Abstract
Inflammaging in male reproductive organs covers a wide variety of problems, including sexual dysfunction and infertility. In this study, the beneficial effects of cordycepin (COR), isolated from potential medicinal fungi Cordyceps militaris, in aging-associated testicular inflammation and serum biochemical changes in naturally aged rats were investigated. Male Sprague Dawley rats were divided into young control (YC), aged control (AC), and COR (5, 10, and 20 mg/kg) treated aged rat groups. Aging-associated serum biochemical changes and inflammatory parameters were analyzed by biochemical assay kits, Western blotting, and real-time RT-PCR. Results showed a significant (p < 0.05) alteration in the total blood cell count, lipid metabolism, and liver functional parameters in AC group when compared with YC group. However, COR-treated aged rats ameliorated the altered biochemical parameters significantly (p < 0.05 and p < 0.01 at 5, 10, and 20 mg/kg, respectively). Furthermore, the increase in the expression of inflammatory mediators (COX-2, interleukin (IL)-6, IL-1β, and tissue necrosis factor-alpha) in aged rat testis was significant (p < 0.05) when compared with YC group. Treatment with COR at 20 mg/kg to aged rats attenuated the increased expression of inflammatory mediators significantly (p < 0.05). Mechanistic studies revealed that the potential attenuating effects exhibited by COR in aged rats was mediated by regulation of NF-κB activation and MAPKs (c-Jun N-terminal kinase, extracellular signal-regulated kinase 1/2, and p38) signaling. In conclusion, COR restored the altered serum biochemical parameters in aged rats and ameliorated the aging-associated testicular inflammation proving the therapeutic benefits of COR targeting inflammaging-associated male sexual dysfunctions.
1. Introduction
Aging is an unavoidable phenomenon of life attributed with severe changes in the different levels from molecules to organs, which carries a possibility of accelerating several aging-related diseases (ARDs) [Citation1,Citation2]. Aging and inflammation also termed as “inflammaging” is an interconnected network with steady development of the inflammatory state in elderly population affecting various organ systems. Inflammaging in male reproductive function covers a wide variety of problems including erectile dysfunction, premature or delayed ejaculation, and a decline of sexual desire ultimately leading to male infertility [Citation3,Citation4]. Many of these changes can be related to or exacerbated by aging-associated inflammatory responses [Citation5,Citation6].
As the life expectancy has been steadily improving, men are seeking to preserve their sexuality and sexual potency into old age. Mounting evidence indicated that pro- and anti-inflammatory molecules and their imbalance contribute to fundamental mechanisms of aging [Citation7,Citation8]. The presence of abnormal and increasing inflammatory molecules in the male reproductive tissues might affect the male physiological sexual functions and become highly detrimental in sperm production [Citation9]. Therefore, it is reasonable to assume that inflammation is usually implicated in the testicular aging process. Although, the impact of anti-inflammatory agents, caloric restriction, and long-term physical exercise on improving the elderly men health status were highlighted [Citation1], the current knowledge on the inflammaging in male reproductive tract and development of appropriate therapeutic anti-inflammaging agents that can help in mitigating male sexual dysfunctions and testicular damage is still unclear [Citation10].
Cordycepin (3′-deoxyadenosine), a purine nucleoside derivative is the main functional component of an entomopathogenic fungus, Cordyceps militaris (Linn.) Fr., used extensively as a crude drug in traditional Oriental medicine and as a folk tonic food in East Asian countries [Citation11,Citation12]. Cordycepin has been known to exhibit many pharmacological properties, including antitumor, antifungal, antiviral, anti-inflammatory, antiatherosclerotic, antioxidant, antiaging, and anticancer and has an ability to enhance immune functions [Citation13–16]. Furthermore, the anti-inflammatory potential of C. militaris and its active constituent cordycepin in lipopolysaccharide (LPS)-stimulated macrophages, inflammatory-associated diabetes regulating genes, and neuroinflammation was also documented [Citation17–20]. Recently, the therapeutic potential of cordycepin as a strong antiviral agent against proteins with SARS-CoV-2 and in the treatment of COVID-19 has been reported [Citation21,Citation22].
Reports from our laboratory and others also revealed that cordycepin enhances sexual function and ameliorates age-related oxidative stress-mediated testicular dysfunction by restoring the antioxidative enzyme status [Citation23–26]. However, the protective effects of cordycepin in inflammaging-associated testicular damage have not been elucidated. Thus, in the present study, we investigated the effects of cordycepin in ameliorating inflammatory responses in the testes of naturally aged rats and explored the underlying mechanisms. In addition, the effect of cordycepin on aging-mediated biochemical changes in blood chemistry parameters was also evaluated.
2. Materials and methods
2.1. Chemicals
Cordycepin () was isolated from C. militaris and purified by butanol partition, silica gel flash chromatography, and recrystallization to obtain 99.5% pure compound named “COR” as reported previously [Citation26]. All chemicals and reagents were purchased from Santa Cruz Biotechnology, Dallas, TX.
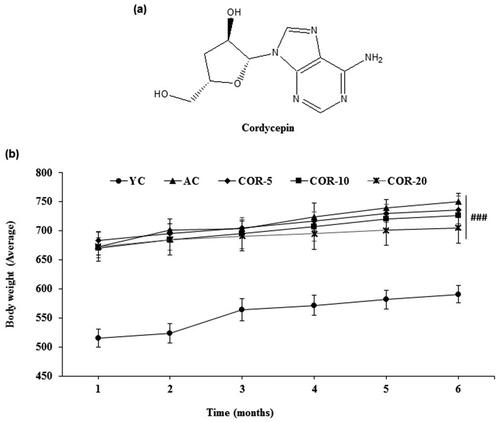
Figure 1. Effect of COR on body weight increments. (a) Structure of cordycepin; (b) Total body weight increments during the course of the study in YC, AC, COR-5, COR-10, and COR-20 groups. Each point represents the mean ± SD (n = 10). ###p < 0.001 compared with YC group. YC: young control, AC: aged control, COR-5: aged rats plus cordycepin 5 mg/kg treated group, COR-10: aged rats plus cordycepin 10 mg/kg treated group and COR-20: aged rats plus cordycepin 20 mg/kg treated group.
2.2. Experimental animals and design
Fifty Sprague Dawley rats (40 rats, 14-month-old 650 ± 20 g and 10 rats, 2-month-old 280 ± 20 g), were obtained from Hanil Experimental Animal Breeding Co. Ltd. (Yeumsung, Chungbuk, Korea), and were maintained at specific pathogen-free animal facility, Regional Innovation Center, Konkuk University, Republic of Korea. Animals were acclimatized at a constant temperature (23 ± 2 °C) and relative humidity (55 ± 10%) on a 12/12-h light/dark cycle for at least 1 week prior to the experiments providing with standard pellet diet and water ad libitum. For experiments, rats were randomly divided into five groups (n = 10): the young control (YC) and aged control (AC) groups received only vehicle (distilled water), whereas the COR-treated groups (COR-5, COR-10, and COR-20) were administrated orally after pelletization at daily doses of 5, 10, and 20 mg/kg body weight of COR, respectively for 6 months. The selection of doses and administration procedures were performed based on our previous study [Citation26]. At the end of the experiment schedule and overnight fasting (only water was provided ad libitum), the animals were sacrificed with carbon dioxide following the National Institutes of Health (NIH) guidelines. The YC group was 8 months old and AC/COR groups were 20 months old at the time of sacrifice. The body weight increments during the course of the study were monitored and testes of each rat were isolated after the experiment removing any adhering adipose tissue. A 10% homogenate of the testis tissue was prepared in Tris-HCl buffer (0.1 M, pH 7.4), centrifuged (2500 rpm for 10 min at 4 °C) to pellet the cell debris, and the clear supernatant was used for Western blotting and other biochemical assays. All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee guidelines of Konkuk University, and the study was approved by the Animal Ethics Committee in accordance with the 14th article of the Korean Animal Protection Law.
2.3. Blood chemistry panels
To measure the blood chemistry parameters, blood samples were collected from the abdominal vein in a SST® gel & clot activator tube (Becton and Dickinson, Franklin Lakes, NJ, USA). Serum was separated by centrifugation at 1500 × g for 10 min at room temperature. An automated chemistry analyzer (Hitachi-747, Hitachi Medical Co., Tokyo, Japan) was used to measure serum biochemical parameters association with lipid metabolism and liver functions including the aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), glutamyl transpeptidase (γ-GTP), albumin (Alb), total cholesterol (T-Cho), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C). A part of blood was collected in a test tube containing ethylenediaminetetraacetic acid (EDTA) (Vacutainer K3E, BD Biosciences, Plymouth, UK) as an anticoagulant to measure the number of blood cell and analyzed using an automated blood cell analyzer (Sysmex NE-8000, TOA Medical Electronics, Kobe, Japan).
2.4. Western blot analysis
The procedure for protein expression analysis was followed by Western blotting technique as described earlier [Citation27]. Briefly, testis protein from each samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Each membrane was incubated for 1 h in Tris-buffered saline containing 0.1% Tween-20 and 5% skim milk to block nonspecific binding. The membranes were then incubated with specific primary antibodies (1:1000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) against inflammatory mediators. The proteins were detected using horseradish peroxidase-conjugated secondary antibodies and a chemiluminescence detection system (GE Healthcare Life Sciences, Little Chalfont, UK). The internal control used was β-actin and the intensity was analyzed using the ImageJ software package (version 1.41o; National Institutes of Health, NIH, Bethesda, MD, USA).
2.5. Reverse transcription-polymerase chain reaction
RNABee reagent (AMS Bio, Abingdon, UK) was used to extract the total RNA according to the manufacturer’s instructions, and 1 µg of extracted RNA was reverse-transcribed as previously described [Citation28]. Polymerase chain reaction (PCR) was performed as described in our previous report [Citation27], and the primers used for the study are shown in . Band intensities were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band analyzed by NIH ImageJ software (version 1.41o; National Institutes of Health, Bethesda, MD).
Table 1. Primers used in the study.
2.6. Statistical analysis
The results are expressed as the mean ± standard deviation (SD). Statistical significance was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons using the Graph-Pad prism software package (version 6.0; GraphPad, Inc., La Jolla, CA) for Windows. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of COR on body weight increments
The changes in body weight gain in all the groups during the course of experiment were observed on a monthly basis. The body weights of the aged rats were significantly higher compared with the young rats (p < 0.001), which is seen as a normal growth pattern and might be considered as an indicator of aging. However, in COR-treated aged rat groups (5, 10, and 20 mg/kg), the body weight gain was lower when compared with untreated AC group but the changes were not significant (; Supplementary Table S1).
3.2. Effect of COR on the blood chemistry panels
As shown in , the numbers of red blood cell (RBC) and white blood cell (WBC) were decreased significantly (p < 0.01) to more than 10% in AC group when compared with YC group. Although the platelet count in AC rats was also markedly decreased when compared with YC group, the results were not significant. In particular, significant changes in the subtypes of WBC were displayed in aged rats. Aging significantly increased the percentage of neutrophil (p < 0.05), eosinophil (p < 0.01), lymphocyte (p < 0.05), and monocyte (p < 0.05) in YC group. COR treatment (5, 10, and 20 mg/kg) to aged rats significantly improved the RBC count and was found to be nearly normal level in COR-20 group (p < 0.01). However, COR treatment did not influence the WBC count. In addition, the altered subtypes of WBC in AC group were considerably recovered by the administration of COR at all doses (5, 10, and 20 mg/kg). However, significant effect was observed in recovering the altered neutrophil (p < 0.5), eosinophil (p < 0.01), lymphocyte (p < 0.05), and monocytes (p < 0.05) with COR treated at 20 mg/kg dose in aged rats.
Table 2. Effect of COR on the blood chemistry panels.
3.3. Effect of COR on serum biochemical parameters related to liver function
With respect to the serum biochemistry parameters associated with liver functions, AC group showed marked alterations when compared with YC group with exception of Alb levels (). Significant changes were observed in parameters including the AST (p < 0.01), ALT (p < 0.05), ALP (p < 0.05), and γ-GTP (p < 0.05) between YC and AC groups. However, COR (5, 10, and 20 mg/kg) treatment to aged rats ameliorated the altered levels of liver function marker enzymes when compared with AC group. Although we observed a dose-dependent effect with COR (5, 10, and 20 mg/kg) in all the parameters tested, a significant effect was shown by COR treated at 20 mg/kg dose in recovering the parameters related to liver function in aged rats (p < 0.01 for AST and ALT; p < 0.05 for ALP and γ-GTP, respectively).
Table 3. Effect of COR on serum biochemical parameters related to liver function.
3.4. Effect of COR on serum biochemical parameters related to glucose and lipid metabolism
Aging significantly decreased the glucose level and increased the T-Cho and TG levels in AC group when compared with YC group (p < 0.05). Treatment with COR to aged rats ameliorated these changes with a significant recovery observed in glucose and TG levels treated at 20 mg/kg (p < 0.05). In addition, the HDL-C and LDL-C contents deviated from the levels in YC group with aging but did not show significant differences. However, COR treatment (5, 10, and 20 mg/kg) to aged rats showed improved HDL-C and LDL-C levels when compared with AC group (p < 0.01 at COR 20 mg/kg). Interestingly, the improved levels were found to be superior to the levels observed in YC group ().
Table 4. Effect of COR on serum biochemical parameters related to glucose and lipid metabolism.
3.5. Effect of COR on aging mediated inflammatory cytokine expression in rat testis
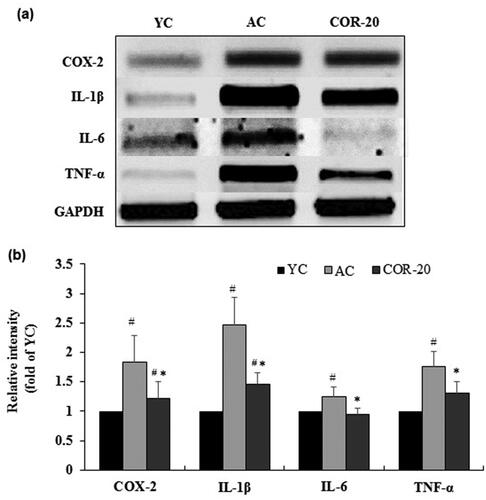
The protein and mRNA expression of inflammatory mediators including COX-2, IL-1β, IL-6, and TNF-α and their corresponding quantification data are shown in and , respectively. The protein expression of cyclooxegenase-2 (COX-2), interleukin (IL)-1β, IL-6, and tissue necrosis factor-alpha (TNF-α) were increased in the testis of AC groups when compared with the YC group. However, treatment with COR at 20 mg/kg attenuated the increased protein expression when compared with AC group (). Quantification data revealed a marked increase in protein level in AC group when compared with YC group and this increase was significantly (p < 0.01) reversed by COR (; Supplementary Table S2). The mRNA expression and the corresponding quantification data showed a similar pattern (; Supplementary Table S3).
Figure 2. Effect of COR on protein expression levels of inflammatory markers. (a) Protein expression of COX-2, IL-1β, IL-6, and TNF-α in YC, AC, and COR-20 groups analyzed by Western blotting in rat testis tissue; (b) Relative intensity levels (fold) in three independent experiments, respectively. β-Actin was used as an internal control. The data are expressed as the mean ± SD. #p < 0.05 compared with YC and *p < 0.05 compared with AC group. COX-2: cyclooxegenase-2, IL: interleukin, TNF-α: tumor necrosis factor-α, YC: young control, AC: aged control, COR-20: aged rats plus cordycepin 20 mg/kg treated group.
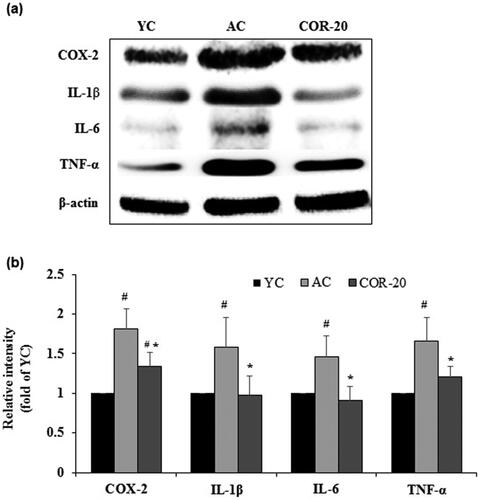
Figure 3. Effect of COR on the mRNA expression levels of inflammatory markers. (a) mRNA expression of COX-2, IL-1β, IL-6, and TNF-α analyzed by RT-PCR analysis in rat testis tissue; (b) Relative intensity levels (fold) in three independent experiments, respectively. GAPDH was used as an internal control. The data are expressed as the mean ± SD. #p < 0.05 compared with YC and *p < 0.05 compared with AC group. COX-2: cyclooxegenase-2, IL: interleukin, TNF-α: tumor necrosis factor-α, YC: young control, AC: aged control, COR-20: aged rats plus cordycepin 20 mg/kg treated group.
3.6. Effect of COR on aging mediated NF-κB signaling in rat testis
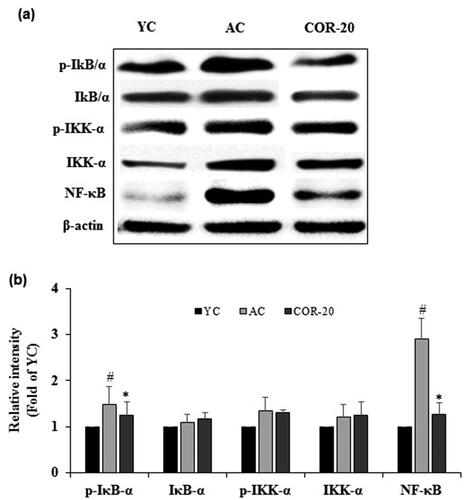
Compared with the YC group, the protein expression of phosphorylated IκB-α, IκB kinase-α (IKK-α), and nuclear factor-κB (NF-κB) were increased in AC group. COR (20 mg/kg) treated aged rats showed a marked decrease in phospo (p)-IκB-α, p-IKK-α, and NF-κB expression () when compared with AC group. Quantification data indicated that the relative intensities of p-IκB-α, p-IKK-α, and NF-κB were increased when compared to YC group, but significant (p < 0.05) increase was observed only in p-IκB-α and NF-κB molecules (Supplementary Table S4). COR treatment to aged rats showed significant (p < 0.05) reduction in the increased levels of p-IκB-α and NF-κB expression (). Although the expression of p-IKK-α was slightly decreased when compared with AC group, the values were not significant. Furthermore, there were no significant changes in the expression level of IκB/α, and IKK-α between the YC, AC, and COR-20 groups.
Figure 4. Effect of COR on expression of NF-κB-mediated inflammatory responses. (a) Protein expression of phospho-IκB-α, IκB-α, phospho-IKK-α, IKK-α, and NF-κB in YC, AC, and COR-20 groups analyzed by Western blotting in rat testis tissue; (b) Relative intensity levels (fold) in three independent experiments, respectively. β-Actin was used as an internal control. The data are expressed as the mean ± SD. #p < 0.05 compared with YC and *p < 0.05 compared with AC group. NF-κB: nuclear factor-κB, IKK-α: IκB kinase-α, IκB-α: NF-κB inhibitor-α, YC: young control, AC: aged control, COR-20: aged rats plus cordycepin 20 mg/kg treated group.
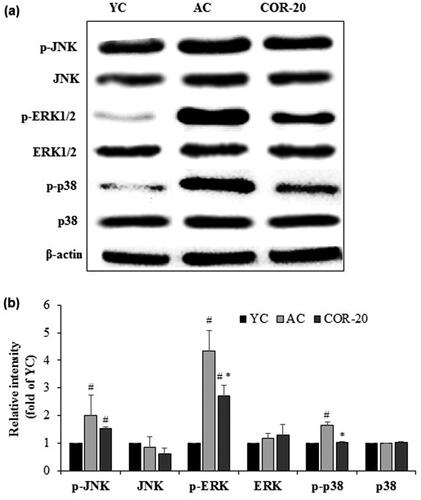
3.7. Effect of COR on aging mediated inflammatory MAPK signaling in rat testis
To understand the mechanistic pathway involved in the protective effect of COR in aging-mediated testicular inflammation, we further assessed the mitogen-activated protein kinase (MAPK) signaling expression including the c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 MAPKs, in testis of aged rats by detecting their phosphorylated forms by Western blot analysis using respective phospho-specific antibodies (). Compared with YC group, the expression of these molecules was increased in AC group. Results showed that the aging-associated inflammatory responses strongly enhanced the phosphorylation of all the three MAPKs tested (p-JNK, p-ERK, and p-p38 MAPKs). However, COR (20 mg/kg) treated aged rats ameliorated the increased expression. Quantification data revealed a marked reduction in the increased expression of these molecules with significant (p < 0.05) levels observed with p-ERK, and p-p38 MAPKs (Supplementary Table S5). Although the increased p-JNK expression observed in AC rats was reduced in COR-20 group, the values were not significant (). Furthermore, there were no significant changes in the expression level of JNK, ERK, and p38 MAPKs between the YC, AC, and COR-20 groups.
Figure 5. Effect of COR on MAPKs-mediated inflammatory signaling. (a) Protein expression of phospho-JNK, JNK, phospho-ERK1/2, ERK1/2, phospho-p38, and p38 MAPKs in YC, AC, and COR-20 groups analyzed by Western blotting in rat testis tissue; (b) Relative intensity levels (fold) in three independent experiments, respectively. β-Actin was used as an internal control. The data are expressed as the mean ± SD. #p < 0.05 compared with YC and *p < 0.05 compared with AC group. YC: young control, AC: aged control, COR-20: aged rats plus cordycepin 20 mg/kg treated group, p-JNK: phospho-c-Jun N-terminal kinase, ERK: extracellular signal-regulated kinase, p-38 MAPK: p38 mitogen-activated protein kinase.
4. Discussion
It is well understood that there is an accelerated inflammatory condition and imbalance in inflammatory homeostasis with increase in age. Aging and inflammation the two closely related processes termed as “inflammaging” can affect multi-organ system contributing to the pathogenesis of various ARDs including the dysfunction in male reproductive system [Citation29,Citation30]. Although decrease in sexual libido and desire can be considered normal during aging process, the impact of inflammaging in male reproductive tract might enhance the sexual problems causing erectile dysfunction, premature ejaculation, impotence, and testicular damage [Citation31,Citation32]. Since the processes of inflammaging are low grade, chronic, and micro-inflammatory in nature, extensive research has been done to identify a potential therapeutic agent in attenuating the inflammaging process in male reproductive system. Delaying the aging of the testis and improving the physiological sexuality in aged men is an important strategy as the testis is the major organ in spermatogenesis.
Earlier report indicated that rats with age range from 6 to 12 months (∼30 years of human age) can be considered as young adults and rats with age range 20–30 months (∼60–75 years of human age) as aged rats [Citation33]. Therefore, in the present study, we used the young control (8 months old) and naturally aged rats (20 months old) to study the effects of COR by evaluating various biochemical aspects. COR-treated aged rats significantly decreased the body weight gain compared with untreated aged rats. Although the changes in body weights might not be considered as potential markers in aging, our observations might support the earlier study that COR possess hypolipidemic and antiadipogenic effects thereby aiding in maintaining the body weights in COR-treated aged rats [Citation34].
Previous studies revealed that aging and inflammation alters the blood cell counts significantly with decreased levels of RBC, WBC, and platelets. Alternatively, elevated levels of almost all subtypes of WBC counts, including eosinophils, monocytes, neutrophils, and lymphocyte were observed [Citation35–37]. In agreement, in the present study, aged rat groups showed decreased RBC, WBC, and platelet count compared with young rats. The WBC subtypes including eosinophils, neutrophils, monocytes, and lymphocytes were elevated in aged rats compared with YC rats. COR treatment alleviated the decreased RBC count significantly but did not show significance in the WBC and platelet count. In general, adult populations show an increased level of WBC and neutrophil count during inflammatory states [Citation38]. However, aged and geriatric patients did not develop an increase in WBC count during inflammatory conditions, which might be due to the misadapted response of the immune system [Citation39]. WBC count, a biomarker of immunological function, is reduced under cardiovascular diseases, smoking, and obesity [Citation40,Citation41]. In the present study, COR treatment to aged rats improved dose-dependently the increased neutrophil count when compared with AC group. The increased eosinophil, lymphocyte, and monocyte counts caused by aging were also reduced. Accordingly, cordycepin is expected to boost immunological function of the elderly people.
The physiological aging of liver is low in comparison with other organs due to its immense regeneration capacity. However, studies indicated that older individuals develop increased liver enzymes and these changes are associated with increasing risk of cardiovascular mortality [Citation42]. Normal levels to slight decrease in serum albumin and elevated levels of AST, ALT, ALP, and γ-GTP were observed with aging [Citation43]. Furthermore, aging and inflammation together elevate liver enzymes and attenuate liver functions thereby accelerating the onset of various liver diseases [Citation44]. In the present study, the liver enzyme (AST, ALT, ALP, and γ-GTP) levels in aged rats were significantly increased in agreement with the reported literature. In the conditions of low-grade chronic inflammation in aging, altered glucose and lipid metabolism was also observed [Citation45]. In the present study, COR improved the altered parameters associated with lipid metabolism suggesting that COR successively recovers liver functions in aged rats.
In the blood chemistry panels and serum biochemical profile evaluations, although we observed a dose-dependent effect at 5, 10, and 20 mg/kg, significant values were observed in all parameters tested with COR treated at 20 mg/kg (COR-20 group). Therefore, COR at 20 mg/kg dose was used to study the effects on aging-associated inflammatory responses in the testis of aged rats. Chronic low-grade inflammation is thought to be a determinant of the aging, and age-related increase in the systemic levels of several gerokines such as chemokines and cytokines was observed when compared with young individuals [Citation46,Citation47]. Upregulation of COX-2 expression induced by IL-1β and its receptors in testicular cells and increased COX-2 mRNA levels in Norway rat Leydig cells was reported [Citation48,Citation49]. Age-related decline of male reproductive functions was directly linked to the upregulation of IL-1β, TNFα, and prostaglandins [Citation50]. In the present study, there is a significant increase in the inflammatory mediators and the proinflammatory cytokine such a COX-2, IL-1β, IL-6, and TNF-α in aged control rat testes when compared with young controls that are predicted to contribute to the inflammaging. COR treatment (20 mg/kg) to aged rats significantly attenuated the aging-associated inflammatory responses by restoring the excessive inflammatory cytokines production in comparison with aged control rats. Our results are in agreement with earlier reported works that ginsenoside Rg1 and a traditional Chinese herbal formula (Liuweidihuang Pills) inhibited the increased levels of proinflammatory cytokines and alleviated inflammation in aged testicular tissues in experimental aged mice and rats [Citation51,Citation52].
The NF-κB signaling pathway and its activation by inflammatory stresses in various tissues has been proposed to be one of the key mediators of aging and associated with several ARDs. NF-κB is a family of transcription factors implicated in numerous stress responses including male testicular cell death [Citation53]. NF-κB activation accelerates the release of proinflammatory molecules, which was observed in aged rats. Earlier report indicated that regulation of NF-κB activity can be helpful in counteracting testicular stress thereby preserving testicular function in aged mice [Citation54]. NF-κB exists in the cytoplasm with IκBα in an inactive state. During aging IKK (IκB kinase) is activated, resulting in the IKKβ-mediated phosphorylation of IκBα. Phosphorylated IκBα is subsequently ubiquitinated and degraded, leading to release and activation of NF-κB complex [Citation55]. NF-κB is then transferred from the cytoplasm to the nucleus, where it induces the expression of downstream proinflammatory factors genes such as IL-1, IL-6, and TNF-α [Citation56,Citation57]. In this study, the expression of NF-κB and phosphorylation of IκB-α increased in aged rat groups and COR treatment to aged rats significantly inhibited the activation of NF-κB and phosphorylation of IκB-α expression indicating that COR might play a potential role in inhibitive proinflammatory activations via the NF-κB signaling system.
MAPKs have been shown to play important roles in inflammation-associated ARDs mediating the inhibition of cell proliferation or cell death. The sub-families including the JNK, ERK, and the p38 MAPKs were activated by mitogens, stress response, and inflammatory cytokines [Citation58,Citation59]. MAPKs have shown to decline the function of male reproductive system by negatively affecting the spermatogenesis, sperm maturation and activation, capacitation, acrosome reaction ultimately reducing the semen quality and fertility [Citation60]. Previous reports indicated that the sperm development in mammalian testis is regulated by MAPKs and abnormal activation of MAPKs can dysfunction Sertoli cells and affect the germ cell development [Citation61]. Furthermore, activation of MAPKs by reproductive toxicants including the bisphenol A, 1–3-dinitrobenzene and 4-nonylphenol isomers in Sertoli TM4 cells was observed [Citation62–64]. Furthermore, reduced ERK and p38 MAPK activities were observed in brains of aged animals and MAPKs signaling is involved in neuronal damage in aged rat brain tissues [Citation65]. MAPKs activity was also involved in dermal inflammaging induced by particulate matter in human keratinocytes and fibroblasts [Citation66]. In the present study, significant increase in the MAPKs expression including the JNK, ERK1/2, and p38 MAPK phosphorylation levels was shown in the testis of aged rats. However, treatment with COR (20 mg/kg) ameliorated these changes indicating that COR might regulate the increase in MAPKs activities thereby increase the reproductive success in aged rats.
5. Conclusions
The present study showed that long-term administration of COR improved the serum biochemical parameters in aged rats and attenuated aging-associated testicular inflammatory responses via regulating the NF-κB/MAPKs signaling pathways indicating its potential benefits in inflammaging-mediated testicular and male sexual dysfunctions.
Supplemental Material
Download MS Word (498.4 KB)Disclosure statement
The authors declare no conflicts of interests in publishing this article.
Additional information
Funding
References
- Frungieri MB, Calandra RS, Bartke A, et al. Ageing and inflammation in the male reproductive tract. Andrologia. 2018;50(11):e13034.
- Xia S, Zhang X, Zheng S, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016; 2016:8426874–8426812.
- Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581–601.
- Costa C, Albersen M. Erectile dysfunction in inflammaging. In: Bagchi BR, editor. Inflammation, advancing age and nutrition. The Netherlands: Elsevier; 2014. p. 287–295.
- Krause W. Male accessory gland infection. Andrologia. 2008;40(2):113–116.
- Rusz A, Pilatz A, Wagenlehner F, et al. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol. 2012;30(1):23–30.
- Jiang H, Zhu W-J, Li J, et al. Quantitative histological analysis and ultrastructure of the aging human testis. Int Urol Nephrol. 2014;46(5):879–885.
- Sibert L, Lacarrière E, Safsaf A, et al. Aging of the human testis. Presse Med. 2014;43(2):171–177.
- Azenabor A, Ekun AO, Akinloye O. Impact of inflammation on male reproductive tract. J Reprod Infertil. 2015;16:123–129.
- Koppula S, Akther M, Haque ME, et al. Potential nutrients from natural and synthetic sources targeting inflammaging—a review of literature, clinical data and patents. Nutrients. 2021;13(11):4058.
- Radhi M, Ashraf S, Lawrence S, et al. A systematic review of the biological effects of cordycepin. Molecules. 2021;26(19):5886.
- Olatunji OJ, Tang J, Tola A, et al. The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316.
- Lee C-T, Huang K-S, Shaw J-F, et al. Trends in the immunomodulatory effects of Cordyceps militaris: total extracts, polysaccharides and cordycepin. Front Pharmacol. 2020;11:575704.
- Wang Z, Chen Z, Jiang Z, et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat Commun. 2019;10(1):2538.
- Xu J-C, Zhou X-P, Wang X-A, et al. Cordycepin induces apoptosis and G2/M phase arrest through the ERK pathways in esophageal cancer Cells. J Cancer. 2019;10(11):2415–2424.
- Jin Y-T, Qi Y-Q, Jin M, et al. Synthesis, antitumor and antibacterial activities of cordycepin derivatives. J Asian Nat Prod Res. 2021;1–11.
- Govindula A, Pai A, Baghel S, et al. Molecular mechanisms of cordycepin emphasizing its potential against neuroinflammation: an update. Eur J Pharmacol. 2021;908:174364.
- Choi YH, Kim G-Y, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Dev Ther. 2014;8:1941.
- Shin S, Lee S, Kwon J, et al. Cordycepin suppresses expression of diabetes regulating genes by inhibition of lipopolysaccharide-induced inflammation in macrophages. Immune Netw. 2009;9(3):98–105.
- Jo WS, Choi YJ, Kim HJ, et al. The anti-inflammatory effects of water extract from Cordyceps militaris in murine macrophage. Mycobiology. 2010;38(1):46–51.
- Verma AK. Cordycepin: a bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID-19. J Biomol Struct Dyn. 2020;1–8. DOI:10.1080/07391102.2020.1850352
- Verma AK, Aggarwal R. Repurposing potential of FDA-approved and investigational drugs for COVID-19 targeting SARS-CoV-2 spike and main protease and validation by machine learning algorithm. Chem Biol Drug Des. 2021;97(4):836–853.
- Chen Y-C, Chen Y-H, Pan B-S, et al. Functional study of Cordyceps sinensis and cordycepin in male reproduction: a review. J Food Drug Anal. 2017;25(1):197–205.
- Chang Y, Jeng K-C, Huang K-F, et al. Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague-Dawley rats. Am J Chin Med. 2008;36(5):849–859.
- Ramesh T, Yoo S-K, Kim S-W, et al. Cordycepin (3'-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012;47(12):979–987.
- Kopalli SR, Cha K-M, Lee S-H, et al. Cordycepin, an active constituent of nutrient powerhouse and potential medicinal mushroom Cordyceps militaris linn., ameliorates age-related testicular dysfunction in rats. Nutrients. 2019;11(4):906.
- Kopalli SR, Cha K-M, Ryu J-H, et al. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis-related molecules. Exp Gerontol. 2017; 90:26–33.
- Won Y-J, Kim B, Shin Y-K, et al. Pectinase-treated panax ginseng extract (GINST) rescues testicular dysfunction in aged rats via redox-modulating proteins. Exp Gerontol. 2014;53:57–66.
- Berdasco M, Esteller M. Hot topics in epigenetic mechanisms of aging: 2011. Aging Cell. 2012;11(2):181–186.
- Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236.
- Chung E. Sexuality in ageing male: review of pathophysiology and treatment strategies for various male sexual dysfunctions. Med Sci. 2019; 7:98.
- Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27(4):557–579.
- Sengupta P. The laboratory rat: relating its age with humans. Int J Prev Med. 2013;4(6):624–630.
- Takahashi S, Tamai M, Nakajima S, et al. Blockade of adipocyte differentiation by cordycepin. Br J Pharmacol. 2012;167(3):561–575.
- Kubota K, Shirakura T, Orui T, et al. Changes in the blood cell counts with aging. Nihon Ronen Igakkai Zasshi. 1991;28(4):509–514.
- Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: Infants to elderly. Scand J Immunol. 2016;83(4):255–266.
- Kounis NG, Soufras GD, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. 2015;21(2):139–143.
- Mardi D, Fwity B, Lobmann R, et al. Mean cell volume of neutrophils and monocytes compared with C-reactive protein, interleukin-6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. Int J Lab Hematol. 2010;32:410–418.
- Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960.
- Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998;31(6):1217–1225.
- Alexander RW. Inflammation and coronary artery disease. N Engl J Med. 1994;331(7):468–469.
- Mahady SE, Wong G, Turner RM, et al. Elevated liver enzymes and mortality in older individuals: a prospective cohort study. J Clin Gastroenterol. 2017;51(5):439–445.
- Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–191.
- Franceschi C, Garagnani P, Morsiani C, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne). 2018;5:61.
- Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590.
- Fulop T, Witkowski JM, Pawelec G, et al. On the immunological theory of aging. Interdiscip Top Gerontol. 2014; 39:163–176.
- Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15(26):3003–3026.
- Matzkin ME, Mayerhofer A, Rossi SP, et al. Cyclooxygenase-2 in testes of infertile men: evidence for the induction of prostaglandin synthesis by interleukin-1β. Fertil Steril. 2010;94(5):1933–1936.
- Syntin P, Chen H, Zirkin BR, et al. Gene expression in brown Norway rat leydig cells: effects of age and of age-related germ cell loss. Endocrinology. 2001;142(12):5277–5285.
- Hales DB. Regulation of leydig cell function as it pertains to the inflammatory response. In Payne AH, Hardy MPH, editors. The Leydig cell in health and disease. Totowa (NJ): Humana Press; 2007. p. 117–131.
- Wang Y, Yang Z, Yang L, et al. Liuweidihuang pill alleviates inflammation of the testis via AMPK/SIRT1/NF-κ B pathway in aging rats. Evid Based Complem Altern Med. 2020;2020:1–9.
- Wang Z, Chen L, Qiu Z, et al. Ginsenoside Rg1 ameliorates testicular senescence changes in D-gal-induced aging mice via anti-inflammatory and antioxidative mechanisms. Mol Med Rep. 2018;17(5):6269–6276.
- Agarwal A. NF-κB in male reproduction: a boon or a bane? TORSJ. 2011;3(1):85–91.
- Zhao X, Bian Y, Sun Y, et al. Effects of moderate exercise over different phases on age-related physiological dysfunction in testes of SAMP8 mice. Exp Gerontol. 2013;48(9):869–880.
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000; 18:621–663.
- Lu L, Wu C, Lu B, et al. BabaoDan cures hepatic encephalopathy by decreasing ammonia levels and alleviating inflammation in rats. J Ethnopharmacol. 2020;249:112301.
- Chen X, Zhang C, Wang X, et al. Juglanin inhibits IL-1β-induced inflammation in human chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47(1):3614–3620.
- Wang X, Martindale JL, Liu Y, et al. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(2):291–300.
- Xia Z, Dickens M, Raingeaud J. L, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331.
- Li MWM, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15(4):159–168.
- Urriola-Muñoz P, Lagos-Cabré R, Moreno RD. A mechanism of male germ cell apoptosis induced by Bisphenol-A and nonylphenol involving ADAM17 and p38 MAPK activation. PLoS One. 2014;9(12):e113793.
- Peretz J, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007-2013. Environ Health Perspect. 2014;122(8):775–786.
- Lee YS, Yoon H-J, Oh J-H, et al. 1,3-Dinitrobenzene induces apoptosis in TM4 mouse sertoli cells: Involvement of the c-Jun N-terminal kinase (JNK) MAPK pathway. Toxicol Lett. 2009;189(2):145–151.
- Liu X, Nie S, Chen Y, et al. Effects of 4-nonylphenol isomers on cell receptors and mitogen-activated protein kinase pathway in mouse sertoli TM4 cells. Toxicology. 2014; 326:1–8.
- Zhen X, Uryu K, Cai G, et al. Age-Associated impairment in brain MAPK signal pathways and the effect of caloric restriction in fischer 344 rats. J Gerontol Ser A: Biol Sci Med Sci. 1999;54(12):B539–B548.
- Kim M, Kim JH, Jeong GJ, et al. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp Dermatol. 2019;28(7):809–815.
