Abstract
Orchids live with mycorrhizal fungi in mutualism. This symbiotic relationship plays an essential role in the overall life cycle of orchids from germination, growth, settlement, and reproduction. Among the 1000 species of the orchid, the Korean lady’s slipper, Cypripedium japonicum, is known as an endangered species. Currently, only five natural habitats of the Korean lady’s slipper remain in South Korea, and the population of Korean lady’s slipper in their natural habitat is not increasing. To prevent extinction, this study was designed to understand the fungal community interacting in the rhizosphere of the Korean lady’s slipper living in the native and artificial habitats. In-depth analyses were performed to discover the vital mycorrhizal fungi contributing to habitat expansion and cultivation of the endangered orchid species. Our results suggested that Lycoperdon nigrescens contributed most to the increase in natural habitats and Russula violeipes as a characteristic of successful cultivation. And the fungi that helped L. nigrescens and R. violeipes to fit into the rhizosphere community in Korean lady’s slipper native place were Paraboeremia selaginellae and Metarhizium anisopliae, respectively. The findings will contribute to restoring and maintaining the endangered orchid population in natural habitats.
1. Introduction
Cypripedium japonicum Thunberg, Korean lady’s slipper, is an orchid species natively inhabiting Korea, China, and Japan. Cypripedium japonicum is classified as a critically endangered species which has been defined at extremely high risk of extinction in the wild by the National Institute of Biological Resources (NIBR) [Citation1] and as an endangered species, defined at very high risk of extinction in the Red List of Threatened Species in the International Union for Conservation of Nature (IUCN) [Citation2]. Currently, in Korea, only five natural habitat sites for Korean lady’s slipper are reminded, and each area has 200 to 1000 wild individual populations [Citation1,Citation2]. One of their endangered causes is habitat destruction, and in this regard, there is an unusual case as follows. In 1989, the C. japonicum habitat had been destroyed by a dam construction (latitude 38°12′37′′, longitude 127°50′46′′). The abandoned C. japonicum was planted in an artificially created garden and settled well and is even propagating by seed germination in the garden, which has become a tourist destination now. However, it is unknown how the settlement and the reproduction were successful. Therefore, an investigation of the fungal community in the garden was also included in this study.
All orchids are mycoheterotrophy during germination and early-stage in the seedling period, and the trophic dependency is maintained or expanded in many cases to the adult stage. Symbiotic mycorrhizal members survive through relationships with the orchid roots. The symbiont fungi are known to help plant growth by improving the root structure, enhancing the detoxification system, and increasing water efficiency [Citation3]. The fungi are primarily classified into two categories. The first group is arbuscular mycorrhizal fungi to penetrate root cortical cells for symbiosis. The second group is named ectomycorrhizal fungi to grow between root epidermal cells for symbiosis [Citation4]. The mycorrhizal fungi have a symbiotic relationship that receives carbon from plants [Citation5] and supplies plants with mineral nutrients [Citation6] which are obtained by a fungal mantle covering the roots or fungal hypha that can extend longer than the root hairs. The term phytobiome means interaction and relationship among the living or non-living objects in the environment of plants [Citation7]. The microbial community in the phytobiome components includes bacteria, fungi, viruses, and nematodes. Most microbes are difficult to cultivate in vitro conditions therefore it was difficult to investigate them naturally. However, the universalization of next-generation sequencing allows us to know the distribution of microorganisms directly from the sample, including those difficult or impossible members to cultivate. Therefore, studies are actively being attempted to investigate microorganism communities from the ecological aspect at the community level of phytobiome.
To prevent Cypripedium japonicum extinction, asexual reproduction methods such as tissue culture and crown division, which could be accelerated by human intervention, are applicable. But currently, no tissue culture protocol for C. japonicum has been reported yet. And only about a thousand populations of C. japonicum are left in natural habitats. Their genetic diversity seems challenging to maintain or develop through human intervention. Therefore, it is critical to understand mycorrhizal fungi, which are expected to intervene naturally in C. japonicum life cycle, and to create an environment where C. japonicum could reproduce by expanding their genetic diversity through seeds. This study utilized C. japonicum rhizosphere samples in the artificial garden and the native habitats. We aimed to understand its fungal community as basic knowledge to prevent the extinction of C. japonicum and explore mycorrhiza to help seed germination or expand using amplicon-metagenomic analysis of fungi.
2. Materials and methods
2.1. Sampling of C. japonicum rhizosphere soils
The native habitats of C. japonicum and the artificial garden location were described in Figure S1. Mt. Dukyu has two spatially separated habitats, and the two habitats have been examined separately (Table S1). The natural habitat information and the average individual record of C. japonicum were provided by Korea National Park Research Institute. Rhizosphere samples were collected at three random points while minimizing the damage to these endangered plants. The artificial garden in Bisugumi was on private land, and it was possible to collect samples only once with the owner’s permission. Except for the garden, the sampling was progressed twice at intervals of 1–3 months (Table S1). The samples were immediately stored and shipped in an icebox, and their DNA extraction proceeded immediately.
2.2. DNA extraction
DNA was extracted using FastDNA™ SPIN Kit for Soil (MP Biomedicals, CA, USA). Tubes with 0.5 g rhizosphere, glass beads, 978 µL sodium phosphate buffer, and 122 µL MT buffer were homogenized by FastPrep-24 (MP Biomedicals) set at 40 s and 6.0 m/s. After removing the supernatant made by the centrifugation, which was set up for 10 min, and at 14,000 g to the new tube, 250 µL of PPS solution was added, mixed 10 times, and centrifugated for 5 min, and at 14,000 g to precipitate proteins. After removing proteins, the supernatant was mixed with 1 mL Binding Matrix Solution by inverting in a 15 mL tube for 2 min and held on a rack for 3 min. The matrix was collected using the matrix binding column included in the kit, and DNA was obtained by dissolving the DNA attached to the matrix.
2.3. Fungi library sequencing and metagenome analyses
ITS2 library of the rhizosphere was amplified by KAPA HiFi HotStart ReadyMix (Kapa Biosystems, MA, USA). The PCR cycle was 25, and the used primer was the Illumina adapter, and the Illumina adapter were linked to the ITS3 (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGCATCGATGAAGAACGCAGC-3′), and ITS4 (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTCCTCCGCTTATTGATATGC-3′). The annealing temperature was 55 °C for 30 s. The library sequencing with MiSeq 2 × 300bp (Illumina, CA, USA) was commissioned to Macrogen (Seoul, Korea). The MiSeq results were saved in FASTQ format. The computer used in the analysis had been composed of 64 GiB DDR4 RAM and AMD Ryzen Threadripper 3970X and installed Ubuntu 20.04 as OS. ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primer regions in the files were trimmed and removed by cutadapt (version 3.4) [Citation8]. Clustering of reads processes was administrated by DADA2 (version 1.16.0) [Citation9], which resulted in an amplicon sequence variant in R (verwion4.0.0). Bad reads (expected errors > 2 at DADA2 optional parameter) were discarded before the reads were merged between forward and reverse reads when minimally 12 bp was overlapped. After removing the chimeric sequences, their taxonomic names were identified by the Naive Bayes classifier based on UNITE database [Citation10]. Ecological approaches such as alpha diversity and beta diversity were evaluated using phyloseq (version 1.32.0) [Citation11] and vegan (version 2.5-0) [Citation12] packages in R. To understand the relationship among the taxonomic groups of the fungal community in the native land rhizosphere, the correlation network was analyzed using the FastSpar (version 0.0.10) [Citation13] which use SparCC algorithm, and DESeq2 (version 1.28.1) [Citation14,Citation15] was used to compare the fungal community of native and cultivation areas. The input of DESeq2 was normalized value using rounded read per million (RPM), rounded at the first place of the decimal point, and added one. The reasons are that DESeq2 only recognizes integers, and the log2 (fold change) cannot be calculated if it was zero when it exists only in natural habitats or only in gardens. And the p-value was adjusted with a stricter Bonferroni correction than FDR (false discovery rate).
3. Results
3.1. Dominant and diversity of fungal community in C. japonicum
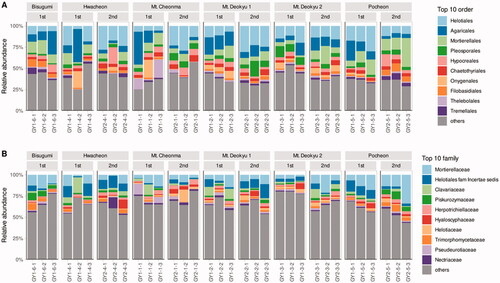
As the first step to detecting the fungal community, microorganisms were identified by amplifying ITS2 in the rhizosphere. The clustered amplicon sequence variant (ASV)s of ITS2 in C. japonicum rhizosphere was well represented because when ITS2 reads were increased, the ASVs had convergence in the rarefaction curve (Figure S2). On average, fungi were identified 97.4% in The ASVs (Figure S3). Generally, in all samples, top 10 orders were Helotiales, Agraicales, Mortierellales, Pleosporales, Hypocreales, Chaetothyriales, Onygenales, Filobasidiales, Thelebolales, and Tremellales. Their sum was composited 61.1% (). The 10 families were Mortierellaceae, Helotiales fam Incertae sedis, Clavariaceae, Piskurozymaceae, Herpotrichiellaceae, Hyaloscyphaceae, Helotiaceae, Trimorphomycetaceae, Pseudeurotiaceae, and Nectriaceae made up 35.0% in the rhizosphere community ().
Figure 1. Relative abundance of top10 order and family. The two top 10 samples are selected based on the average of all samples in order (A) and in family (B).
Analysis was to observe in terms of ecological diversity. Alpha diversities of 33 samples were not different in 11 groups divided into time and space (Figure S4). The beta diversities in the natural habitats were evaluated by principal coordinates analysis (PCoA) by the summarized relative abundance by lowest taxonomy (). Because it was challenging to find meaningful discoveries connected to the habitat information and the individual or community of fungi information, the PCoA results were evaluated by permutational multivariate analysis of variance (PERMANOVA) and pairwise PERMANOVA as post hoc (). Beta diversities were different in habitat location, population, average shoot length, and habitat wideness in PERMANOVA. In habitat location, “Mt. Cheonma between Mt. Deokyu 2.” and “Mt. Cheonma between Hwacheon” were different beta-diversity significantly. However, the samples according to shoot length showed no significant difference in post hoc “8 vs 16” and “18 vs 196” in population, and “0.8 vs 0.6” and “0.8 vs 10” in the habitat location showed differences in post hoc, but the regularity was not observed in these number sizes.
Figure 2. Principal coordinates analysis in national habitat. The panels are emphasized by (A) habitat location, (B) sampling time, (C) shoot length, (D) area, (E) population. The place and sampling times are indicated by different colors and the same location samples in different times connect the lines. The characteristics of the measured habitats are indicated by a blue and black gradation.
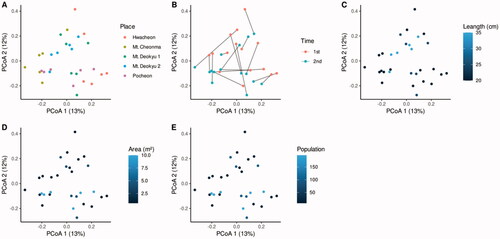
Table 1. PERMANOVA of the PCoA results in natural habitats of Cypripedium japonicum.
3.2. Comparison of the fungal community of C. japonicum between the habitat types
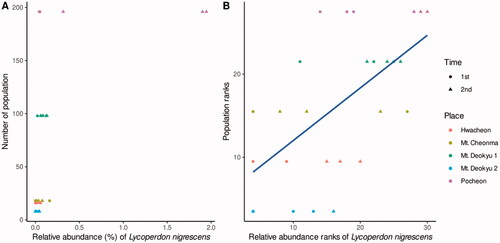
Spearman’s rank correlation analyses were performed to find beneficial fungi between measurements which were the information of the habitat location, individual number, average shoot length, and sums that were the relative abundance of which the lowest taxonomic name was the same in each of the ASVs (, Table S2). Among the three information and the 81 taxonomies, the results show that population was positively correlated with Lycoperdon nigrescens ().
Figure 3. Correlation between Lycoperdon nigrescens and Cypripedium japonicum Thunberg population. (A) real values. (B) Ranks. The blue line indicates a regression in ranks. Among 243 Spearman’s ranking correlations of taxonomy and habitat indicators, only L. nigrescens were positively correlated with population. Bonferroni corrected p-value is 0.033 and p-value meaning correlation is 0.634.
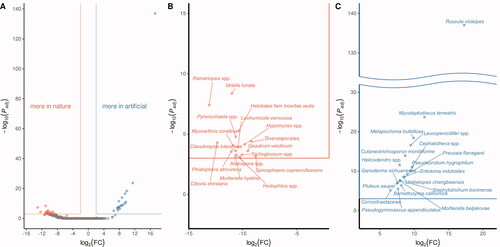
Differential abundance analysis was conducted to determine which microorganisms are more inhabited between the natural habitats and the artificial garden (). To include fungi that only live in the natural habitats or the artificial garden, log2 (foldchange) was obtained by using the RPM normalization +1. It was Alatospora spp., Ciboria shiraiana, Clavulinopsis luteonana, Diversisporales, Geastrum velutinum, Helotiales fam Incertae sedis, Hodophilus spp., Hypomyces spp., Idriella lunata, Leohumicola verrucosa, Mortierella hyalina, Mycoarthris corallina, Phialophora atrovirens, Pyrenochaeta spp., Ramariopsis spp., Spirosphaera cupreorufescens, and Trichoglossum spp. that lived more in the natural habitat significantly (). And Barnettozyma californica, Cephalotheca spp., Coniochaetaceae, Cutaneotrichosporon moniliiforme, Entoloma indutoides, Ganoderma sichuanense, Helicodendron spp., Leucopenicillifer spp., Metapochonia bulbillosa, Mortierella beljakovae, Mycoleptodiscus terrestris, Pluteus saupei, Preussia flanaganii, Pseudeurotium hygrophilum, Pseudogymnoascus appendiculatus, Russula violeipes, Staphylotrichum boninense, and Umbelopsis changbaiensis were more lived in Bisugumi, artificial garden (). Especially, Russula violeipes differed greatly and were very significant.
Figure 4. Difference abundance between the native habitats and the artificial garden. This analysis was run by DESeq2 package in R. The adjusted p-values were conducted by Bonferroni adjustment. (A) all of the analyzed is zoom in and separated to (B) more in nature, and (C) more in the garden. The criteria for the significant are padj < 0.001, and │log2(fold change)│ > 2.
3.3. Interaction network of fungal community in C. japonicum
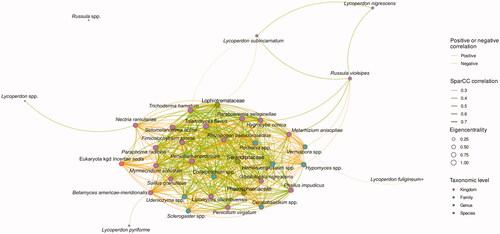
To comprehend the interaction between fungi, network analyses were performed. Because the garden sample was only three samples, and there were too many cases that did not include fungi of the natural habitats or only lived in the garden, it is dangerous to be overinterpreted in correlation, the Bisugumi samples were excepted. Because of too many significant results (Figure S5), these were calculated to eigencentrality, which represents the centrality of the relationships, and the top 30 were selected as the network hubs. The hubs were Talaromyces flavus, Paraboeremia selaginellae, Penicillium improvisum, Lophiotremataceae, Paraphoma radicina, Pochonia spp., Trichoderma hamatum, Colletotrichum spp., Serendipitaceae, Phaeosphaeriaceae, Myrmecridium schulzeri, Hemileucoglossum spp., Setomelanomma holmii, Hypomyces spp., Phallus impudicus, Fimicolochytrium jonesii, Lipomyces chichibuensis, Penicillium virgatum, Hygrocybe conica, Udeniozyma spp., Metarhizium anisopliae, Ceratobasidium spp., Nectria ramulariae, Sclerogaster spp., Betamyces americae-meridionalis, Suillus granulatus, Vermispora spp., Gibellulopsis nigrescens, Rhizopogon pseudoroseolus, and Eukaryota kgd incertae sedis. Also, Russula, which was very significant in the volcano plot, and Lycoperdon, which was more common as the population was higher, were selectively described as nodes ().
Figure 5. Correlation network among taxonomic groups in the natural habitats. When correlation p-value is under 0.05, top 30 of eigencentrality, Lycopedon and Rusulla are selectively described. Green and yellow lines mean positive and negative correlation and their thickness mean correlation magnitude. The size of the point represents the eigencentrality of a significant network, including all taxonomic groups not plotted in this figure. The top 30 is described by red edge at the point. The filled colors of the points represented the taxonomic levels of the groups.
4. Discussion
Korean lady’s slipper, C. japonicum, is an orchid that grows naturally in Korea, China, and Japan. Cypripedium japonicum is designated as a critical endangered (CR) by the National Institute of Biological Resource (NIBR) in the Republic of Korea and classified as endangered (EN) in the Red List of International Union of Conservation Nature (IUCN) [Citation1,Citation2]. In Korea, a total of five natural habitats of C. japonicum have been reported, and the number of C. japonicum inhabiting ranges from about 200 to 1000 [Citation1]. Therefore, research on their population proliferation is needed. In general, crown segmentation, tissue culture, and seed germination are known for the artificial proliferation of orchid plants. It is a way to minimize human intervention and maintain genetic diversity on its own. The ideal method among them is seed sexual reproduction. However, C. japonicum has no known proliferation protocol by seed or tissue culture. Therefore, this study was planned to understand the fungal community, considering that mycorrhizal fungi are essential for the germination and growth of C. japonicum.
ITS2 region library of C. japonicum rhizosphere fungal community was clustered at 9296 sequences in a total of 33 samples. The alpha diversity was not different among the 33 samples (Figure S4). Beta diversity analyses were performed without Bisugumi samples, the artificial garden, due to the sampling being allowed only once, and the C. japonicum numerical information was not obtained. The beta diversity significantly differed according to the habitant location, area, hoot length, and population of C. japonicum (, ). Between Mt. Cheonma and Mt. Deokyu 2, and between Mt. Cheonma and Hwacheon had differences, but the sampling time was the difference post hoc. In the post hoc of population, and the habitant area, there were significant difference results but no regularity according to the values of that, so it was presumed that the results of the location had accidentally been affected. These habitats and the growth information of C. japonicum living in the habitats were also used to find correlation analyses with the relative abundance. Spearman’s rank correlation analyses were conducted 243 times among 81 phylogeny groups. Only one fungus, L. nigrescens, showed a strong positive correlation with the C. japonicum population (). Genus Lycoperdon has been reported to help plant growth as an ectomycorrhizal fungus [Citation16,Citation17] and accumulate selenium [Citation18–20]. This finding indicates indirect evidence that the larger population in the habitat, the more selenium supplied by the ectomycorrhizal fungi or the higher the selenium concentration. Further research will be needed to clarify, but it was presumed to be associated with the reports that root growth had been promoted when appropriate selenium treatment had been performed on orchids [Citation21].
The comparison between the artificial and the natural habitats () indicated that 17 fungi species were more abundant in the natural habitats (), and 18 other fungi were more lived in the Bisugumi (). The 17 fungi species could be classified as a natural group that is not essential for the artificial propagation of C. japonicum. The latter 18 fungi could be classified as an artificial group that may be important in the artificial garden or replaces important something in the natural habitat. In particular, Russula violeipes were overwhelmingly more abundant in the artificial garden than in the natural habitats. Genus Russula is also known as the ectomycorrhiza, which promotes plants growth [Citation16,Citation17] and is critically important in orchid germination [Citation22]. Russula violeipes is considered the important bioresource candidate available for restoring C. japonicum.
Network analysis was planned to understand the correlation among the fungi taxonomic groups (Figure S5). The network was so complex that it was necessary to re-plot the meaningful part of which the criteria were 30 groups with high network influence as an eigencentrality standard, Lycoperdon, which was necessary for populations size of C. japonicum, and Russula, which was highly abundant in the Bisugumi. In this data, Lycoperdon subcarnatum, Lycoperdon pyriforme, Lycoperdon pyriforme, and other unknown Lycopedon spp. were thought to be relatively unimportant because they had a negative correlation with the top 30 or had a positive correlation with the hub which had many negatively correlated other hubs. And unknown Russula was not directly relative to the hubs. On the other hand, L. nigrescens, important for the population size of C. japonicum, and R. violeipes, more living in the garden, were suggested to be important members of the community. Because L. nigrescens was a positive correlation with the R. violeipes. And R. violepes was a positive correlation with Paraboeremia selaginellae and Metarhizium anisopliae, which had a positive correlation with other hubs, and a negative correlation with Myrmecridium schulzeri and Hypomyces spp., which are negative correlations with other herbs. Also, the Hypomyces spp. were grouped in not essential for cultivation at the volcano plot ().
This study suggests that the ectomycorrhizal fungi that directly benefited the breeding or habitat of C. japonicum in the Korean topography were R. violeipes and L. nigrescens. Furthermore, it suggests that the ectomycorrhizal fungi that helped them settle in the Korean lady’s slipper rhizosphere community were P. selaginellae and M. anisopliae.
Supplemental Material
Download MS Word (3.1 MB)Disclosure statement
No potential conflict of interest was reported by author(s).
Additional information
Funding
References
- Kim SB, Suh MH, Lee BY, et al. Korean red list of threatened species. 2nd ed. Incheon (South Korea): National Institute of Biological Resources; 2014.
- Rankou H. Cypripedium japonicum. The IUCN red list of threatened species 2014 [Internet]. Cambridge: IUCN Global Species Programme Red List Unit; 2014 [cited 2022 January 26]. Available from: https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T13188414A16672875.en
- Sathiyadash K, Muthukumar T, Karthikeyan V, et al. Orchid mycorrhizal fungi: structure, function, and diversity. In: Orchid biology: recent trends & challenges. Singapore (Singapore): Springer; 2020. p. 239–280.
- Bonfante P, Genre A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1(1):1–11.
- Schüssler A, Martin H, Cohen D, et al. Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature. 2006;444(7121):933–936.
- Selosse MA, Richard F, He X, et al. Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol. 2006;21(11):621–628.
- Leach JE, Triplett LR, Argueso CT, et al. Communication in the phytobiome. Cell. 2017;169(4):587–596.
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10–12.
- Callahan BJ, McMurdie PJ, Rosen MJ, et al. Dada2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581–583.
- Nilsson RH, Larsson KH, Taylor AFS, et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47(D1):D259–D264.
- McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217.
- Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14(6):927–930.
- Watts SC, Ritchie SC, Inouye M, et al. FastSpar: rapid and scalable correlation estimation for compositional data. Bioinformatics. 2019;35(6):1064–1066.
- Lee C, Lee S, Park T. 2017. A comparison study of statistical methods for the analysis metagenome data. Paper presented at the IEEE International Conference on Bioinformatics and Biomedicine; 2017 November 13–16; Kansas City, MO.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550–521.
- Arteaga-León C, Pérez-Moreno J, E1spinosa-Victoria D, et al. Ectomycorrhizal inoculation with edible fungi increases plant growth and nutrient contents of Pinus ayacahuite. Rev Mex Biodiv. 2018;89(4):1089–1099.
- Pyasi A, Soni KK, Verma RK. Effect of ectomycorrhizae on growth and establishment of Sal (Shorea robusta) seedlings in Central India. Nusantara Biosci. 1970;5(1):44–49.
- Mandic M, Grgic J, Grgic Z, et al. The natural level of selenium in wildlife mushrooms in Eastern Croatia. Prehrambeno Technol Biotechnol. 1991;29:159–161.
- Quinche JP. Lycoperdon perlatum, un champignon accumulateur de metaux lourds et de selenium. Mycologia Helvetica. 1990;3:477–486.
- Stijve T. Selenium content of mushrooms. Z Lebensm Unters Forsch. 1977;164(3):201–203.
- Seliem MK, Abdalla N, El-Ramady HR. Response of phalaenopsis orchid to selenium and bio-nano-selenium: in vitro rooting and acclimatization. Env Biodivers Soil Sec. 2020;4:277–290.
- Tĕšitelová T, Tĕšitel J, Jersáková J, et al. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am J Bot. 2012;99(6):1020–1032.
