Abstract
A new fungus isolated from the leaves of Eleutharrhena macrocarpa in southern Yunnan, China is described using morphological and molecular evidence. Phylogenetic trees based on the combined nuclear ribosomal DNA internal transcribed spacer (ITS), translation elongation factor-1α (TEF1), and β-tubulin gene (TUB2) sequences showed that Diaporthe eleutharrhenae sp. nov. is sister to Diaporthe chinensis N.I. de Silva, Lumyong & K.D. Hyde and morphologically differs in shorter alpha conidia (5–8.5 × 1.5–2 µm) and the presence of beta conidia. This study also resolves a nomenclatural problem, as two taxa were published using the same name. To avoid confusion, the unrelated D. chinensis H. Dong, J. W. Xia & X. G. Zhang is here renamed as D. dongii (H. Dong, J. W. Xia & X. G. Zhang) S. J. Song & Landrein, sp. nov. in honor of the author that described this species. Study and description of fungi associated with threatened tropical species could help to understand their ecology as well as the potential spread of fungi onto cultivated crop species.
1. Introduction
With more than 19,000 plant species, Yunnan is a biodiversity hotspot [Citation1]. The southern Yunnan flora, including Eleutharrhena macrocarpa (Diels) Forman, has strong affinities with the southeast flora of Asia [Citation2] and is threatened by climate change and loss of native forests. E. macrocarpa is a critically endangered species in the Menispermaceae family and also a plant species with extremely small populations (PSESP) with less than 100 individuals remaining [Citation3]. Menispermaceae species have high alkaloid and tannin content and are frequently used as medicinal plants including a promising treatment for cancer [Citation4]. Few associated fungi have been reported from this family [Citation5,Citation6], and to date, no fungi associated with E. macrocarpa have been described.
Diaporthe is a very large genus including more than 1000 published species names (http://www.indexfungorum.org/Names/Names.asp). The genus is widely distributed throughout the world, includes many pathogens, epiphytes, endophytes, and saprobes and is associated with a wide range of plant hosts [Citation7]. Some pathogenic Diaporthe species are responsible for serious economic damage and can cause severe cases of leaf spot, blight, wilt, dieback, or canker [Citation8].
Diaporthe classification was previously based solely on morphological characters resulting in many duplicate names and nomenclature problems. A combination of morphology and phylogenetic data has recently become prevalent and the nomenclature within the genus is now more stable [Citation9]. Internal transcribed spacer (ITS), translation elongation factor-1α (TEF1), and β-tubulin gene (TUB2) sequences are widely used for identification within Diaporthe [Citation10].
During an investigation of the E. macrocarpa phyllosphere in southern Yunnan, we found a new species that is published here. Diaporthe eleutharrhenae sp. nov. is described and compared to sister taxa, using morphology and phylogenetic results based on ITS, TEF1, and TUB2 sequences.
2. Material and methods
2.1. Morphological observations
The specimens used in this study were deposited in the herbarium of China Forestry Culture Collection Center and China General Microbiological Culture Collection Center. Sterilized potato dextrose agar (PDA) produced by Acmec Tech. Co., Ltd (Shanghai, China) was prepared (46 g/L) for isolating fungi and purifying fungi from E. macrocarpa. Synthetic low-nutrient agar medium (SNA) produced by Coolaber biochemical Tech. Co., Ltd (Beijing, China) was prepared (3.15 g/L) for generating conidia. Observations were carried out using a 1000X microscope (Eclipse Ci, Nikon, Co., Ltd, Tokyo, Japan) after 15 d growth on a SPH-310A shaker (Shiping Tech. Co., Ltd, Beijing, China) at 150 rpm and in the dark.
2.2. DNA extraction, DNA sequencing, and phylogenetic analysis
Mycelium DNA was collected and extracted using the DNA extraction kit (SK8259, Sangon Biotech. Co., Ltd, Shanghai, China). The nuclear rDNA ITS was amplified with primers ITS1/ITS4 [Citation11]; Nuclear rDNA TEF-1, and TUB2 were amplified using the primers EF1–728/EF1– 986 [Citation12] and BT2a/BT2b [Citation13], respectively. PCR was carried out in a 25 µL volume, including 12.5 µL PCR mix, 1 µL of each primer, 1 µL template DNA, and 9.5 µL ddH2O, following the protocol described here: one 5 min cycle of initial denaturation at 95 °C, 30 cycles of denaturation (94 °C) and annealing (57 °C), one cycle of extension at 72 °C, and a 10 min final extension cycle at 72 °C. PCR products were purified and sequenced by Sangon Biotechnology Co., Ltd (Shanghai, China) and sequences were then uploaded to the NCBI database ().
Table 1. ITS, TEF1, and TUB2 sequences used for the identification of Diaporthe eleutharrhenae.
Taxa for phylogenetic analysis and outgroups were chosen according to previous studies [Citation14,Citation15] and additional related species were added according to the ITS, TEF1, and TUB2 blast results. Sequences were then downloaded from the NCBI database ().
The sequences were aligned and trimmed in Geneious and then concatenated in PhyloSuite [Citation16]. Diaporthella corylina was used as the outgroup [Citation14,Citation17]. The best nucleotide substitution model was chosen using ModelFinder [Citation17] and a Bayesian analysis was performed using MrBayes [Citation18] with two parallel runs of 500,000 generations. Maximum likelihood analysis was performed using IQ-tree [Citation19] with 5000 bootstraps.
3. Taxonomy
Diaporthe eleutharrhenae S. J. Song & Landrein, sp. nov.
Etymology: The epithet eleutharrhenae refers to the free stamens in the flowers of the genus Eleutharrhena, the host of Diaporthe eleutharrhenae.
Diagnosis: Diaporthe eleutharrhenae can be distinguished from its closest related sister species D. chinensis N. I. de Silva, Lumyong & K. D. Hyde [Citation14] by the shorter alpha conidia (5–8.5 µm, 10–14 in D. chinensis) and the presence of beta conidia (beta conidia are absent in D. chinensis) (; ).
Figure 1. (A) Pycnidia of Diaporthe eleutharrhenae isolated from synthetic low-nutrient agar medium (SNA) on day 15. (B) Secretion of D. eleutharrhenae on the PDA Petri dish and on day 20. (C and D) Colony morphology of D. eleutharrhenae observed from the top and below the PDA Petri dish on day 7. (E and F) Alpha conidia. (G) beta conidia. Conidia were isolated from synthetic low-nutrient agar medium (SNA). Scale bars in A, E, F, G are 5 µL. Scale bar in B is 50 µL. Scale bars in C and D are 1 cm. Shorter alpha conidia (5–8.5 µm) and the presence of beta conidia are a key character for distinguishing D. eleutharrhenae from the most closely related species D. chinensis N. I. de Silva, Lumyong & K. D. Hyde.
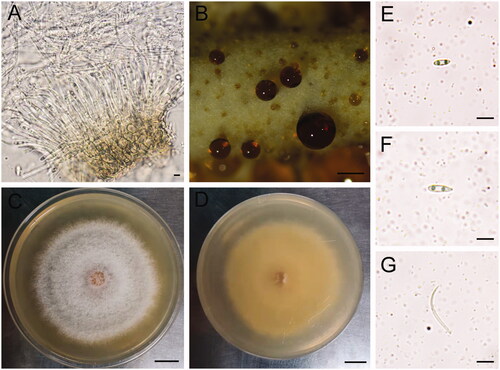
Table 2. Synoptic characters of Diaporthe eleutharrhenae and its most closely related species.
Description: Conidiomata pycnidia, globose or irregular, solitary or aggregated together, dark brown to black. Conidia exuding from the pycnidia in white to cream drops. Conidiophores cylindrical, straight or slightly curved. Alpha conidia 5–8.5 × 1–2.8 µm (n = 60), hyaline, obovate to clavate with both ends obtuse. Beta conidia 16.5–56.1 × 0.67–3.33 µm (n= 0) curved and hyaline, one end obtuse, another end acute.
Cultures: Colonies can reach 60 mm in diameter after 7 d at 25 °C. Colonies on PDA are flat, with abundant dirty white and yellowish pigmented mycelium showing in the later stages. Pale to dark brownish pigmented rings can be seen. Brown secretion can be observed after ca.14 d PDA.
Typus: Yunnan Province, China, 1.5 km from Manbian road (21°57′N, 101°15′E) Menglun town, Xishuangbanna Dai Autonomous prefecture, May 2021.
Known distribution: Currently known from a single site in Xishuangbanna Dai Autonomous prefecture in Yunnan, China.
Host: Currently only known from E. macrocarpa.
4. Result and discussion
The combined ITS, TEF1, and TUB2 matrix is 1452 bp in length and the phylogenetic tree included 42 samples. TIMEF + G (ITS), TVM + I +G (TEF1), and HKY + G (TUB2) substitution models were selected for maximum likelihood and Bayesian analyses. Phylogenetic trees based on each region were congruent and indicate that Diaporthe eleutharrhenae is sister to Diaporthe chinensis N. I. de Silva, Lumyong & K.D. Hyde with high support (Maximum likelihood bootstrap = 94%, Bayesian posterior probability = 1) ().
Figure 2. Maximum likelihood tree based on two collections of Diaporthe eleutharrhenae and its closest related species using ITS, TEF1, and TUB2 sequences. “–” means no support. Maximum likelihood bootstrap value and Bayesian posterior probabilities are shown next to the branch node. Diaporthe dongii (H. Dong, J. W. Xia & X. G. Zhang) S. J. SONG & Landrein is the suggested new name of Diaporthe chinensis H. Dong, J. W. Xia & X. G. Zhang. Results strongly support Diaporthe chinensis N. I. de Silva, Lumyong & K. D. Hyde as the closest related species of Diaporthe eleutharrhenae.
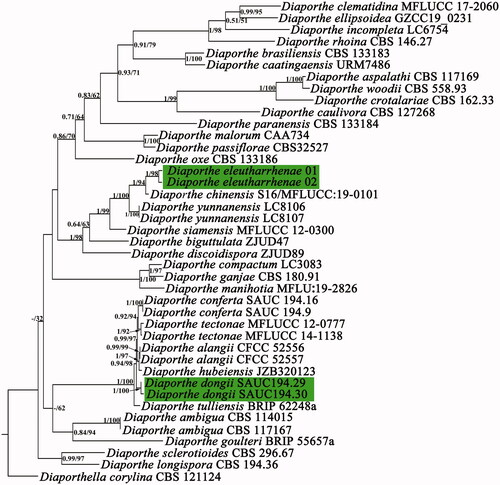
During the preparation of sequences for phylogenetic analysis, we found two different fungi taxa had the same name, Diaporthe chinensis N. I. de Silva, Lumyong & K.D. Hyde [Citation14] and Diaporthe chinensis H. Dong, J. W. Xia & X. G. Zhang [Citation15].
D. chinensis N. I. de Silva, Lumyong & K. D. Hyde is sister to Diaporthe yunnanensis Y. H. Gao & L. Cai isolated from healthy leaves of Magnolia candolli and published on March 25 2021 [Citation14]. It was described based on large alpha conidia (10–14 × 3–6 µm) and its phylogenetic position. Diaporthe chinensis H. Dong, J. W. Xia & X. G. Zhang is sister to D. conferta isolated from Litchi chinensis leaves and published on March 22 2021 [Citation15]. Its description was based on the host identity, distribution, the existence of beta conidia and gamma conidia, as well as its phylogenetic position. To avoid confusion and following the International Code of Nomenclature for algae, fungi, and plants [Citation22], we propose the renaming of Diaporthe chinensis H. Dong, J. W. Xia & X. G. Zhang to Diaporthe dongii (H. Dong, J. W. Xia & X. G. Zhang) S. J. Song & Landrein.
Diaporthe species are associated with diverse hosts. Their life cycle is variable, and their distribution can be narrow or wide. This variability is reflected by our results; D. dongii is a pathogenic species found on cultivated Litchi chinensis, and D. eleutharrhenae is an epiphytic species growing on the leaves of the native and endangered liana E. macrocarpa. It is not known if D. eleutharrhenae occurs onto cultivated species. The population of E. macrocarpa is located at the margin of a fragmented forest and is surrounded by grapefruit and rubber plantations. It is not known at present if D. eleutharrhenae is specific to its host or it could be more widespread and be associated with other rainforest species. To our knowledge, no fungi have been reported to be associated with E. macrocarpa before. These results will contribute to the research in the conservation of E. macrocarpa as well as our understanding of the potential spread of native fungi onto cultivated crops.
Acknowledgements
The authors thank Jo Osborne for manuscript review and editing, and thank to Pang ZhiQiang and Hao Chunhui and Shao ShiCheng for providing suggestions on conducting this study.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Qian LS, Chen JH, Deng T, et al. Plant diversity in Yunnan: current status and future directions. Plant Divers. 2020;42(4):281–291.
- Zhu H, Yan LC. 2012. Native seed plants in Xishuangbanna of Yunnan. Beijing, China: Science Press.
- Hou ZQ, Zhou D, Hou SN, et al. Present situation of Eleutharrhena macrocarpa in China. Plant Divers Resour. 2015;37(5):640–646.
- Muharini R, Enawaty E. Cytotoxic activity of stem of Pycnarrhena cauliflora through apoptosis induction on human breast cancer cell line T47D. Pharm Sci Res. 2019;6(3):5.
- Mishra A, Gond SK, Kumar A, et al. Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb Ecol. 2012;64(2):388–398.
- Tanda S. Two new species of powdery mildew fungi from Japan. Mycoscience. 1994;35(1):49–52.
- Yang Q, Fan XL, Guarnaccia V, et al. High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. MycoKeys. 2018;39:97–149.
- Gao YH, Liu F, Duan WJ, et al. Diaporthe is paraphyletic. Ima Fungus. 2017;8(1):153–187.
- Gomes RR, Glienke C, Videira SIR, et al. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41.
- Sun XD, Cai XL, Pang QQ, et al. 2021. First report of Diaporthe longicolla causing leaf spot on Kalanchoe pinnata in China. Plant Dis. 2021;105(11):3739.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocol. 1990;18(1):315–322.
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–556.
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330.
- De Silva NI, Maharachchikumbura SSN, Thambugala KM, et al. Morpho-molecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere. 2021;12(1):163–237.
- Dong H, Mu TC, Zhang Z, et al. Molecular phylogenetic analysis reveals two new species of Diaporthe from Yunnan province, southwestern China. Mycosystema. 2021;40:436–446.
- Zhang D, Gao FL, Jakovlic I, et al. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2020;20(1):348–355.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, et al. Model finder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14(6):587–589.
- Ronquist F, Teslenko M, Van Der Mark P, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542.
- Minh BQ, Schmidt HA, Chernomor O, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37(5):1530–1534.
- Udayanga D, Liua X, Mckenzie EH, et al. Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptog Mycol. 2012;33(3):295–309.
- Huang F, Udayanga D, Wang X, et al. Endophytic Diaporthe associated with citrus: a phylogenetic reassessment with seven new species from China. Fungal Biol. 2015;119(5):331–347.
- Turland NJ, Wiersema JH, Barrie FR, et al. 2018. International code of nomenclature for algae, fungi, and plants (Shenzhen code) adopted by the nineteenth international botanical congress Shenzhen, China. Glashütten, Germany: Koeltz Botanical Books.
