Abstract
The genus Mitrula (Mitrulaceae, Helotiales), as also known as swamp beacons, inhabits submerged, decaying vegetation in standing or decaying needles, twigs, leaves, and shallow water. They play an important role in carbon cycling in some freshwater ecosystems. In the herbarium of the Korea National Arboretum (KH), seven Mitrula specimens were collected during mushroom forays in the period from 2019 to 2021. The Korean collections were found to be macromorphologically closely related to M. paludosa and M. elegans, but micromorphologically they could be distinguished by characteristics of slightly narrower asci and aseptate ascospores. Our molecular phylogenetic analyses of the internal transcribed spacer (ITS) and 28S rDNA regions also revealed that our specimens were related to M. paludosa and M. elegans, but formed a distinct clade. Based on these results, we reported our specimens as new to science and discussed the phylogeny and diversity of Mitrula species.
1. Introduction
Aero-aquatic fungi play an important role in carbon cycling, specifically, during the decomposition of wood debris and leaf litter in woodland streams and rivers [Citation1,Citation2]. Most aero-aquatic fungi inhabiting freshwater habitats are known to form only asexual morphs (e.g., aquatic hyphomycetes) [Citation3–5]. However, some species belonging to the families Mitrulaceae and Vibrissaceae are characterized by the formation of only the sexual morph in nature [Citation5].
The family Mitrulaceae includes only one genus, namely Mitrula [Citation6]. Approximately 80 Mitrula species have been recorded to date (ref. Index Fungorum; www.indexfungorum.org, accessed on March 18 2022), but after revisions, 12 species remained (namely M. lunulatospora, M. luteola, M. microspore, M. multiformis, M. norvegica, M. omphalostoma, M. paludosa, M. pistillaris, M. roseola, M. serpentine, M. sphaerocephala, and M. ushuaiae) [Citation2,Citation5,Citation7]. Most Mitrula species have yellow to orange and club-shaped apothecia and occur on submerged, decaying vegetation in shallow standing waters [Citation2]. However, some Mitrula species have white to flesh-pinkish apothecia and occur on sandy soil associated with dead plant material [Citation1,Citation6].
Only one species, M. paludosa, has been recorded in South Korea [Citation8,Citation9]. It was reported that the species closely resembles M. paludosa, although its microscopic features differ slightly from those of European M. paludosa. However, they did not perform molecular phylogenetic analysis or detailed morphological comparisons with related taxa.
In the present study, we reevaluated the Korean Mitrula species based on morphological observations and molecular analysis. We described the Korean Mitrula species and proposed a species new to science.
2. Materials and methods
2.1. Sample collection
In 2019, 100 Mitrula ascocarps were collected in woodland streams in South Korea. Since then, several samples of Mitrula species have been collected during mycological surveys in Korea. These samples were collected in the period from April to May, and most of the ascocarps emerged above the needles of Pinus densiflora or leaf litter in streams and rivers (). Both yellow and whitish apothecia (KA21-0148) were collected from the same location. The detailed collection dates and localities of the Korean samples are listed in . Type specimens have been deposited in the herbarium of the Korea National Arboretum (KH), and MycoBank numbers have been provided for the new species.
Figure 1. Phylogenetic tree of Mitrula species based on the RAxML analysis of their ITS and 28S regions. Specimens identified during this study are indicated in bold. Bootstrap values higher than 60% are shown in the branches. The scale bar equals the number of nucleotide substitutions per site.
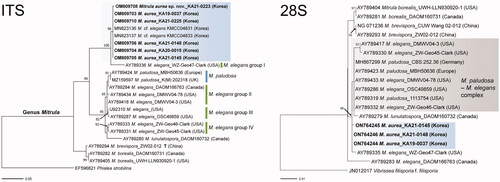
Table 1. A list of species, specimens, and GenBank accession numbers of sequences used for phylogenetic trees in this study.
2.2. Morphological observations
Macro-morphological characteristics were examined using a dissecting microscope. For micro-morphological characterization, the asci, paraphyses, and ascospores were examined using a compound microscope (Zeiss Plan-Apochromat, Oberkochen, Germany) and photographed with an Axiocam 506 color camera (Zeiss). Microscopic parameters were measured and calculated using the ZEN 3.1 blue edition software (Zeiss). Whenever possible, 30 representative images were acquired for each microscopic characteristic. Morphological characteristics of M. elegans, M. lunulatospora, and M. paludosa were from Redhead [Citation1].
2.3. DNA isolation, PCR, sequencing, and phylogenetic analysis
Genomic DNAs was extracted from six specimens using a DNeasy Plant Mini DNA Extraction Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. The genomic DNA was used for PCR amplification. The internal transcribed spacer (ITS) and 28S regions of rDNA were amplified. The obtained PCR amplicons were purified using a QIAquick purification Kit (Qiagen Inc., Germantown, MD, USA) and sequenced at the Macrogen sequencing facility (Macrogen Inc., Seoul, South Korea). A Blastn search against the NCBI database (http://www.ncbi.nlm.nih.gov) was performed, and new sequences were deposited in GenBank (www.ncbi.nlm.nih.gov/genbank/). For phylogenetic analyses, the dataset was aligned using MAFFT v. 7.475 [Citation13], selecting the auto option. The DNA sequences were assembled and manually edited in BioEdit v. 7.2.5 [Citation14] and MEGA v. 7.0.26 [Citation15]. A phylogenetic tree was constructed using RAxML on the CIPRES web server (https://www.phylo.org). The relative robustness of individual branches was estimated by bootstrapping with 1000 replicates. Phialea strobilina (EF596821) and Vibrissea filisporia f. filisporia (JN012017) were used as the basal taxa.
3. Results
3.1. Phylogenetic analysis
Six sequences from the Korean collections were newly generated and deposited in GenBank. GenBank accession numbers for the ITS and 28S regions are listed in . In the phylogenetic tree of Mitrulaceae obtained based on the ML analysis, the Korean collections formed a supported monophyletic group which was distant from the rest of Mitrula species, with 100% bootstrap support (). In the ITS tree, M. paludosa and M. elegans groups II, III, and IV were closely related, except for M. elegans group I. However, M. elegans group I was closely related to the Korean collections (). In the 28S tree, the Korean collections formed a monophyletic group, but M. paludosa and M. elegans formed a complex group.
3.2. Taxonomy
Mitrula aurea sp. nov.: S-E. Cho, H.S. Kim, Y-N Kwag & C.S. Kim [#MB843007] ( and ).
Figure 2. Morphological characteristics of Mitrula aurea sp. nov. (A–C) Most of our specimens have yellow apothecia, but one collection (KA21-0148) produced white apothecia (D); (E) Paraphyses; (F, G) Asci; (H) Ascospores. Scale bars: A = 5 cm; B–D = 1 cm; E–H = 10 μm.

Holotype: Found on the soil or dead foliage of Pinus densiflora in Goesan-gun, Chungcheongbuk-do, Korea, on May 16 2019; collected by H.S. Kim and Y-N. Kwag, KA19-0037. GenBank Nos.: OM809703 (ITS sequence) and ON764244 (28S sequence).
Etymology: From the Latin word “aureus”, meaning “gold-colored”, because of the goldish or golden yellow cap-color of the ascocarps.
Description: Ascocarps gregarious, solitary to caespitose, fleshy, 1.5–4.0 cm high. Cap ovoid to irregularly pyriform, cylindrical or clavate, smooth to rugose, slightly tremellose, mostly luteous to yellow-luteous, but rarely whitish to pale pinkish, 5–8 mm wide. Stipes unbranched, glabrous, and slightly lubricous above, 1–3 mm side above, hyaline; basal part occasionally enlarged, matted hyphal hairs below moderately, developing reddish-brown stains when damaged. Asci eight-spored, elongate to clavate, 95–130 × 5.5–7.0 μm. Paraphyses filiform, slightly enlarged above, 100–150 × 2.5–3 μm. Ascospores elliptical to ovoid, broadly cylindrical, 12.0–17.0 × 2.3–3.0 μm, not septate.
Habitat: Gregarious in shallow water, on decaying needles, twigs, and leaves of Pinus densiflora.
Sporulating period: April–May (spring).
Specimens examined: KOREA: Chungcheongbuk-do, Goesan-gun, May 16 2019 (KA19-0037); April 30 2021 (KA21-0145); May 3 2021 (KA21-0148), Gangwon-do, Yangyang-gun; May 7 2020 (KA20-0015), Gangwon-do, Inje-gun; May 15 2021 (KA21-0188), Gangwon-do, Pyeongchang-gun; May 29 2021 (KA21-0223); May 30 2021 (KA21-0225).
Notes: The asci of the Korean collections were slightly narrower (95–130 × 5.5–7.0 μm) than those of M. paludosa (asci: 119–133 × 6.5–7.5 μm [Citation1]). Microscopic features of the samples from the Korean collections were similar to those of M. elegans. However, samples from the Korean collections had a shorter stipe and no septate of ascospores compared with those of M. elegans. In addition, the Korean collections were clearly distinct from M. paludosa and M. elegans in the ITS and 28S trees (), and geographical distribution and sporulating period ().
Table 2. Morphological comparison between Mitrula aurea sp. nov. and morphologically related Mitrula species.
4. Discussion
The taxonomic position of the genus Mitrula among the Helotiales families is controversial [Citation2,Citation16]. Until 1994, the genus Mitrula was identified as a member of the family Geoglossaceae based on its clavate apothecia [Citation1]. However, based on the observation of ascus ultrastructures, Verkley [Citation17] concluded that the genus Mitrula (specifically, M. paludosa) is closely related to members of the family Sclerotiniaceae. This placement was accepted until 2004 [Citation18]. In the early 1990s, with the introduction of molecular phylogeny into fungal taxonomy, Wang et al. [Citation2] placed the genus Mitrula within Leotiomycetes based on ITS rDNA sequences. Currently, based on the comprehensive classification of Leotiomycetes by Ekanayaka et al. [Citation6], the genus Mitrula is placed within the family Mitrulaceae and characterized as saprobic, with white to flesh-pinkish or yellow-orange hymenium, clavate to stipitate apothecia, eight-spored asci, and no recorded asexual morphs in nature.
Mitrula paludosa is a type species of the genus Mitrula. It was first described by Elias Magnus Fries in 1816. However, this species never had a type specimen designated until Redhead provided a neotype based on European specimens [Citation1]. In South Korea, Park et al. [Citation9] reported the Korean Mitrula species as M. paludosa. However, the present study revealed that the Korean Mitrula species are phylogenetically distinct from M. paludosa and other related Mitrula species (see ). According to Redhead [Citation1], M. paludosa and related North American Mitrula species (M. borealis, M. elegans, and M. lunulatospora) are clearly distinctive based on their geographic distribution and sporulation period. Similarly, in our ITS and 28S trees, M. aurea, including two Korean Mitrula samples (KMCC04831 and KMCC04833 in ITS tree only, collected from the National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong, South Korea), was geographically very far from other reported Mitrula species except for M. elegans group I (see ), and its sporulating period is in spring ().
In the present study, M. elegans was not monophyletic (). It was divided into four groups in ITS tree, and all specimens were reported from North America. M. elegans is characterized by slender, cylindrical to clavate ascospores. Although M. elegans groups I, II, III, and IV are morphologically very similar, they can be divided into four species based on the phylogenetic concept of fungal taxonomy; this is especially true for M. elegans group I, which was distinct from the other M. elegans groups in the ITS tree [Citation2]. Due to the lack of microscopic differences, further morphological, ecological, and molecular studies are required to clarify the relationship between M. elegans groups. The Korean Mitrula species was found to be morphologically similar to M. elegans; however, they were clearly distinct in the ITS and 28S trees (; ).
Interestingly, one specimen of Korean Mitrula, KA21-0148, had white apothecia (see ). Although this morphological characteristic is significantly different from that of the other Korean specimens, it was well-supported by a clade in phylogenetic trees (). Similarly, M. elegans and M. paludosa have yellowish apothecia, but they become pinkish or whitish when aging or submerged () [Citation1]. Therefore, we concluded that KA21-0148 is the same Korean Mitrula species (M. aurea) because apothecial color may not be an important feature in Mitrula taxonomy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Redhead SA. The genus Mitrula in North America. Can J Bot. 1977;55(3):307–325.
- Wang Z, Binder M, Hibbett DS. Life history and systematics of the aquatic discomycete Mitrula (Helotiales, Ascomycota) based on cultural, morphological, and molecular studies. Am J Bot. 2005;92(9):1565–1574.
- Raja H, Miller A, Shearer C. Freshwater ascomycetes: Aquapoterium pinicola, a new genus and species of Helotiales (Leotiomycetes) from Florida. Mycologia. 2008;100(1):141–148.
- Gulis V, Baschien C, Marvanová L. Two new Tricladium species from streams in Alaska. Mycologia. 2012;104(6):1510–1516.
- Johnston PR, Quijada L, Smith CA, et al. A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus. 2019;10:1.
- Ekanayaka AH, Hyde KD, Gentekaki E, et al. Preliminary classification of Leotiomycetes. Mycosphere. 2019;10(1):310–489.
- Schoch CL, Wang Z, Townsend JP, et al. Geoglossomycetes cl. nov., Geoglossales ord. nov. and taxa above class rank in the Ascomycota Tree of Life. Persoonia. 2009;22:129–138.
- Seok SJ, Lim YW, Kim CM, et al. List of mushrooms in Korea. Seoul: Korea Society of Mycology; 2013.
- Park Y-W, Koo C-D, Hong D-E, et al. Note on the new record of Mitrula paludosa (Geoglossaceae) in Korea. Kor J Mycol. 2009;37(1):104–107.
- Gernandt DS, Camacho FJ, Stone JK. Meria laricis, an anamorph Rhabdocline. Mycologia. 1997;89(5):735–744.
- Vu D, Groenewald M, Vries MD, et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol. 2019;92:135–154.
- Hustad VP, Miller AN. Phylogenetic placement of four genera within the Leotiomycetes (Ascomycota). N Am Fungi. 2011;6(9):1–13.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
- Wang Z, Binder M, Schoch CL, et al. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Mol Phylogenet Evol. 2006;41(2):295–312.
- Verkley GJM. Ultrastructure of the apical apparatus in Leotia lubrica and some Geoglossaceae (Leotiales, Ascomycotina). Persoonia. 1994;15:405–430.
- Eriksson OE, Baral HO, Currah RS, et al. Outline of Ascomycota-2004. Myconet. 2004;10:1–99.
