Abstract
Agaricales species form pileate-stipitate fruiting bodies and play important roles in maintaining the terrestrial ecosystem as decomposers, symbionts, and pathogens. Approximately 23,000 Agaricales species have been known worldwide, and 937 species have been recorded in the Republic of Korea. However, most of them were identified solely based on morphological characteristics that often led to misidentifications. The specimens collected from 2018 to 2020 in the Republic of Korea were identified based on phylogenetic analysis of the internal transcribed spacer (ITS) sequences. Their identities were confirmed by microscopic characteristics. As a result, 14 Agaricales species were discovered for the first time in the Republic of Korea. They belonged to nine genera: Agaricus, Calocybe, Cortinarius, Hygrocybe, Inocybe, Lepista, Leucoagaricus, Marasmius, and Psathyrella. Detailed macroscopic and microscopic descriptions were provided to help distinguish these species. The morphological and molecular data provided in this study will serve as reliable references for the identification of Agaricales species.
1. Introduction
The Agaricales is the largest order in Agaricomycetes, Basidiomycota. Most species in the Agaricales form mushrooms with gilled hymenophore, pileus, and stipe. Pileate-stipitate forms are correlated to the elevated diversification rate [Citation1,Citation2], and Agaricales is the most succeeding group with c.a. 23,000 species worldwide [Citation3]. Agaricales species play various roles as decomposers, symbionts, and pathogens, helping to maintain the ecosystem. In addition, they are consumed as foods, nutrient supplements, and medicines [Citation4]. However, misidentification of Agaricales species often causes poisoning accidents [Citation5,Citation6] and misinterpretations in scientific research [Citation7].
Species in the Agaricales have traditionally been identified based on their macroscopic and microscopic characteristics. Unfortunately, except for some species, morphological characteristics are often not sufficient to identify Agaricales specimens to the species level. Morphological characteristics can vary within species according to geographic conditions, surrounding environments, and developmental stages [Citation8,Citation9]. Vice versa, different species may have similar morphology if they are closely related or have undergone convergent evolution [Citation10,Citation11].
DNA sequence-based classification and identification are now being widely used to overcome the limitations of morphology-based identification. Since the 1990s, DNA sequences have become widely used for fungal phylogenetics [Citation12] and have significantly changed the classification system of Agaricales [Citation13–16]. Following the revision of the higher-order classification system, Agaricales are now identified to the species level using the internal transcribed spacer (ITS) sequences. Among diverse genetic markers, the ITS region has been proposed as a genetic marker for species-level fungal identification because it is easily amplified, has sufficient resolution to distinguish species, and has the most abundant data deposited in public databases [Citation17–19].
According to the National species list, 937 Agaricales species are present in the Republic of Korea [Citation20]. However, most of them were identified based on morphological characteristics, which often resulted in misidentifications. Recent studies based on molecular phylogeny revealed that many Agaricales species in the Republic of Korea were new or unrecorded species [Citation21–25]. As a part of the project hosted by the National Institute of Biological Resources (NIBR) to investigate the true fungal diversity in the Republic of Korea, we found 14 unrecorded Agaricales species by analyzing the morphology and ITS sequences. In the current study, we provided detailed morphological descriptions and sequence information of the unrecorded species.
2. Materials and methods
2.1. Sampling and morphological observation
Specimens used in this study were obtained from the herbarium of the Seoul National University Fungus Collection (SFC). Eighteen fruiting bodies were collected from diverse regions of the Republic of Korea from 2018 to 2020. The location information for each specimen is presented in the taxonomy section. Photographs were taken and macroscopic characters were recorded in the field. Colors were described based on the ‘Methuen Handbook of Colour’ [Citation26].
To observe microscopic characteristics, dried specimens were mounted on 5% KOH and stained with 1% Congo red. Photographs were taken using the Eclipse 80i light microscope (Nikon, Tokyo, Japan). Innately colored structures were also observed without staining. The size of basidia, basidiospores, and different types of cystidia was measured for at least 20 individuals per specimen using Image J2 [Citation27]. Q values indicate the length-to-width ratio.
2.2. Molecular identification
DNA was extracted from the specimens using the CTAB protocol [Citation28]. ITS region was amplified through PCR using ITS1F/ITS4 [Citation29] or ITS1F/ITS4B primers [Citation30]. PCR was performed as follows: 5 min initial denaturation at 95 °C, followed by 35 cycles of 40 s at 95 °C, 40 s at 55 °C and 60 s at 72 °C with a final extension step for 5 min at 72 °C. The PCR product was visually checked on 1% agarose gel (BIOFACT, Daejeon, the Republic of Korea) stained with the EcoDye DNA staining solution (SolGent Co., Daejeon, the Republic of Korea) and purified using the Expin™ PCR SV Kit (GeneAll Biotechnology, Seoul, the Republic of Korea). Sequences were read in both directions through Macrogen (Seoul, the Republic of Korea) using an ABI3730 automated DNA Sequencer. The sequences from both directions were edited, merged, and deposited in GenBank under the accession numbers ON059509–ON059528 (). ITS sequences newly generated were compared with the reference sequences in GenBank using BLAST. Multiple sequence alignment was performed for each genus with the reference sequences using MAFFT v. 7.450 [Citation31]. The sequences of type specimen were included if present. Neighbor-joining (NJ) phylogenetic trees were constructed using the TN93 model with 1,000 bootstrap replications [Citation32]. Sequence merging, editing, alignment, and phylogenetic analysis were performed using Geneious Prime® 2020.2.2 (https://www.geneious.com).
Table 1. Specimen information and BLAST results of ITS sequences were generated in this study.
3. Results
The 18 specimens used in this study were classified to be eight families and nine genera in Agaricales based on fruiting body morphology: Agaricus, Calocybe, Cortinarius, Hygrocybe, Inocybe, Lepista, Leucoagaricus, Marasmius, and Psathyrella (). For accurate identification, the ITS regions of all specimens were sequenced. The length of the edited ITS sequences ranged from 548 to 644 bp. The sequence alignments for each genus varied in length from 563 to 782 bp. Molecular identification was supported by high pairwise similarity with the reference sequences (99.1–100%) () and high bootstrap values (≥67), which showed distinct monophyletic groups (Figure S1–S9). Each group was identified to the species level according to the reference sequences. Ten of the fourteen unrecorded species formed a monophyletic clade with type specimens of each corresponding species. The morphological characteristics of the observed specimens were in good agreement with that of the reference specimens, but variations existed for some. Detailed macroscopic and microscopic descriptions were provided for all 14 species. Korean names were also provided (Table S1). Remarks were made for each species with reference to previous descriptions of the same species, sister species, or morphologically similar species.
Figure 1. Fruiting bodies of the unrecorded Agaricales species from the Republic of Korea. (A) Agaricus atrodiscus; (B) A. dilatostipes; (C) A. karstomyces; (D) A. xanthodermulus; (E) Calocybe convexa; (F) Cortinarius rubellus; (G) Hygrocybe singeri; (H) Inocybe venustissima; (I) Lepista panaeola; (J) Leucoagaricus subpurpureolilacinus; (K, L) Marasmius macrocystidiosus; (M) Psathyrella amaura; (N) P. squamosa; (O) P. subsingeri.

4. Discussion
In this study, we discovered 14 unrecorded Agaricales species in the Republic of Korea through morphological and molecular identification. They belong to nine genera of the eight families in Agaricales. They were identified to the generic level in the field according to their distinctive morphological features. However, identifying them to the species level solely based on fruiting body morphology remained a challenge. The unrecorded species were more frequently found in Agaricus (four species) and Psathyrella (three species). Some of the congeneric species in these genera have very similar fruiting body morphology; thus, it is difficult to distinguish between species by macroscopic features. In these cases, a combination of microscopic characteristics and phylogenetic analysis of ITS sequences can increase the accuracy of identification to the species level.
Among the unrecorded Agaricus species, three species belonged to the monophyletic clade Agaricus section Xanthodermatei. Only two species from this section have been reported in the Republic of Korea: A. moelleri Wasser and A. placomyces Peck [Citation20]. Species in this section generally exhibit phenolic odor and yellow discoloration on the surface from bruises, cuts, and KOH reactions [Citation33]. In addition, all five species in the Republic of Korea possess a white pileus covered with fine, appressed, and grayish scales concentrated at the center. Due to their morphological similarity, these species may be easily misidentified as one another unless a molecular analysis is performed.
Psathyrella species are not easy to distinguish between species as they share morphological characteristics such as fragile and frequently hygrophanous pileus that never melts into goo, gilled hymenophore that changes in color from pale pink to dark brown with age, and dark, smooth basidiospores [Citation34,Citation35]. In addition, previous morphology-based identification and classification of Psathyrella species have turned out to be incongruent with DNA sequence-based phylogenetic analysis [Citation36,Citation37]. As such, molecular identification is essential to accurately classify and identify Psathyrella species.
The increasing number of molecular taxonomic studies revealed that many Agaricales species in the Republic of Korea have been misidentified and were in fact new or unrecorded species [Citation22,Citation23,Citation38–40]. In line with the previous sequence-based taxonomic studies, this study also discovered many unrecorded Agaricales species belonging to diverse genera such as Calocybe, Cortinarius, Hygrocybe, Inocybe, Lepista, Leucoagaricus, and Marasmius. Given that the number of species in Inocybe and Cortinarius is rapidly increasing through sequence-based phylogenetic studies [Citation41–44], it is expected that more ectomycorrhizal species are to be discovered in the Republic of Korea.
Among the unrecorded species, several species were lethal or putatively poisonous. Cortinarius rubellus, also known as the deadly webcap, belongs to Cortinarius subgenus Orellanus, section Orellani [Citation45]. Species in this section contain orellanine, which causes irreversible kidney failure in humans [Citation46,Citation47]. Three Agaricus species, A. atrodiscus, A. karstomyces, and A. xanthodermulus, belong to sect. Xanthodermatei, and species in this section harbor toxic phenolic compounds including phenol and p-quinol [Citation48,Citation49]. According to the Marseille Poison Control Center in France, approximately 20% of mushroom poisonings had been caused by species in A. sect. Xanthodermatei from 1994 to 1998 [Citation50]. Many species in Inocybe contain large doses of muscarine [Citation51,Citation52], thus I. venustissima may also be toxic. To prevent and cure mushroom poisoning, studies on toxic compounds should be conducted based on accurate species identification through both morphological and molecular analyses.
In conclusion, 14 Agaricales species were introduced for the first time in the Republic of Korea through morphological and molecular identification. The morphology and sequence data presented in this study will improve the reliability of public databases for the identification of Agaricales species. In addition, morphological descriptions may be useful in distinguishing several poisonous species in the Republic of Korea.
5. Taxonomy
Agaricus atrodiscus Linda J. Chen, Callac, R.L. Zhao & K.D. Hyde, Fungal Diversity 75: 185 (2015)
Specimens examined: Republic of Korea, Jeollanam-do, Sinan-gun, Docho-myeon, U-ido-ri, 34°36′41"N 125°50′03"E, Sep 2018, Tae Heon Kim, SFC20180911-10
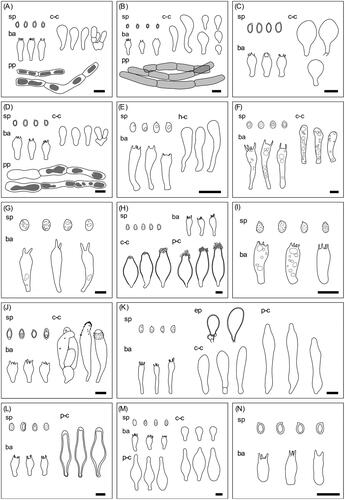
Pileus 64–85 mm in diam., plano-convex to convex, obtuse or subumbonate, white background covered with fine, appressed, grayish brown (5E3) squamules densely arranged at the center, sparse toward the margin. Lamellae free, crowded, grayish red (7B3) to dark brown (7F7). Stipe 127–194 × 8–15 mm, cylindrical, straight to flexuous, white, turning lightly yellowish (4A5) when bruised, smooth. Annulus pendant, white, membranous. Basidiospores 4.6–5.6 × 2.8–3.4 μm (av. 5.1 × 3.1 μm), Q = 1.5–1.8 (av. 1.7), ellipsoid to oblong, smooth, thick-walled, brown. Basidia 14.7–20.6 × 5.3–7.1 μm, narrowly clavate to clavate, 4-spored. Cheilocystidia 11.2–22.1 × 5.1–9.6 μm, narrowly clavate to broadly clavate, sometimes catenulate. Pleurocystidia absent. Pileipellis hyphae 4.8–9.2 μm wide, cylindrical, containing brown vacuolar pigments, and smooth ( and ).
Figure 2. Microscopic structures of the unrecorded Agaricales species from the Republic of Korea. (A) Agaricus atrodiscus; (B) A. dilatostipes; (C) A. karstomyces; (D) A. xanthodermulus; (E) Calocybe convexa; (F) Cortinarius rubellus; (G) Hygrocybe singeri; (H) Inocybe venustissima; (I) Lepista panaeola; (J) Leucoagaricus subpurpureolilacinus; (K) Marasmius macrocystidiosus; (L) Psathyrella amaura; (M) P. squamosa; (N) P. subsingeri. sp: basidiospores, ba: basidia, h-c: hymenial cystidia, c-c: cheilocystidia, p-c: pluerocystidia, pp: pileipellis, scale bars = 10 μm.
Habitat: Gregarious on the ground of Pinus densiflora forests.
Remarks: The catenulate cheilocystidia were observed in Korean specimens but were not observed in the holotype [Citation53]. Agaricus atrodiscus and A. moelleri belong to the same section Xanthodermatei and are highly similar in both macroscopic and microscopic characteristics including the yellow discoloration on the stipe [Citation54]. Nevertheless, A. atrodiscus can be distinguished from A. moelleri as hyphae composing pileipellis of A. moelleri do not contain vacuolar pigments [Citation54]. A. atrodiscus has no sister species, forming an unbranched phylogenetic lineage [Citation53].
Agaricus dilatostipes M.Q. He & R.L. Zhao, Scientific Reports 7 (5122): 14 (2017)
Specimens examined: Republic of Korea, Jeollanam-do, Goheung-gun, Bongnae-myeon, Changpo-gil, 34°26′54"N 127°30′14"E, Jul 2017, Jae Young Park, Komsit Wisitrassameewong & Nam Kyu Kim, SFC20170713-09. Republic of Korea, Gyeongsangnam-do, Hapcheon-gun, Gaya-myeon, 35°47′58.7"N 128°5′46.2"E, Jul 2017, Jae Young Park, Komsit Wisitrassameewong & Namwhi Kim, SFC20170726-82. Republic of Korea, Gyeongsangnam-do, Hapcheon-gun, Gaya-myeon, 35°47′42"N 128°05′02"E, Sep 2018, Hyun Lee, Jae Young Park & Abel Severin Lupala, SFC20180907-90.
Pileus 57–131 mm in diam., paraboloid at first, applanate when mature, center obtuse, white background covered with appressed, fibrillose, grayish brown (8D3) squamules densely arranged at the center, sparse toward the margin, background turning red when wet. Lamellae free, crowded, dull red (10B3) to grayish brown (8F3). Stipe 60–116 × 8–21 mm (16–41 mm at the base), cylindrical to tapering upward, straight or flexuous, bulbous at the base, white, floccose. Annulus pendant, white, membranous, upper side smooth, lower side floccose. Basidiospores 4.1–5.8 × 2.8–3.7 μm (av. 4.7 × 3.1 μm), Q = 1.4–1.7 (av. 1.5), ellipsoid to oblong, smooth, thick-walled, brown. Basidia 10.5–17.8 × 6.1–8.1 μm, clavate, 2 or 4-spored. Cheilocystidia 10.6–47.0 × 6.4–12.1 μm, narrowly clavate to clavate, obovoid, sometimes with long stipe. Pleurocystidia absent. Pileipellis hyphae 4.9–9.8 μm wide, cylindrical, hyaline to light brown, and smooth ( and ).
Habitat: Solitary or gregarious on the ground of broad-leaved or mixed forests.
Remarks: The Korean specimen differed from the holotype by having a floccose stipe but other features were consistent, including the most characteristic bulbous base of the stipe [Citation55]. Similarly, Agaricus dulcidulus Schulzer possesses a stipe with a bulbous base but has smaller (3.6–4.8 × 2.6–3.3 μm) basidiospores than that of the A. dilatostipes [Citation56]. Agaricus blatteus M.Q. He & R.L. Zhao is phylogenetically closely related to A. dilatostipes but has a smaller-sized pileus (13–28 mm in diam.) [Citation55].
Agaricus karstomyces R.L. Zhao, Phytotaxa 257 (2): 112 (2016)
Specimens examined: Republic of Korea, Jeollanam-do, Haenam-gun, Bugil-myeon, Heungchon-gil, 34°29′02"N 126°38′51"E, Jul 2018, Hyun Lee, Namwhi Kim, Seung-Yoon Oh & Seon Woo Kim, SFC20180705-35.
Pileus 92 mm in diam., applanate, slightly depressed, white to dull red (10B4) background covered with fine, appressed, brownish gray (9D2) squamules densely arranged at the center, sparse toward the margin. Lamellae free, crowded, reddish brown (9D5) to dark brown (9F8). Stipe 119 × 7–17 mm, cylindrical to tapering upwards, straight to flexuous, white, slightly striate. Annulus pendant, white, membranous. Basidiospores 5.7–7.4 × 3.7–4.5 μm (av. 6.7 × 4 μm), Q = 1.5–1.9 (av. 1.7), ellipsoid to oblong, smooth, thick-walled, brown. Basidia 11.7–15.6 × 5.7–8.1 μm, clavate, 2 or 4-spored. Cheilocystidia 12.3–24.8 × 8.0–16.5 μm, spheropendunculate to clavate. Pleurocystidia absent. Pileipellis not observed ( and ).
Habitat: Solitary on the ground of a broad-leaved forest.
Remarks: Basidiospores of this specimen are more ellipsoidal than that of the holotype (mean Q value = 2) [Citation57]. Agaricus karstomyces is morphologically and phylogenetically similar to A. moelleri but they can be differentiated by the size of basidia. Basidia of A. karstomyces is shorter than that of the A. moelleri (15–22 × 5–7 μm) [Citation54].
Agaricus xanthodermulus Callac & Guinb., Mycologia 97 (2): 421 (2005)
Specimens examined: Republic of Korea, Jeollanam-do, Jindo-gun, Jodo-myeon, Maenggoldo-ri, 34°12′21"N 125°51′41"E, Jul 2018, Jae Young Park, SFC20180704-90.
Pileus 39–55 mm in diam., applanate to plano-convex, subumbonate, white background covered with fine, appressed, grayish brown (8E3) squamules densely arranged at the center, sparse toward the margin. Lamellae free, crowded, vivid brown (10E6) when old. Stipe 59–86 × 5–8 mm, cylindrical, flexuous, yellowish gray (4B2) to grayish beige (4C2), smooth to slightly striate. Annulus flaring, white, membranous. Basidiospores 5.1–6.9 × 3.2–5.2 μm (av. 5.9 × 4.1 μm), Q = 1.3–1.7 (av. 1.5), ellipsoid to oblong, smooth, thick-walled, brown. Basidia 16.1–21.6 × 6.2–9.6 μm, clavate, 2 or 4-spored. Cheilocystidia 12.8–21.0 × 6.5–8.5 μm, clavate, sometimes catenulate. Pleurocystidia absent. Pileipellis hyphae 4.6–14.0 μm wide, cylindrical, containing brown vacuolar pigments, and smooth ( and ).
Habitat: Gregarious on the ground of a mixed forest.
Remarks: Basidia and basidiospores of Korean A. xanthodermulus had smaller than those of the references [Citation58]. Microscopically, A. xanthodermulus is distinguished from a similar species A. laskibarii L.A. Parra & P. Arrill. by the shape of cheilocystidia. The former species has clavate cheilocystidia, whereas the latter species harbor versiform (capitate, clavate, cylindrical, fusiform, lageniform, utriform) cheilocystidia [Citation33,Citation59].
Calocybe convexa X.D. Yu & J.J. Li, Mycologia 109 (1): 61 (2017)
Specimens examined: Republic of Korea, Gangwon-do, Jeongseon-gun, Gohan-eup, 37°8′56"N 128°54′3"E, Jul 2020, Jun Won Lee, Shinnam Yoo & Yoonhee Cho, SFC20200716-04.
Pileus 14–44 mm in diam., plano-convex to convex, margin undulate, reddish yellow (4B8) to light brown (6D8), smooth, dull. Lamellae adnate to subdecurrent, crowded, crinkled, forking, white. Stipe 40–55 × 3–7 mm, flattened, straight to flexuous, grayish brown (6D3) to yellowish brown (5E3), striate, hollow. Basidiospores 2.9–4.2 × 1.6–2.6 μm (av. 3.6 × 2.2 μm), Q = 1.4–1.9 (av. 1.6), ellipsoid to oblong, smooth, hyaline. Basidia 15.2–20.3 × 3.9–5.6 μm, narrowly clavate to clavate, 2 or 4-spored. Hymenial cystidia 11.3–26.4 × 2.2–5.3 μm, narrowly cylindrical to clavate ( and ).
Habitat: Gregarious on the ground near Dianthus superbus.
Remarks: Baisidospores of Korean Ca. convexa were longer than that of the holotype (2.0–3.0 × 1.8–2.5 μm) [Citation60]. Calocybe convexa is easily distinguishable from its closely related monophyletic relatives. Calocybe convexa has a yellow pileus, whereas Ca. ionides (Bull.) Donk and Ca. obscurissima (A. Pearson) M.M. Moser have violet pilei [Citation61,Citation62] and Ca. gambosa (Fr.) Donk has a white pileus [Citation62]. Calocybe ionides are the sister of Ca. convexa.
Cortinarius rubellus Cooke, Grevillea 16 (78): 44 (1887)
Specimens examined: Republic of Korea, Gyeongsangbuk-do, Bonghwa-gun, Seokpo-myeon, 37°4′5"N 128°58′54"E, Sep 2020, Ki Hyeong Park, Shinnam Yoo & Yoonhee Cho, SFC20200916-40.
Pileus 26 mm in diam., campanulate, umbonate, brownish orange (6C5) to grayish orange (5B3), fibrillose squamules evenly distributed. Lamellae emarginate, close, brownish orange (7C6) Stipe 74 × 10–12 mm, cylindrical, flexuous, golden brown (5D7) to brown (7E8), fibrose, solid. Cortina yellowish white (3A2), thread-like. leaves the distinct band on the stipe. Basidiospores 8.4–10.2 × 5.7–7.2 μm (av. 9.1 × 6.5 μm), Q = 1.2–1.5 (av. 1.4), subglobose to amygdaliform, verrucose, with a large drop, brownish orange. Basidia 30.8–51.5 × 9.1–10.7 μm, narrowly clavate, 2 or 4-spored. Cheilocystidia 23.8–38.1 × 5.3–11.0 μm, narrowly clavate to clavate. Pleurocystidia absent ( and ).
Habitat: Solitary on the stump of a broad-leaved tree.
Remarks: Cortinarius rubellus has been reported to reside in North Korea based on macroscopic characters but there were no official records in the Republic of Korea [Citation63]. This specimen agrees with the previous macroscopic and microscopic descriptions of Co. rubellus [Citation64,Citation65]. Cortinarius rubellus differs from its sister species Co. orellanus Fr., where Co. rubellus has band of veil remnants on its stipe and more reddish stipe [Citation64,Citation66]. Among the species reported from the Republic of Korea, Co. distans is morphologically similar to Co. rubellus. Co. rubellus can be distinguished from Co. distans by its denser lamellae and larger spores [Citation67].
Hygrocybe singeri (A.H. Sm. & Hesler) Singer, Sydowia 11: 355 (1958)
Specimens examined: Republic of Korea, Gangwon-do, Jeongseon-gun, Gohan-eup, 37°08′53"N 128°54′11"E, Sep 2021, Ki Hyeong Park, Shinnam Yoo & Yoonhee Cho, SFC20210902-20.
Pileus 21–28 mm in diam., conical to plano-convex, subumbonate, margin striate, yellowish brown (5F8) at the center, grayish yellow (2B4) toward the margin, blackening with age, glutinous. Lamellae adnexed to free, subdistant, pale yellow (3A3) when young, dark brown (9F4) when old. Stipe 50–60 × 3–4 mm, cylindrical, straight, concolorous with the pileus, base white, glutinous Basidiospores 8.4–12.2 × 6.2–9.0 μm (av. 9.7 × 7.3 μm), Q = 1.2–1.7 (av. 1.3), subglobose to ellipsoid, smooth, with multiple small drops, hyaline. Basidia 33.5–44.6 × 9.5–11.7 μm, narrowly clavate to clavate, 2-spored. Hymenial cystidia are absent ( and ).
Habitat: Gregarious on the ground of an oak forest.
Remarks: The isotype of H. singeri differs from the Korean H. singeri in having narrower basidiospores and 4-spored basidia. However, the two specimens are conspecific as they are molecularly almost identical and for the other morphological aspects [Citation68]. Glutinous stipe is the main characteristic of H. singeri, which differentiates the species from closely related H. conica (Schaeff.) P. Kumm. species complex [Citation68].
Inocybe venustissima Bandini & B. Oertel, Mycological Progress 18 (1–2): 262 (2018)
Specimens examined: Republic of Korea, Gangwon-do, Gangwon-do, Jeongseon-gun, Gohan-eup, 37°9′6"N 128°54′12"E, Jul 2020, Jun Won Lee, Shinnam Yoo & Yoonhee Cho, SFC20200716-08.
Pileus 53–55 mm in diam., campanulate to convex, umbonate, margin incurved, grayish orange (5B4), dry, shiny, fibrillose. Lamellae emarginate to free, close, grayish yellow (4B3). Stipe 76–95 × 8–9 mm, cylindrical, straight, yellowish gray (4B2), striate. Basidiospores 5.9–9.5 × 4.2–6.3 μm (av. 8.0 × 4.9 μm), Q = 1.4–1.9 (av. 1.6), ellipsoid to amygdaliform, smooth, hilar appendage mostly depressed, sometimes germ pore indistinctly present, with a large drop, yellowish-brown. Basidia 21.1–27.2 × 8.4–10.6 μm, narrowly clavate to clavate, 2 or 4-spored. Pleurocystidia 49.5–61.7 × 16.0–23.2 μm, fusiform to utriform, slightly thick-walled, apex covered with crystalline material. Cheilocystidia 46.2–57.4 × 15.6–23.3 μm, similar in shape to pleurocystidia ( and ).
Habitat: Gregarious on the ground near Larix kaempferi.
Remarks: Inocybe venustissima is morphologically distinguished from its sister species I. chalcodoxantha Grund & D.E. Stuntz which has smaller pileus (25 mm in diam.) and larger basidiospores (7.5–10 × 5–6.5 μm) than those of I. venustissima [Citation69].
Lepista panaeola (Fr.) P. Karst., Bidrag till Kännedom av Finlands Natur och Folk 32: 481 (1879)
Specimens examined: Republic of Korea, Jeollabuk-do, Muju-gun, Seolcheon-myeon, 35°53′26"N 127°46′33"E, Sep 2018, Hae Jin Cho, SFC20180915-03.
Pileus 24–35 mm in diam., convex, center applanate to slightly depressed, margin regular or slightly undulate, white to brownish gray (5C2), moist, smooth. Lamellae adnate, crowded, grayish beige (4C2). Stipe 26–31 × 7–8 mm, cylindrical, straight to flexuous, white to grayish brown (5E3), smooth. Basidiospores 4.6–7.1 × 3.0–4.8 μm (av. 5.6 × 3.6 μm), Q = 1.3–1.8 (av. 1.5), ellipsoid to oblong, verrucose, hyaline. Basidia 14.2–21.5 × 5.2–8.2 μm, shape, 4-spored ( and ).
Habitat: Densely cespitose on the ground of a Pinus densiflora forest.
Remarks: Basidia of this specimen are shorter compared to the previous description of Lp. panaeola (22.8–26.4 × 6–7 μm) but other morphological features were consistent [Citation70]. Lepista densifolia (J. Favre) Singer & Clémençon and Lepista irina (Fr.) H.E. Bigelow can be confused as Lp. panaeola since they all have whitish basidiomata and verrucose basidiospores. However, Lp. densifolia has deeply decurrent lamellae and smaller basidiospores (3.5–4.5 × 2.5–3.5 μm) [Citation71,Citation72], and Lp. irina has larger basidiospores (7–9 × 4–5 μm) [Citation73–75] compared to Lp. panaeola.
Leucoagaricus subpurpureolilacinus Z. W. Ge & Zhu L. Yang, Mycologia 107 (5) (2015)
Specimens examined: Republic of Korea, Jeollanam-do, Gurye-gun, Masan-myeon, 35°14′29"N 127°29′17"E, Sep 2015, Young Woon Lim, SFC20150904-39.
Pileus 23 mm in diam., plano-concave, center umbonate, white background covered with brown (7C5) appressed, fibrillose squamules densely arranged at the center, sparse toward the margin. Lamellae free, crowded, white. Stipe 45 × 2–4 mm, tapering upward, flexuous, white, smooth. Annulus flaring, white, membranous, smooth. Basidiospores 7.3–10.8 × 4.7–5.5 μm (av. 8.4 × 5.0 μm), Q = 1.5–2.2 (av. 1.7), amygdaliform to citriniform, smooth, thick-walled, constricted at the apex, hyaline. Basidia 15.2–21.0 × 7.6–10.0 μm, clavate, 4-spored. Cheilocystidia 26.8–62.5 × 8.6–12.5 μm, narrowly utriform, narrowly clavate, oblong, sometimes fusiform, crystals present sometimes with the gelatinous covering ( and ).
Habitat: Solitary on the ground of a broad-leaved forest.
Remarks: Leucoagaricus subpurpureolilacinus is characterized by brown appressed fibrillose squamules, crystals with gelatinous covering, and amygdaliform basidiospores with constriction at the apex [Citation76]. Leucoagaricus purpureolilacinus Huijsman has similar basidiomata to La. subpurpureolilacinus but lacks gelatinous covering on its cystidia [Citation77]. Leucoagaricus rubrotinctus (Peck) Singer is phylogenetically similar to La. subpurpureolilacinus. Despite their close relationships, La. rubrotinctus have basidiospores without constriction near the apex and cystidia without crystals or gelatinous covering [Citation78].
Marasmius macrocystidiosus Kiyashko & E.F. Malysheva, Mycosphere 186(1): 9 (2014)
Specimens examined: Republic of Korea, Seoul, Gwanak-gu, 37°27′22"N 126°56′46"E, Sep 2018, Myung Soo Park, Jaya Seelan Sathiya Seelan & Namwhi Kim, SFC20180905-71. Republic of Korea, Gyeongsangbuk-do, Yecheon-gun, Bomun-myeon, 36°40′33"N 128°35′32"E, Aug 2013, Young Woon Lim, SFC20130807-33. Republic of Korea, Jeollabuk-do, Jinan-gun, Jeongcheon-myeon, 35°53′43"N 127°25′41"E, Jul 2014, Young Woon Lim, SFC20140723-12.
Pileus 10–69 mm in diam, convex to hemispherical, brownish orange (8D6) and smooth when moist, orange white (5A2) and wrinkled when dry, hygrophanous. Lamellae adnexed, subdistant, white Stipe 27–68 × 4–7 mm, cylindrical to tapering upward, straight to flexuous, striate, twisted, white tomentose at the base, white. Basidiospores 3.7–7.4 × 2.4–3.7 μm (av. 5.8 × 3.1 μm), Q = 1.6–2.3 (av. 1.9), ellipsoid to amygdaliform, often phaseoliform, smooth, hyaline. Basidia 22.7–33.5 × 3.8–7.4 μm, cylindrical to narrowly clavate, 4-spored. Pleurocystidia 41.8–58.3 × 5.2–10.2 μm, fusiform to ventricose, thin-walled. Cheilocystidia 21.1–31.9 × 6.5–10.7 μm, (narrowly) clavate to fusiform, thin-walled. Elements of pileus surface 17.4–24.2 × 10.0–12.5 μm, spheropedunculate to obovoid, sometimes broadly clavate, thick-walled. Clamp connections are present in all tissues ( and ).
Habitat: Solitary or gregarious on the coniferous leaves.
Remarks: Marasmius macrocystidiosus specimens from the Republic of Korea differ from the holotype by having an orangish pileus and smaller sizes of all micro-structures [Citation79]. Marasmius macrocystidiosus is phylogenetically closely related to M. albimyceliosus Corner. Marasmius albimyceliosus differs from M. macrocystidiosus in possessing a thinner stipe (1–4 mm), Globulares-type cells of pileipellis, and in the absence of pleurocystidia and caulocystidia [Citation80,Citation81].
Psathyrella amaura (Berk. & Broome) Pegler, Kew Bulletin Additional Series 12: 382 (1986)
Specimens examined: Republic of Korea, Gangwon-do, Jeongseon-gun, Gohan-eup, 37°8′55"N 128°54′9"E, Jul 2020, Jun Won Lee, Shinnam Yoo & Yoonhee Cho, SFC20200716-19.
Pileus 26–27 mm in diam., broadly conical, margin striate to sulcate, light brown (5D4) at center to light reddish brown (7E5) toward the margin, fibrillose. Lamellae adnate, ventricose, subdistant, dark brown (10F8). Stipe 37 × 3–4 mm, cylindrical to tapering upward, light orange-brown (5D3), smooth, finely fibrous toward the cap. Basidiospores 7.0–9.0 × 3.8–5.3 μm (av. 8.1 × 4.5 μm), Q = 1.6–2.0 (av. 1.8), subcylindrical to phaseoliform, brown, smooth, thick-walled, germ pore indistinctly present, with drops. Basidia 14.4–19.5 × 7.1–8.4 μm, clavate, 4-spored. Pleurocystidia 47.4–56.1 × 10.8–16.8 μm, lageniform to fusiform with an obtuse apex, distinctly thick-walled ( and ).
Habitat: Solitary on the ground near Pinus koraiensis.
Remarks: Pleurocystidia of Korean P. amaura were smaller compared to that of the previously described specimens (55–80 × 15–20 μm) [Citation35]. Psathyrella amaura and its sister species P. olympiana A.H. Sm. have distinctly thick-walled pleurocystidia and have no or indistinct germ pore, unlike many other Psathyrella species [Citation35,Citation82]. Psathyrella olympiana can be distinguished from P. amaura by distinct veils on the stipe [Citation83].
Psathyrella squamosa (P. Karst.) A.H. Sm., Memoirs of the New York Botanical Garden 24: 220 (1972)
Specimens examined: Republic of Korea, Gangwon-do, Taebaek-si, Sodo 2-gil, 37°06′47"N 128°56′50"E, Jun 2019, Abel Severin Lupala & Jae Young Park, SFC20190619-05.
Pileus 13–32 mm in diam., convex to broadly conical, center obtuse, margin striate to sulcate, appendiculate, bright brown (6D4) to light brown (7D8), beige (4C3) when dry, white floccose scales scattered, hygrophanous. Lamellae sinuate, subdistant, light brownish orange (5C4). Stipe 29–41 × 2–4 mm, cylindrical, white to grayish yellow (3C3), covered with white floccose scales. Basidiospores 6.6–7.8 × 3.2–4.2 μm (av. 7.1 × 3.6 μm), Q = 1.8–2.2 (av. 2.0), subcylindrical, brown, smooth, thick-walled, germ pore indistinctly present, with drops. Basidia 13.2–19.4 × 6.4–8.6 μm, clavate, 4-spored. Cheilocystidia 13.0–16.9 × 7.4–9.5 μm, clavate. Pleurocystidia-like cheilocystidia 34.9–42.3 × 11.7–15.1 μm, similar in shape to pleurocystidia Pleurocystidia 33.8–42.9 × 11.2–15.0 μm, narrowly utriform, thin-walled ( and ).
Habitat: Gregarious on the ground of a Pinus densiflora forest.
Remarks: Psathyrella squamosa from the Republic of Korea had more cylindrical basidiospores and generally smaller cystidia compared to the previous records [Citation78,Citation84]. The description of thin-walled pleurocystidia was in line with the P. squamosa from China [Citation82]. Psathyrella gossypina (Bull.) A. Pearson & Dennis is macroscopically and microscopically very similar to P. squamosa and had been considered synonymous. However, P. gossypina is clearly distinguished from P. squamosa in phylogenetic analysis of the ITS region and by the presence of one or two large oil drops within cystidia, which are not observed in P. squamosa [Citation78,Citation85]. Psathyrella fimiseda Örstadius & E. Larss. are closely related to P. squamosa but have smaller pileus (3–6 mm in diam.) and stipe (10–20 × 0.5–1 mm) [Citation37].
Psathyrella subsingeri T. Bau & J.Q. Yan, MycoKeys 33: 94 (2018)
Specimens examined: Republic of Korea, Incheon, Ongjin-gun, Daecheong-myeon, 37°46′14"N 124°45′36"E, Sep 2018, Changmu Kim, Jin Sung Lee & Jae Young Park, SFC20180905-83
Pileus 22–25 mm in diam., hemispherical to convex, center obtuse to subumbonate, margin indistinctly striate, white to brownish gray (6C2), light brown (7D4) and wrinkled when dry, hygrophanous. Lamellae emarginate, close, white to dull red (8C3), brown (7F7) and wrinkled when dry. Stipe 27 × 3 mm, cylindrical, flexuous, white to pale yellowish brown (4A2), smooth to slightly striate, hollow. Basidiospores 4.4–6.2 × 2.8–4.3 μm (av. 5.1 × 3.4 μm), Q = 1.3–1.7 (av. 1.5), ellipsoid, golden brown, smooth, thick-walled, germ pore absent. Basidia 10.8–26.2 × 5.0–12.3 μm, broadly cylindrical, 2 or 4-spored. Cheilocystidia not observed. Pleurocystidia not observed ( and ).
Habitat: Gregarious on the ground of a mixed forest.
Remarks: Because of the poor condition of the dried specimen, it was difficult to observe the microscopic characteristics except for the basidiospores and basidia. Korean specimen differed from the original description of P. subsingeri in the size and color of basidiospores. According to the original description, this species has nearly hyaline to slightly yellow-colored basidiospores ranging in size from 5.8–7.8 (–8.8) × 3.9–4.4 (–5.0) μm [Citation82]. Psathyrella candolleana is closely related to P. subsingeri [Citation82] and resides in the Republic of Korea [Citation20]. They can be distinguished by the presence of germ pore as only P. candolleana has germ pore on its basidiospores [Citation78]. Psathyrella luteopallida A.H. Sm. is morphologically similar to P. subsingeri but has larger basidiospores (9–12 × 5–6 μm) [Citation34,Citation82].
Supplemental Material
Download MS Excel (11.3 KB)Supplemental Material
Download MS Power Point (178.4 KB)Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Sánchez-García M, Ryberg M, Khan FK, et al. Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi. Proc Natl Acad Sci USA. 2020;117(51):32528–32534.
- Varga T, Krizsán K, Földi C, et al. Megaphylogeny resolves global patterns of mushroom evolution. Nat Ecol Evol. 2019;3(4):668–678.
- Roskov Y, Ower G, Orrell T, et al. Species 2000. ITIS Catalogue of Life, 2019 Annual Checklist; 2019. [cited 2022 Mar 1]. Available from: http://www.catalogueoflife.org/annual-checklist/2019.
- Wani BA, Bodha R, Wani A. Nutritional and medicinal importance of mushrooms. J Med Plants Res. 2010;4(24):2598–2604.
- Brandenburg WE, Ward KJ. Mushroom poisoning epidemiology in the United States. Mycologia. 2018;110(4):637–641.
- Park MS, Bahk GJ. Estimate of the prevalence and burden of food poisoning by natural toxic compounds in South Korea. Food Res Int. 2015;78:108–113.
- Raja HA, Miller AN, Pearce CJ, et al. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod. 2017;80(3):756–770.
- Frøslev TG, Jeppesen TS, Laessøe T, et al. Molecular phylogenetics and delimitation of species in Cortinarius section Calochroi (Basidiomycota, Agaricales) in Europe. Mol Phylogenet Evol. 2007;44(1):217–227.
- Justo A, Minnis AM, Ghignone S, et al. Species recognition in Pluteus and Volvopluteus (Pluteaceae, Agaricales): morphology, geography and phylogeny. Mycol Prog. 2011;10(4):453–479.
- Ashraf A, Hyde KD, Zhao R, et al. Inter-and intra-specific diversity in Agaricus endoxanthus and allied species reveals a new taxon, A. punjabensis. Phytotaxa. 2016;252(1):1–16.
- Garnica S, Weiß M, Oertel B, et al. A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Can J Bot. 2005;83(11):1457–1477.
- Bruns TD, White TJ, Taylor JW. Fungal molecular systematics. Annu Rev Ecol Syst. 1991;22(1):525–564.
- Moncalvo J-M, Lutzoni FM, Rehner SA, et al. Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Syst Biol. 2000;49(2):278–305.
- Moncalvo J-M, Vilgalys R, Redhead SA, et al. One hundred and seventeen clades of euagarics. Mol Phylogenet Evol. 2002;23(3):357–400.
- Matheny PB, Curtis JM, Hofstetter V, et al. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia. 2006;98(6):982–995.
- Hibbett DS, Pine EM, Langer E, et al. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc Natl Acad Sci USA. 1997;94(22):12002–12006.
- Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA. 2012;109(16):6241–6246.
- Hibbett DS, Abarenkov K, Kõljalg U, et al. Sequence-based classification and identification of fungi. Mycologia. 2016;108(6):1049–1068.
- Osmundson TW, Robert VA, Schoch CL, et al. Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLOS One. 2013;8(4):e62419.
- National Institute of Biological Resources. National species list of Korea; 2021. [cited 2022 Mar 01]. Available from: http://kbr.go.kr.
- Kim NK, Kim M, Lee JS, et al. Six new recorded species of macrofungi on Gayasan National Park in Korea. Korean J Mycol. 2021;49(3):385–392.
- Cho S-E, Kwag Y-N, Han S-K, et al. Seven newly recorded macrofungi of Inocybaceae (Agaricales, Basidiomycota) in Korea. Korean J Mycol. 2021;49(2):139–153.
- Lee H, Park MS, Park J-H, et al. Seventeen unrecorded species from Gayasan National Park in Korea. Mycobiology. 2020;48(3):184–194.
- Cho HJ, Lee H, Park MS, et al. Two new species of Laccaria (Agaricales, Basidiomycota) from Korea. Mycobiology. 2020;48(4):288–295.
- Lee H, Park JY, Wisitrassameewong K, et al. First report of eight milkcap species belonging to Lactarius and Lactifluus in Korea. Mycobiology. 2018;46(1):1–12.
- Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London, England: Eyre Methuen; 1967.
- Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18(1):529.
- Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoort RA, editors. (eds) Plant molecular biology manual. Dordrecht, Netherlands: Springer; 1994; p. 183–190.
- White TJ, Bruns TD, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Innis MA, Gelfand. DH, Sninsky JJ (eds). PCR protocols: a guide to methods and applications. London, England: Academic Press; 1990. p. 315–322.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10(3):512–526.
- Kerrigan RW, Callac P, Guinberteau J, et al. Agaricus section Xanthodermatei: a phylogenetic reconstruction with commentary on taxa. Mycologia. 2005;97(6):1292–1315.
- Smith AH. North American species of Psathyrella. New York City, United States: The New York Botanical Garden Press; 1972.
- Kits van Waveren E. The Berkeley & Broome species of Psathyrella in the Kew Herbarium. Kew Bull. 1995;50(2):307–325.
- Padamsee M, Matheny PB, Dentinger BTM, et al. The mushroom family Psathyrellaceae: evidence for large-scale polyphyly of the genus Psathyrella. Mol Phylogenet Evol. 2008;46(2):415–429.
- Larsson E, Örstadius L. Fourteen coprophilous species of Psathyrella identified in the Nordic countries using morphology and nuclear rDNA sequence data. Mycol Res. 2008;112(10):1165–1185.
- Lee JS, Choi SY, Kim C, et al. Twelve undescribed species of macrofungi from Korea. Korean J Mycol. 2016;44(4):233–239.
- Kwon SL, Jang S, Kim C, et al. Note of five unrecorded mushrooms including three rare species on Mount Juwang in Korea. Mycobiology. 2020;48(3):157–168.
- Antonín V, Ryoo R, Shin H-D. Marasmioid and gymnopoid fungi of the Republic of Korea. 2. Marasmius sect. Globulares. Persoonia. 2010;24(1):49–59.
- Pradeep CK, Vrinda KB, Varghese SP, et al. New and noteworthy species of Inocybe (Agaricales) from tropical India. Mycol Prog. 2016;15(3):24.
- Bandini D, Oertel B, Ploch S, et al. Revision of some central European species of Inocybe (Fr.: Fr.) Fr. subgenus Inocybe, with the description of five new species. Mycol Prog. 2019;18(1–2):247–294.
- Ammirati J, Liimatainen K, Bojantchev D, et al. Cortinarius subgenus Leprocybe, unexpected diversity and significant differences in species compositions between Western and Eastern North America. Persoonia. 2021;46(1):216–239.
- Liimatainen K, Niskanen T, Dima B, et al. The largest type study of Agaricales species to date: bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia. 2014;33(1):98–140.
- Høiland K, Holst-Jensen A. Cortinarius phylogeny and possible taxonomic implications of ITS rDNA sequences. Mycologia. 2000;92(4):694–710.
- Moser M. Neuere erkenntnisse über pilzgifte und giftpilze. Z Pilzkunde. 1971;37:41–56.
- Keller-Dilitz H, Moser M, Ammirati JF. Orellanine and other fluorescent compounds in the genus Cortinarius, section Orellani. Mycologia. 1985;77(5):667–673.
- Gill M, Strauch RJ. Constituents of Agaricus xanthodermus Genevier: the first naturally endogenous azo compound and toxic phenolic metabolites. Z Naturforsch C Biosci. 1984;39(11–12):1027–1029.
- Wood WF, Watson RL, Largent DL. Phenol, the odour compound from Agaricus praeclaresquamosus. Biochem Syst Ecol. 1998;26(7):793–794.
- de Haro L, Prost N, Perringue C, et al. Intoxications par champignons Expérience du Centre anti-poisons de Marseille en 1994 et 1998. J de Pediatrie et de Puericulture. 2000;13(1):58–61.
- Catalfomo P, Eugster CH. Muscarine and muscarine isomers in selected Inocybe species. Helv Chim Acta. 1970;53(4):848–851.
- Malone MH, Brown JK, Stuntz DE, et al. Paper chromatographic determination of muscarine in Inocybe species. J Pharm Sci. 1962;51(9):853–856.
- Boonmee S, Yang ZL, Cai Q, et al. Fungal diversity notes 111-252-taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015;75(1):27–274.
- Mahdizadeh V, Safaie N, Goltapeh EM, et al. Agaricus section Xanthodermatei in Iran. Phytotaxa. 2016;247(3):181–196.
- He M-Q, Chen J, Zhou J-L, et al. Tropic origins, a dispersal model for saprotrophic mushrooms in Agaricus section Minores with descriptions of sixteen new species. Sci Rep. 2017;7(1):5122.
- Parra LA. Fungi europaei, volume 1A, Agaricus L. Allopsalliota, Nauta & Bas (parte II). Alassio, Savona, Italy: Edizioni Candusso; 2013.
- Zhou J-L, Su S-Y, Su H-Y, et al. A description of eleven new species of Agaricus sections Xanthodermatei and Hondenses collected from Tibet and the surrounding areas. Phytotaxa. 2016;257(2):99–121.
- Callac P, Guinberteau J. Morphological and molecular characterization of two novel species of Agaricus section Xanthodermatei. Mycologia. 2005;97(2):416–424.
- Parra LA, Arrillaga P. Agaricus laskibarii. A new species from French coastal sand-dunes of seignosse. Documents Mycologiques. 2002;31(124):33–38.
- Li J-J, Wu S-Y, Yu X-D, et al. Three new species of Calocybe (Agaricales, Basidiomycota) from northeastern China are supported by morphological and molecular data. Mycologia. 2017;109(1):55–63.
- Pearson A. New records and observations. III. Trans Br Mycol Soc. 1946;29(4):191–210.
- Bon M, Wilkinson J, Ovenden D. The mushrooms and toadstools of Britain and North-Western Europe. London, England: Hodder & Stoughton; 1987.
- Ban S-E, Cho D-H. Notes on higher fungi of Mt. Backdu (I). Korean J Nat Conserv. 2012;10(3–4):193–220.
- Robertson CP, Wright L, Gamiet S, et al. Cortinarius rubellus Cooke from British Columbia, Canada and Western Washington, USA. PNW Fungi. 2006;1(1):1–7.
- Shibata H. Cortinarius rubellus, a poisonous species new to Japan. Mycoscience. 2004;45(6):395–397.
- Breitenbach J, Kränzlin F. Fungi of Switzerland, vol. 5, agarics 3rd part. Lucerna, Switzerland: Verlag Mykologia; 2000.
- Bessette AE, Bessette AR, Fischer DW. Mushrooms of northeastern North America. Syracuse, New York, United States: Syracuse University Press; 1997.
- Hesler LR, Smith AH. North American species of hygrophorus. Knoxville, Tennessee, United States: University of Tennessee Press; 1963.
- Grund DW, Stuntz DE. Nova Scotian Inocybes. I. Mycologia. 1968;60(2):406–425.
- Gulden G. Studies in Lepista (FT.) WG smith section Lepista (Basidiomycotina, Agaricales). Sydowia. 1983;36:59–74.
- Singer R, Clémençon H. Notes on some leucosporous and rhodosporous European agarics. Nova Hedwigia; 1972.
- Bigelow HE. North American species of Clitocybe. Part I. Stuttgart, Germany: Schweizerbart Science Publishers; 1982.
- Bigelow HE, Hesler LR. Clitocybe in Tennessee and North Carolina. J Elisha Mitchell Sci Soc. 1960;76(1):155–167.
- Bon M. Tricholomataceae de France et d′Europe occidentale (6 Ème Partie: Tribu Clitocybeae Fay.). clé monographique. Documents Mycologiques. 1983;13(51):1–53.
- Bon M, L Clitocybes. Omphales et ressemblants. Flore Mycologique D’Europe. Documents Mycologiques. 1997;4:1–173.
- Ge Z-W, Yang ZL, Qasim T, et al. Four new species in Leucoagaricus (Agaricaceae, Basidiomycota) from Asia. Mycologia. 2015;107(5):1033–1044.
- Vellinga EC. Leucoagaricus. In: MENoordeloos ME, Kuyper TW, Vellinga EC, editors. Flora agaricina neerlandica, vol. 5. Rotterdam, Netherlands: A.A. Balkema Publishers; 2001. p. 85–108..
- Breitenbach J, Kränzlin F. Fungi of Switzerland, vol. 4, agarics 2nd part. Lucerna, Switzerland: Verlag Mykologia; 1995.
- Kiyashko AA, Malysheva EF, Antonín V, et al. Fungi of the Russian far east 2. New species and new records of Marasmius and Cryptomarasmius (Basidiomycota). Phytotaxa. 2014;186(1):1–028.
- Corner EJH. The agaric genera Marasmius, Chaetocalathus, Crinipellis, Heimiomyces, Resupinatus, Xerula and Xerulina in Malesia. Nova Hedwigia. 1996;111:1–175.
- Wannathes N, Desjardin DE, Hyde KD, et al. A monograph of Marasmius (Basidiomycota) from Northern Thailand based on morphological and molecular (ITS sequences) data. Fungal Divers. 2009;37:209–306.
- Yan JQ, Bau T. The Northeast Chinese species of Psathyrella (Agaricales, Psathyrellaceae). Mycokeys. 2018;33:85–102.
- Vašutová M. Taxonomic studies on Psathyrella sect. Spadiceae. Czech Mycol. 2008;60(2):137–171.
- Hausknecht A, Pidlich-Aigner H, Forstinger H. Ergebnisse des Mykologischen Arbeitstreffens in Langschlag (Waldviertel, Niederösterreich) im September/Oktober 2005. Österreiches Zeitschrift Für Pilzkunde. 2006;15:149–179.
- Örstadius L, Ryberg M, Larsson E. Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycol Prog. 2015;14(5):25.
