Abstract
Aspicilia humida Lee is described as a new lichen-forming fungus from a wetland forest, South Korea. The new species is distinguishable from Aspicilia aquatica (Fr.) Körb., the most similar species, by the absence of prothallus, black disk without green color in water, olive-brown epihymenium, shorter hymenium, hymenium I + yellowish blue-green, wider paraphysial tips without a vivid pigment, smaller asci, smaller ascospores, and the presence of stictic acid. Molecular analyses employing internal transcribed spacer (ITS) and mitochondrial small subunit (mtSSU) sequences strongly support A. humida as a distinct species in the A. cinerea group. A surrogate key is provided to assist in the identification of all 28 aspicilioid species of Korea.
1. Introduction
As the Aspicilia was comprising more than 300 species, the large genus has been classified into several infrageneric groups and some of them were finally splitted into some new genera or resurrected as old genera [Citation1–6]. The genus Megaspora was newly introduced by the characteristics of large ascospores with thick walls and anastomosing paraphyses [Citation7,Citation8]. The genus Lobothallia was raised up from the A. radiosa group [Citation9]. The old genus Circinaria (previously the A. contorta/calcarea group) was reintroduced by the characteristics of diverse thalli (crustose, foliose to subfruiticose), broad-ellipsoid to globose ascospores which are shown generally less than eight per ascus, and particularly the presence of aspicilin [Citation3,Citation4]. The old genus Sagedia was reintroduced based on the molecular analysis [Citation3]. The genus Teuvoa was newly introduced by the characteristics of the lack of lobate, radiating thalli, generally absence of algal layer underlining hypothecium, the absence of secondary metabolites, and the substrate preference to barks or woods but not rocks, in comparing with Lobothallia [Citation10]. The genus Oxneriaria (previously the A. mashiginensis group) was newly introduced by defining the characteristics of radiating thalli with wrinkled or lobate periphery, small ascospores, presence of substictic acid, and the habitat preference to polar and alpine areas [Citation5]. The old genus Aspiciliella was reintroduced by the characteristics of consistently ellipsoid ascospores, small conidia, and the presence of norstictic acid in all species [Citation11]. The infrageneric groups are further categorized into seven groups in Aspicilia s. str. [Citation6]. The Aspicilia (200 spp.) is still considered the main genus of the family Megasporaceae (243 spp.) [Citation12].
Hue, a French lichenologist, first described the aspicilioid lichens of Korea including 15 species (Aspicilia adamanticola Hue, A. asteria Hue, A. chinnampoana Hue, A. dimorphodes Hue, A. exserta Hue, A. fauriana Hue, A. geographica Hue, A. leucera Hue, A. microsporeta Hue, A. stellata Hue, A. stenospora Hue, A. tofacea Hue, A. tumens Hue, A. umbrinella Hue, and A. vulcanica Hue) [Citation13]. After a century, Kondratyuk discovered A. contorta ssp. hoffmanniana S. Ekman & Fröberg ex R. Sant. (syn. Circinaria hoffmanniana (S. Ekman & Fröberg ex R. Sant.) A. Nordin) in 2013 [Citation14], and Aptroot and Moon recorded A. cinerea (L.) Körb., A. grisea Arnold, Circinaria caesiocinerea (Nyl. ex Malbr.) A. Nordin, Savić & Tibell, and C. leprosescens (Sandst.) A. Nordin, Savić & Tibell in 2014 [Citation15]. Kondratyuk focused on the aspicilioid lichens of Korea in 2016 and eight species were introduced from Korea (A. pseudoabbasiana S.Y. Kondr., Lőkös & Hur, A. pseudovulcanica S.Y. Kondr., Lőkös & Hur, A. subepiglypta S.Y. Kondr., Lőkös & Hur, A. subgeographica S.Y. Kondr., Lőkös & Hur, A. subgoettweigensis S.Y. Kondr., Lőkös & Hur, A. submamillata S.Y. Kondr., Lőkös & Hur [Citation16], A. geumodoensis S.Y. Kondr., Lőkös & Hur (syn. Rimularia geumodoensis (S.Y. Kondr., Lőkös & Hur) S.Y. Kondr., Lőkös & Hur and R. badioatra (Kremp.) Hertel & Rambold) [Citation17]. Particularly, Paukov reclassified both A. dimorphodes and A. fauriana to A. intermutans (Nyl.) Arnold, and both A. geographica and A. microsporeta to Lecanora oreinoides (Körb.) Hertel & Rambold in 2017 [Citation18]. Kondratyuk detected Rimularia gibbosa (Ach.) Coppins, Hertel & Rambold [Citation19] and Yakovchenko introduced two Rimularia species such as R. badioatra and R. limborina Nyl. in 2018 [Citation20]. Overall 27 species of the aspicilioid lichens were recorded in Korea.
This study aimed to describe a new lichen-forming fungus in the genus Aspicilia. One of the field surveys for the lichen biodiversity in the forested wetlands of South Korea was carried out in a wetland forest of a high mountain, Gangwon Province in 2020, and two specimens of aspicilioid lichens were collected (). The specimens were comprehensively analyzed in ecology, morphology, chemistry and molecular phylogeny and did not correspond to any previously known species. We describe them as a new species, Aspicilia humida, and this discovery contributes to the taxonomy with overall 28 taxa in the genus Aspicilia of Korea. The specimens are deposited in the herbarium of the Baekdudaegan National Arboretum (KBA, the herbarium acronym in the Index Herbariorum), South Korea.
2. Materials and methods
2.1. Morphological and chemical analyses
Specimen sections were prepared manually with a razor blade under a stereomicroscope (Olympus optical SZ51; Olympus, Tokyo, Japan), scrutinized under a compound microscope (Nikon Eclipse E400; Nikon, Tokyo, Japan) and pictured using a software program (NIS-Elements D; Nikon) and a DS-Fi3 camera (Nikon) mounted on a Nikon Eclipse Ni-U microscope (Nikon). The ascospores were examined at 1000× magnification in water. The length and width of the ascospores were measured and the range of spore sizes was shown with average, standard deviation (SD), length-to-width ratio, and the number of measured spores. Thin-layer chromatography (TLC) was performed using solvent systems A and C according to standard methods [Citation21].
2.2. Isolation, DNA extraction, amplification, and sequencing
Hand-cut sections of 10–20 ascomata with thallus from the collected specimens were prepared for DNA isolation and DNA was extracted with a NucleoSpin Plant-II Kit in line with the manufacturer’s instructions (Macherey-Nagel, Düren, Germany). PCR amplifications for the internal transcribed spacer region (ITS1-5.8S-ITS2 rDNA), the mitochondrial small subunit, and the nuclear large subunit ribosomal RNA genes was achieved using the primers ITS5 and ITS4 [Citation22], mrSSU1 and mrSSU3R [Citation23], and LR0R and LR5 [Citation24], respectively. The PCR thermal cycling parameters used were 95 °C (15 sec), followed by 35 cycles of 95 °C (45 sec), 54 °C (45 sec), and 72 °C (1 min), and a final extension at 72 °C (7 min) based on Ekman [Citation25]. The annealing temperature was occasionally altered by ±1 degree in order to get a better result. PCR purification and DNA sequencing were accomplished by the Macrogen (Seoul, Korea).
2.3. Phylogenetic analyses
All ITS and mtSSU sequences () were aligned and edited manually using ClustalW in Bioedit V7.2.6.1 [Citation26]. All missing and ambiguously aligned data and parsimony-uninformative positions were removed and only parsimony-informative regions were finally analyzed in MEGA X [Citation27]. The final alignment comprised 1163 (ITS) and 1058 (mtSSU) columns. In them, variable regions were 171 (ITS) and 120 (mtSSU). Finally, the phylogenetically informative regions were 444 (ITS) and 271 (mtSSU). Phylogenetic trees with bootstrap values were obtained in RAxML GUI 2.0 beta [Citation28] using the maximum likelihood method with a rapid bootstrap with 1000 bootstrap replications and GTR GAMMA for the substitution matrix. The posterior probabilities were obtained in BEAST 2.6.4 [Citation29] using the GTR 123141 (ITS) and the GTR 121323 (mtSSU) models, as the appropriate models of nucleotide substitution produced by the Bayesian model averaging methods with bModelTest [Citation30], empirical base frequencies, gamma for the site heterogeneity model, four categories for gamma, and a 10,000,000 Markov chain Monte Carlo chain length with a 10,000-echo state screening and 1000 log parameters. Then, a consensus tree was constructed in TreeAnnotator 2.6.4 [Citation29] with no discard of burnin, no posterior probability limit, a maximum clade credibility tree for the target tree type, and median node heights. All trees were displayed in FigTree 1.4.2 [Citation31] and edited in Microsoft Paint. The bootstrapping and Bayesian analyses were repeated three times for the result consistency and no significant differences were shown for the tree shapes and branch values. The phylogenetic trees and DNA sequence alignments are deposited in TreeBASE under the study ID 28153. Overall analyses in the materials and methods were accomplished based on Lee and Hur [Citation32].
Table 1. Species list and DNA sequence information employed for phylogenetic analysis.
3. Results and discussion
3.1. Phylogenetic analyses
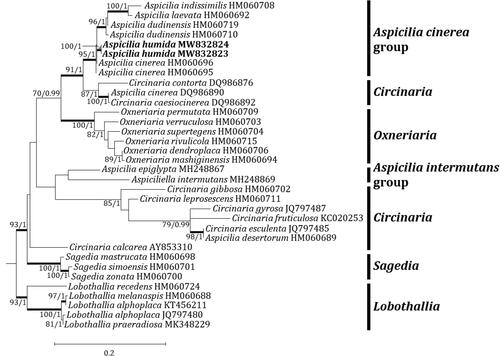
Two independent phylogenetic trees for the genus Aspicilia and related genera were produced from 98 sequences (66 for ITS, and 32 for mtSSU) from GenBank and four new sequences (each two for ITS and mtSSU) from the new species (). The new species was positioned in the A. cinerea group in both trees. The ITS tree describes that the new species is located in a clade with A. subfarinosa (J. Steiner) Şenkard. & Sohrabi and Circinaria hispida (Mereschk.) A. Nordin, Savić & Tibell, represented by a bootstrap value of 98 and a posterior probability of 0.7 (not shown) for the branch. Other species, such as A. abbasiana S.Y. Kondr., Lőkös, Ismayil & S.Y. Guo, A. blastidiata Paukov, A. Nordin & Tibell, A. cinerea, A. dudinensis (H. Magn.) Oxner, A. pseudoabbasiana, A. pseudovulcanica, A. subepiglypta, A. subdepressa Arnold, A. subgeographica, A. subgoettweigensis, and A. submamillata, are closely located to the new species in the A. cinerea group, represented just by a bootstrap value of 79 for the branch (). The mtSSU tree shows that the new species is solely located in the A. cinerea group. Closely positioned species to the new species are A. cinerea, A. dudinensis, A. indissimilis (H. Magn.) Räsänen, and A. laevata (Ach.) Arnold, represented by a bootstrap value of 95 and a posterior probability of 1 for the branch (). The phylogenetic analyses did not designate any species identical to the new species in the genus Aspicilia.
Figure 2. Phylogenetic relationship among available species in the genus Aspicilia based on a maximum likelihood analysis of the dataset of ITS sequences. The tree was rooted with six Lobothallia and Teuvoa sequences. Maximum likelihood bootstrap values ≥ 70% and posterior probabilities ≥ 95% are shown above internal branches. Branches with bootstrap values ≥ 90% are shown in bold. The new species Aspicilia humida is presented in bold, and all species names are followed by the Genbank accession numbers. Reference provides the species related to the specific GenBank accession numbers and voucher information.
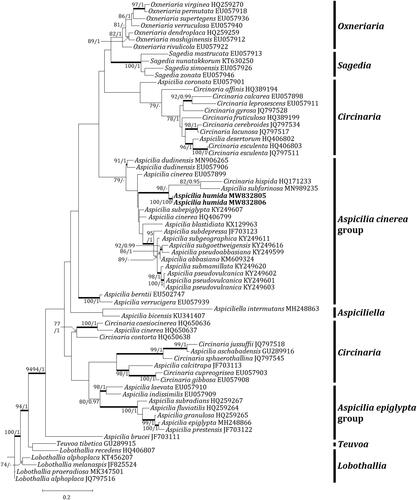
Figure 3. Phylogenetic relationships among available species in the genus Aspicilia based on a maximum likelihood analysis of the dataset of the mitochondrial small subunit (mtSSU) sequences. The tree was rooted with five Lobothallia sequences. Maximum-likelihood bootstrap values ≥ 70% and posterior probabilities ≥ 95% are shown above internal branches. Branches with bootstrap values ≥ 90% are shown in bold. The new species Aspicilia humida is presented in bold, and all species names are followed by the GenBank accession numbers. Reference provides the species related to the specific GenBank accession numbers and voucher information.
3.2. Taxonomy
Aspicilia humida B.G. Lee sp. nov.
No: MB839181
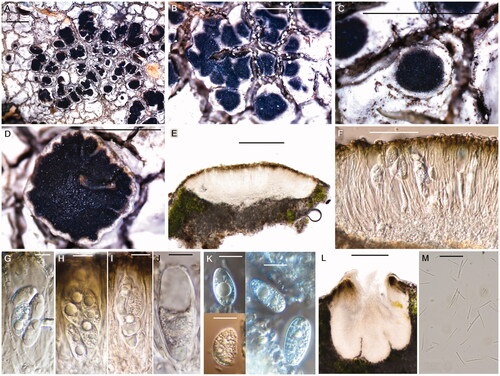
Figure 4. Aspicilia humida (BDNA-L-0000703, holotype for A–K; BDNA-L-0000711, paratype for L & M) in morphology. (A–D): Habitus and apothecia emerging single to several per an areole; (E): Adnate apothecia without constriction at the base in section; (F): Epihymenium in olive-brown pigment; (G–J): Clavate asci with eight spores; (K): Ellipsoid or globose ascospores with no septation; (L): Immersed pycnidia; (M): Thread-like pycnoconidia. Bars: A–D 1 mm; E 200 μm; F 50 μm; G–K 10 μm; L 100 μm; M 10 μm.
3.2.1. Diagnosis
Aspicilia humida differs from A. aquatica by the absence of prothallus (vs. thick gray prothallus), black disk without green color in water (vs. black disk with translucent green when wet), olive-brown epihymenium (vs. olive-green epihymenium), shorter hymenium (50–60 μm vs. 150–170 μm), hymenium I + yellowish blue-green (vs. hymenium I + blue or turning dark red-brown), wider paraphysial tips without vivid pigment (4.5–6 μm wide vs. blackened tips in 2–5 μm wide), smaller asci (64–72 × 17–27 μm vs. 80–140 × 25–35 μm), smaller ascospores (10.5–23 × 6–13.5 μm vs. 20–35 × 13–20 μm), and the presence of stictic acid (vs. no substance).
3.2.2. Type
South Korea, Gangwon Province, Pyeongchang-gun, Daegwallyeong-myeon, Hoenggye-ri, a forest wetland, 37°46'0.02"N, 128°42'19.58"E, 1,047 m alt., on siliceous rock, 03 June 2020, B. G. Lee & H. J. Lee 2020-000503, with Diplotomma alboatrum (Hoffm.) Flot. and Endocarpon maritimum Y. Joshi & Hur (holotype: BDNA-L-0000703!; GenBank MW832805 for ITS, MW832823 for mtSSU, and MW832826 for LSU); same locality, on siliceous rock, 03 June 2020, B. G. Lee & H. J. Lee 2020-000511, (paratype: BDNA-L-0000711; GenBank MW832806 for ITS, MW832824 for mtSSU, MW832827 for LSU).
Thallus saxicolous, crustose, mainly areolate and partially rimose, pale gray to white, margin determinate, not pruinose, 175–300 μm thick; cortex hyaline, 25–30 μm thick; medulla 25–30 μm thick; photobiont coccoid, algal layer 35–50 μm thick, cells globose to subglobose, 5–15 μm. Small crystals in cortex, medulla and between algal cells, not dissolving in K. Prothallus inconspicuous.
Apothecia abundant, generally rounded but subangular or even irregular when several apothecia contiguous or coalescent, emerging single to several per an areole, adnate when mature, not constricted at the base, 0.2–1.7 mm diam. Disk flat or somewhat concave, smooth or slightly rugose, not pruinose, black from the beginning and partially paler when old, 100–130 μm thick; lecanorine, thalline margin present and same color to thallus or slightly darker, proper margin indistinct. Amphithecium well-developed, with small crystals in both cortical layer and medulla, crystals extending to the base, not dissolving in K, 90–100 μm wide laterally, 50–60 μm wide at periphery. Parathecium inconspicuous, hyaline but olive-brown at periphery, 10–15 μm wide laterally, 15–25 μm wide at periphery, disappearing to the base. Epihymenium olive green to brown, smooth and not granular, brown pigment dissolving in K, 10–15 μm high. Hymenium hyaline, 50–60 μm high, I + yellowish blue-green. Hypothecium hyaline, 25–50 μm high. Oil droplets present mainly in hypothecium and also along paraphyses in hymenium. Paraphyses septate, anastomosing, 2–2.5 μm wide, simple or branched at tips, tip cells somewhat bead-like (moniliform), bead-like formation clearer in staining, swollen but not pigmented, 4.5–6 μm wide. Asci clavate, 8-spored, 64–72 × 17–27 μm (n = 5). Ascospores constantly simple, ellipsoid or somewhat globose, 10.5–23 × 6–13.5 μm (mean = 17.3 × 9.8 μm; SD = 2.6 (L), 1.5 (W); L/W ratio 1.2–2.7, ratio mean = 1.8, ratio SD = 0.3; n = 106). Pycnidia immersed, ostiolar region slightly projected, rounded, black, 250–275 × 200–230 μm. Pycnoconidia thread-like, straight, slightly curved or v-shaped, 5.5–28 × 0.5–1.0 μm (mean = 15.1 × 0.7 μm; SD = 3.7 (L), 0.1 (W), n = 110)
3.2.3. Chemistry
Thallus K–, KC–, C–, Pd–. Medulla K + yellow, I–. UV + gray to dull white. Stictic acid was detected by TLC.
3.2.4. Distribution and ecology
The species occurs on a siliceous rock nearby a stream in an open wetland forest of a high mountain. The species is currently known from the type collections.
3.2.5. Etymology
The species epithet indicates the lichen’s geography, namely a humid wetland.
3.2.6. Notes
The new species is similar to A. aquatica, A. vulcanica and A. pseudovulcanica in having white to gray thallus with negative reaction in K among saxicolous species. However, the new species differs from A. aquatica by the absence of prothallus, black disk without green color in water, olive-brown epihymenium, shorter hymenium, hymenium I + yellowish blue-green, wider paraphysial tips without vivid pigment, smaller asci, smaller ascospores, and the presence of stictic acid [Citation33,Citation34].
The new species is different from A. vulcanica by apothecia emerging one to several per single areole, larger apothecia, narrower paraphyses, shorter and wider asci, and the substrate preference to siliceous rock [Citation13,Citation16].
The new species is distinguished from A. pseudovulcanica by thicker, larger apothecia without pruina, shorter hymenium, smaller ascospores [Citation16]. Reference provides the key characteristics distinguishing A. humida from the compared species above.
Table 2. Comparison of Aspicilia humida with closely-related species.
The new species is further compared with A. straminella Hue and A. verrucigera Hue in having grayish, areolate thallus and K + yellow medulla in saxicolous species. However, A. straminella is different from the new species by thicker and straw-gray thallus, smaller apothecia, taller hymenium and wider ascospores [Citation13,Citation16]. Aspicilia verrucigera differs from the new species by thicker and darker thallus occasionally with brown color, smaller apothecia, taller hymenium, wider ascospores and the presence of norstictic acid [Citation16,Citation33].
3.9 Key to aspicilioid species of Korea (28 taxa)
Overall 28 species have been recorded for the aspicilioid lichens including the genera Aspicilia, Circinaria, Lecanora, and Rimularia in Korea, except for synonyms. For synonyms after taxonomic revision, A. adamanticola is corresponded to A. cinerea [Citation18], A. contorta ssp. hoffmanniana is reclassified to C. hoffmanniana [Citation35], A. geographica and A. microsporeta are conspecific to L. oreinoides [Citation18], A. dimorphodes and A. fauriana are indistinguishable to A. intermutans [Citation18], and A. geumodoensis is reclassified to R. geumodoensis [Citation18]. This key is revised from Kondratyuk’s work [Citation16] only for Korea territory, and seven more species are included such as A. grisea, A. humida, A. intermutans, C. caesiocinerea, C. hoffmanniana, L. oreinoides, and R. limborina. A. exserta is corrected from A. excerta of Kondratyuk’s work [Citation16].
Paraphysis cells not bead-like, disk umbonate or gyrose..2
Paraphysis cells bead-like (moniliform), disk generally smooth ..5
Thallus C– or C ± pink (not or containing ± gyrophoric acid), hymenium up to 150 μm, ascospores 18–30 × 10–18 μm ..Rimularia limborina
Thallus C + red (containing gyrophoric acid), hymenium up to 120 μm, ascospores 10–25 × 7–13 μm..3
Thallus lighter, whitish gray, ascospores 20–25 × 9–13 μm....Rimularia geumodoensis
Thallus darker, pinkish brown to gray brown, ascospores 10–25 × 7–13 μm..4
Thallus pinkish brown to dark gray-brown, hymenium 70–100 μm, apothecia 0.2–0.4 mm diam., disk concave to umbonate, ascospores 10–22 × 7–13 μm....Rimularia badioatra
Thallus pale gray-brown to beige, hymenium up to 75 μm, apothecia 0.5–0.8 mm diam., disk slightly convex, ascospores 16–25 × 10–13 μm....Rimularia gibbosa
Thallus or medulla K + red or K + yellow turning to red (containing norstictic acid) ....6
Thallus or medulla K– red or K + yellow (containing aspicilin or stictic acid, but not containing norstictic acid) ..16
Thallus with farinose-erose soredia..A. grisea
Thallus without soredia7
Apothecia rare or solitary per areole when present..8
Apothecia occurring one to several per areole..12
Thallus whitish..9
Thallus grayish to brownish..11
Thallus thin, apothecia 0.2–0.3 mm diamA. tofacea
Thallus thick, apothecia 0.5–1.5 mm diam. ..10
Asci 120 × 24 μm, ascospores 15–20 × 9–10 μm..A. exserta
Asci 80 × 16 μm, ascospores 17–20 × 7–8 μm.A. leucera
Apothecia 0.3–0.5 mm diam., ascospores 16–20 × 6–7 μm..A. stenospora
Apothecia 0.4–1.0 mm diam., ascospores 12–19 × 7–11 μm..A. tumens
Thallus white without grayish or brownish color..13
Thallus white-gray or gray to gray-brown..14
Thallus thin 0.16–0.22 mm thick, apothecia occurring one to two per areole, 0.4–0.8 mm diam. ..A. chinnampoana
Thallus thick 0.5–0.6 mm thick, apothecia occurring one to several per areole, 0.3–0.5 mm diam. ..A. stellata
Apothecia 0.2–0.3 mm diam., occurring three to five per areole, conidia 15–20 × 0.7–0.9 μm..A. subepiglypta
Apothecia 0.4–1.2 mm diam., occurring one to three per areole, conidia 7–16 × 1 μm..15
Apothecia occurring one to two per areole, ascospores 12–22 × 6–13 μm, conidia 11–16 × 1 μm..A. cinerea
Apothecia occurring two to three per areole, ascospores 22–28 × 12–14 μm, conidia 7–11 × 1 μm..A. intermutans
On calcareous or volcanic rocks..17
On siliceous rocks..18
On calcareous rocks, thallus with pruina, apothecia prominent, black, asci 4-spored....Circinaria hoffmanniana
On volcanic rocks, thallus without pruina, apothecia immersed, flesh-colored (beige), asci 8-spored..A. vulcanica
Thallus with soredia or isidia, containing aspicilin..Circinaria leprosescens
Thallus without soredia or isidia..19
Thallus areolate, thick (0.4–0.6 mm thick) ..20
Thallus areolate to rimose or subsquamulose, thin or thick..23
Thallus with pruina..21
Thallus without pruina..22
Apothecia 0.2–0.5 mm diam., disk blackish with grayish pruina..A. subgoettweigensis
Apothecia 0.4–1.0 mm diam., disk gray to brownish with white pruina.A. submamillata
Thallus dark gray to lead-gray, apothecia 0.25–0.3 mm diam., ascospores 17–22 × 8–12 μm, conidia 13–17 × 0.7–0.8 μm..A. pseudoabbasiana
Thallus light gray to slightly brownish gray, apothecia 0.9–1 mm diam., ascospores 19–22 × 12–16 μm, conidia 3.5–5.5 × 0.7–0.8 μm..A. subgeographica
Thallus with pruina..24
Thallus without pruina..26
Thallus thick (up to 0.4 mm thick), white to cream white, or pale yellow, apothecia occurring one to three per areole, ascospores 9–14 × 4–6.5 μm...........Lecanora oreinoides
Thallus thin, white, light gray or pale brown, apothecia occurring one to two per areole, ascospores 16–24 × 9–14 μm..25
Apothecia 0.2–0.5 mm diam., thallus white or pale brown......................A. umbrinella
Apothecia 0.5–0.9 mm diam., thallus white-gray to light gray.................A. pseudovulcanica
Thallus subsquamulose in center, ascospores 14–30 × 7–16 μm, containing aspicilin........Circinaria caesiocinerea
Thallus areolate to rimose only, ascospores 10–23 × 6–14 μm, not containing aspicilin27
Thallus bluish gray, apothecia mostly solitary per areole, rarely two, 0.2–0.5 mm diam., asci 100–132 × 22–26 μm, ascospores 16–20 × 10–14 μm......A. asteria
Thallus pale gray to white, apothecia occurring one to several per areole, 0.2–1.7 mm diam., asci 64–72 × 17–27 μm, ascospores 10.5–23 × 6–13.5 μm, containing stictic acid..A. humida
Acknowledgements
This work was supported by a grant from the Korean National Research Resource Center Program (NRF-2017M3A9B8069471) and the Korean Forest Service Program through the Korea National Arboretum (KNA-202003127AF-00) for the forested wetland conservation of Korea.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Magnusson AH. Studies in species of Lecanora, mainly the Aspicilia gibbosa group. Kungl Svenska Vetenskapsakad Årsbok. 1939;3(17):1–182.
- Lumbsch HT, Schmitt I, Lücking R, et al. The phylogenetic placement of Ostropales within Lecanoromycetes (Ascomycota) revisited. Mycol Res. 2007;111(3):257–267.
- Nordin A, Savić S, Tibell L. Phylogeny and taxonomy of Aspicilia and Megasporaceae. Mycologia. 2010;102(6):1339–1349.
- Sohrabi M, Owe-Larsson B, Nordin A, et al. Aspicilia tibetica, a new terricolous species of the himalayas and adjacent regions. Mycol Prog. 2010;9(4):491–499.
- Haji Moniri M, Gromakova AB, Lőkös L, et al. New members of the Megasporaceae (Pertusariales, lichen-forming Ascomycota): Megaspora iranica spec. nova and Oxneriaria gen. nova. Acta Bot Hung. 2017;59(3-4):343–370.
- Wheeler TB. Multilocus phylogeny of the lichen family Megasporaceae [MS thesis]. Missoula, MT: University of Montana; 2017.
- Clauzade G. Les genres Aspicilia Massal. et Bellemerea Hafellner et Roux. Bull Soc Bot Cent.-Ouest. 1984;15:127–141.
- Wirth V. Die flechten Baden-Württembergs: Verbreitungsatlas. Stuttgart: E. Ulmer; 1987.
- Hafellner J. Die Gattung Aspicilia, ihre Ableitungen nebst Bemerkungen über cryptolecanorine Ascocarporganisation bei anderen Genera der Lecanorales (Ascomycetes lichenisati). ABM. 1991;16:133–140.
- Sohrabi M, Leavitt SD, Halici MG, et al. Teuvoa, a new lichen genus in Megasporaceae (Ascomycota: Pertusariales), including Teuvoa junipericola sp. nov. Lichenologist. 2013;45(3):347–360.
- Zakeri Z, Divakar PK, Otte V. Taxonomy and phylogeny of Aspiciliella, a resurrected genus of Megasporaceae, including the new species A. portosantana. Herzogia. 2017;30(1):166–176.
- Lücking R, Hodkinson BP, Leavitt SD. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–approaching one thousand genera. Bryologist. 2017;119(4):361–416.
- Hue A. Lichenes morphologice et anatomice. Genus 48 – Aspicilia. [ser. 5.]. Nouv Arch Mus Hist Nat. 1910;2:1–120.
- Kondratyuk S, Lőkös L, Tschabanenko S, et al. New and noteworthy lichen-forming and lichenicolous fungi. Acta Bot Hung. 2013;55(3-4):275–349.
- Aptroot A, Moon KH. 114 New reports of microlichens from Korea, including the description of five new species, show that the microlichen flora is predominantly Eurasian. Herzogia. 2014;27(2):347–365.
- Kondratyuk SY, Lőkös L, Park JS, et al. New Aspicilia species from South Korea proved by molecular phylogeny with a key to the Eastern Asian aspicilioid lichens. Studia Bot Hung. 2016;47(2):227–249.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 5. Acta Bot Hung. 2016;58(3-4):319–396.
- Paukov A, Nordin A, Roux C, et al. Lectotypification and synonymization of some Aspicilia species (Megasporaceae, Ascomycota) described by A. Hue from Korea and Japan. Phytotaxa. 2017;291(1):94–98.
- Kondratyuk SY, Lőkös L, Halda JP, et al. New and noteworthy lichen-forming and lichenicolous fungi 6. Acta Bot Hung. 2017;59(1-2):137–260.
- Yakovchenko L, Davydov EA, Paukov A, et al. New lichen records from Korea–I. Mostly arctic-alpine and tropical species. Herzogia. 2018;31(2):965–981.
- Orange A, James PW, White FJ. Microchemical methods for the identification of lichens. London: British Lichen Society; 2001.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a Guide to Methods and Applications. 1990;18(1):315–322.
- Zoller S, Scheidegger C, Sperisen C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist. 1999;31(5):511–516.
- Rehner SA, Samuels GJ. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res. 1994;98(6):625–634.
- Ekman S. Molecular phylogeny of the Bacidiaceae (Lecanorales, lichenized Ascomycota). Mycol Res. 2001;105(7):783–797.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Stecher G, Tamura K, Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol. 2020;37(4):1237–1239.
- Edler D, Klein J, Antonelli A, et al. raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol. 2021;12(2):373–377.
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. BEAST 2.5: an advanced software platform for bayesian evolutionary analysis. PLoS Comput Biol. 2019;15(4):e1006650
- Bouckaert RR, Drummond AJ. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol Biol. 2017;17(1):42.
- Rambaut A. FigTree v1.4.2 [Internet]. Edinburgh: University of Edinburgh; 2014. [cited 2020 Jul 4]. Available from: http://tree.bio.ed.ac.uk/software/figtree.
- Lee BG, Hur JS. A new lichenized fungus, Lecanora baekdudaeganensis, from South Korea, with a taxonomic key for korean Lecanora species. MycoKeys. 2020;70:39–58.
- Nash TH, III Gries C, Bungartz F. Lichen flora of the greater sonoran desert region., Vol. III. Tempe, AZ, U.S.A.: Lichens Unlimited/Arizona State University; 2007.
- Smith CW, Aptroot A, Coppins BJ, et al. The lichens of great britain and Ireland. London, UK: The British Lichen Society; 2009.
- Roux C, Bertrand M, Nordin A. Aspicilia serenensis Cl. Roux et M. Bertrand sp. nov., espèce nouvelle de lichen (groupe d’A. calcarea, Megasporaceae. Bull Soc Linn Provence. 2016;67:165–182.