colonies were gray to dark gray with dense aerial mycelia, reaching 90 mm at 7 days after inoculation. On BMA: Conidiomata formed within 7 days, solitary or aggregate, dark brown to dark gray, covered with white, yellowish-green, to dark green hyphae, 287–635 × 266–597 µm (MUCC 2900).
Holotypus: on Leucothoe catesbaei, Japan, Aichi, Nagoya, 18 Jul 2006, by I. Araki (MUMH 10420, ex-type culture MUCC 237).
Host: Aucuba japonica, Leucothoe catesbaei (this study).
Materials examined: on Leucothoe catesbaei, Japan, Aichi, Nagoya, 18 Jul 2006, by I. Araki (MUMH 10420, culture MUCC 237); from the fruit gall induced by Asphondylia aucubae on Aucuba japonica, Japan, Ibaraki, Tsukuba, May 23, 2019, by N. Uechi (culture MUCC 2900 = 18-2).
Note: On the resultant tree of molecular and phylogenetic analyses, this species formed a single clade. The clade composed of the two examined isolates was moderately supported by the statistical values of ML and BI (ML BS: 68, BI PP: 0.98). This species is phylogenetically closely related to B. auasmontanum, B. dothidea, B. minutispermatia, B. sinensis, and B. wangensis. However, the L/W ratio of B. tenuispora was bigger than that of the hitherto known species in the same clade (). Moreover, the size of the conidia of B. tenuispora (23–32 × 4–6.7 µm) was larger than that of B. auasmontanum [(8.1) 8.8–11.3 (13) × (2.5) 2.9–3.9 (5) µm] and B. minutispermatia (8–14 × 3–4; [Citation25,Citation26]).
Botryosphaeria sp., .
Figure 3. Morphological features of Botryoshaeria sp. (A and B: MUCC 2899). (A) fruit galls by Asphondylia aucabae on Aucuba japonica. (B) Conidiomata formation on the BMA after 7 d. (C) Vertical section of pycnidium in the leaf tissue. (D–E) Conidia and conidiophores. (F–G) Conidia. Scale bars, 200 μm (C) and 25 μm (D–G).

Teleomorphic state: Unknown.
Anamorphic state formed on the host: Conidiomata formed on BMA within 7 days, solitary or aggregate,dark brown to dark gray, covered with white to dark green hyphae, globose to ellipsoid, 252–712 × 208–422 µm; pycnidial wall composed of depressed or irregular cells in five to eight layers, brown to dark brown, blackish around the ostiole, paler toward the conidiogenous region; paraphyzes hyaline, rounded at the apex, septate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, smooth, phialidic conidiogenesis with periclinal thickening, or holoblastic conidiogenesis after percurrent proliferation at the tip, 8.5–16.8 × 1.3–3.1 µm. Conidia fusiform to cylindro-clavate, rounded at the apex, convex to truncate at the base with fine frill, hyaline, aseptate, smooth, with granular contents, 14.6–29 × 3.2–6.2 µm, L/W = 4.38 (min 3.39, max 5.88; n = 100).
Cultural characteristics on PDA: Colonies were gray to dark gray with dense aerial mycelia, reaching 90 mm at 7 days after inoculation.
Host: Aucuba japonica, Castanea crenata.
Materials examined: from a pupa of Asphondylia aucubae, Japan, Ibaraki, Tsukuba, April 29, 2019, by N. Uechi (culture MUCC 2897 = 2); fruit galls induced by Ashpondylia aucubae on Aucuba japonica, ibid, May 23, 2019, by N. Uechi (culture MUCC 2898 = 17-2); fruit galls induced by Ashpondylia aucubae on Aucuba japonica, ibid, May 23, 2019, by N. Uechi (culture MUCC 2899 = 21-1); fruit galls induced by Ashpondylia aucubae on Aucuba japonica, ibid, May 23, 2019, by N. Uechi (MUCC 2901 = 23-1); and on C. crenata, Japan, Kumamoto, Uki, 8 Sep 2017, by Y. Fukunaga (culture MUCC 2754 = K-018).
Note: All isolates, except MUCC 2754, were obtained from the insect (Asphondylia aucubae) galls and pupa, which were induced on fruit of A. japonica. In contrast, MUCC 2754 was isolated from diseased chestnuts. The relationship among these isolates is unclear. On the Bayesian tree, these isolates were recognized as an independent clade but were supported with a somewhat weak PP value (0.79). This suggested that it should be treated as a species.
4. Discussion
In this study, isolates of the genus Botryosphaeria in Japan were reexamined for their taxonomical position based on molecular phylogeny and morphology. As a result, these isolates were divided into five clades: B. dothidea, B. qingyuanensis, B. sinensis, B. tenuispora, and Botryosphaeria spp. Botryosphaeria qingyuanensis and B. sinensis have been newly added to the Japanese mycoflora. Botryosphaeria tenuispora was described as a new species based on its phylogenetic position and morphological characteristics of the conidia. Although B. dothidea is known as a polyxenic species, it was confirmed that plural Botryosphaeria sp. were sharing one host plant species, B. dothidea and B. sinensis infected and established the habitat on Prunus sp., B. dothidea and Botryosphaeria sp. were from C. crenata, B. tenuispora and Botryosphaeria sp. were from A. japonica, and B. dothidea and B. tenuispora were from Leucothoe fontanesiana. In contrast, the current taxonomic position of the hitherto known Japanese species, such as B. laricina that causes the shoot blight of genus Larix [Citation29,Citation30] and B. yedoensis that inhabits Prunus spp. [Citation31], are still unclear. More detailed studies based on phylogeny and morphology are required.
Botryosphaeria spp. are often isolated from the insect gall. The relationships between gall midges and host plants have been discussed. Asphondylia species on Acacia and B. dothidea [Citation8] and Asphondylia prosopidis on Prosopis tree and B. dothidea have been studied [Citation9]. In Italy and Poland, B. dothidea isolated from the Asphondylia gall on Lamiaceae had identical sequences. In contrast, the fungus isolated from the gall collected in the Southern Hemisphere showed mutations in those sequences [Citation10]. In this study, the isolates from galls and pupa on the fruit of A. japonica affected by A. aucubae and one isolate from C. crenata formed a single clade on the BI tree with a weak PP value (0.79; Botryosphaeria spp. on ). The morphological characteristics of conidia and the ecological niche of the isolates suggested that it should be treated as a new species.
In this study, three strains of Botryosphaeria that were isolated from the galls and twigs of A. japonica, a native plant in East Asian countries, were recognized (). The species diversity of Botryosphaeria on Aucuba and its origin is interesting. The insect gall on Aucuba is formed by monophagous gall midge, A. aucubae [Citation32]. This indicates that the monophagous midge does not act as a vector of Botryosphaeria from plants belonging to different plant genera. In contrast, as described above, B. dothidea has often been reported to be related to the gall, and its dispersal has been discussed [Citation8,Citation9]. In Japan, the warty stem blight of A. japonica by Botryosphaeria sp. has been reported [Citation33]. However, its taxonomical position in the current species criteria based on phylogeny is unknown. Furthermore, MUCC 2533 isolated from the branch of A. japonica was identified as B. sinensis. In the future, it is necessary to clarify the relationship among Botryosphaeria sp. related to diseases, galls, Asphondylia species, and host plants. These studies would contribute to revealing the interspecific interaction, such as the cospeciation and expansion of niches, of fungi.
Botryosphaeria dothidea is distributed worldwide and has many hosts. According to the U.S. Department of Agriculture fungal host database, B. dothidea has been recorded to infest 403 plant species [Citation34]. In this study, 13 Japanese isolates from nine plant species in seven families were identified as B. dothidea. In recent years, some new species, B. sinensis [Citation28], B. minutispermatia [Citation26], B. wangensis [Citation27], and B. guttulata [Citation13] have been described as closely related species of B. dothidea. These species are distinguished from other closely related species by their phylogenetic positions and morphological characteristics. However, the phenotypic characteristics of the conidia of B. dothidea have been reported to be various and unstable [Citation10]. In this study, the morphology of the conidia of 13 isolates of B. dothidea was examined. The combined characteristic phylogeny and morphology is useful and stable for the recognition of the species.
Botryosphaeria tenuispora, proposed as a new species in this study, is closely related to B. dothidea. It formed an independent clade as an inner clade of B. dothidea () and had a typically higher L/W ratio than the hitherto known species (). This taxon is recognized using the combined data of ITS + TEF1-α + TUB2 regions, which are regions that are currently used regions for the molecular recognition of Botryosphaeria sp. [Citation4,Citation27]. In phylogenetic analyses, including those of Japanese strains, statistical support values for clades of B. dothidea and the hitherto known species closely related to B. dothidea were generally low. It is necessary to find stable phenotypic characters in morphology and the additional loci to analyze the phylogenetic relationships. Moreover, as the reports of the new species of the genus Botryosphaeria are eccentrically located in East Asia, a more global taxonomic, ecological, and phylogenetic survey of this genus is required in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cesati V, Notaris G. d. Schema di classificazione degli sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persoon. Commentario Soc Crittogamologica Ital. 1863;4:177–240.
- Crous PW, Slippers B, Wingfield MJ, et al. Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol. 2006;55:235–253.
- Liu JK, Phookamsak R, Doilom M, et al. Towards a natural classification of Botryosphaeriales. Fungal Divers. 2012;57(1):149–210.
- Phillips AJL, Alves A, Abdollahzadeh J, et al. The Botryosphaeriaceae: genera and species known from culture. Stud Mycol. 2013;76(1):51–167.
- Phillips AJL, Hyde KD, Alves A, et al. Families in Botryosphaeriales: a phylogenetic, morphological, and evolutionary perspective. Fungal Divers. 2019;94(1):1–22.
- Slippers B, Wingfield MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Revol. 2007;21(2-3):90–106.
- Marsberg A, Kemler M, Jami F, et al. Botryosphaeria dothidea: a latent pathogen of global importance to woody plant health. Mol Plant Pathol. 2017;18(4):477–488.
- Adair RJ, Burgess T, Serdani M, et al. Fungal associations in Asphondylia (Diptera: Cecidomyiidae) galls from Australia and South Africa: Implications for biological control of invasive acacias. Fungal Ecol. 2009;2(3):121–134.
- Park I, Sanogo S, Hanson SF, et al. Molecular identification of Botryosphaeria dothidea as a fungal associate of the gall midge Asphondylia prosopidis on mesquite in the United States. BioControl. 2019;64(2):209–219.
- Zimowska B, Okoń S, Becchimanzi A, et al. Phylogenetic characterization of Botryosphaeria strains associated with Asphondylia galls on species of Lamiaceae. Diversity. 2020;12(2):41.
- Slippers B, Crous PW, Denman S, et al. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia. 2004;96(1):83–101.
- Dissanayake AJ, Phillips AJL, Hyde KD, et al. Botryosphaeriaceae: current status of genera and species. Mycosphere. 2016;7(7):1001–1073.
- Chen Y, Dissanayake AJ, Liu Z, et al. Additions to Karst Fungi 4: Botryosphaeria spp. associated with woody hosts in Guizhou Province, China including B. guttulata sp. nov. Phytotaxa. 2020;454(3):186–202.
- The database of the common names of plant diseases in Japan [internet]. Ibaraki: The Genetic Resources Center, NARO (National Agriculture and Food Research Organization); 2020. [cited 2020 Sep 20]. Available from: https://www.gene.affrc.go.jp/databases-micro_pl_diseases.php.
- Hattori Y, Akiba M, Nakashima C. Taxonomic re-examination of Botryosphaeriaceae causing tree disease. Tree Forest Health. 2019;23:42–43.
- White TJ, Bruns T, Lee S, et al. Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, 1990. p. 315–322.
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologica. 1999;91(3):553–556.
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330.
- Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 1999;16(12):1799–1808.
- Ohata K, Araki T, Kiso A, et al. 1995. Sakumotsu byougenkinn kenkyu gihou no kiso -bunri・baiyou・sessyu. Tokyo: Japan Plant Protection Association; 1995.
- Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20(4):1160–1166.
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791.
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2019;15(4):e1006650.
- Slippers B, Roux J, Wingfield MJ, et al. Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia. 2014;33:155–168.
- Ariyawansa HA, Hyde KD, Liu JK, et al. Additions to Karst Fungi 1: Botryosphaeria minutispermatia sp. nov., from Guizhou Province, China. Phytotaxa. 2016;275(1):35–44.
- Li GQ, Liu FF, Li JQ, et al. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia. 2018;40:63–95.
- Zhou Y, Dou Z, He W, et al. Botryosphaeria sinensia sp. nov., a new species from China. Phytotaxa. 2016;245(1):43–50.
- Sawada K. Fungi inhabiting conifers in the Tohoku district. II. Fungi on various conifers except ‘Sugi. Bulletin of the Government Forest Experimental Station Meguro. 1950;46:144–148.
- Shang YZ. Taxonomic study on the pathogen fungus of shoot blight of larch. Acta Mycol Sinica. 1987;6:248–249.
- Sawada K. Descriptive catalogue of Taiwan (Formosan) fungi. Part XI. Special Publication College of Agriculture National Taiwan University. 1959;8:1–268.
- Yukawa J, Ohsaki N. Separation of the Aucaba fruit midge, Asphondylia aucubae sp. nov. from the ampelopsis fruit midge, Asphondylia baca MONZEN (Diptera, Cecidomyiidae). Kontyu, Tokyo. 1988;56:365–376.
- Harada Y, Usuta Y, Yoshida Y. Warty Stem Blight of Aucuba japonica, a new disease caused by Botryosphaeria sp. (abstracts presented at the Meeting of the Tohoku Division). Jpn J Phytopathol. 1996;62:601.
- The U.S. Department of Agriculture fungal host database [internet]. Washington, D.C: United States Department of Agriculture; 2020. [cited 2020 Sep 20]. Available from: https://nt.ars-grin.gov/fungaldatabases/fungushost/fungushost.cfm.