Abstract
Wound care has become increasingly important over the years. Various synthetic products for wound care treatment have been reported to cause toxic side effects and therefore natural products are in significant demand as they have minimal side effects. The presence of bioactive compounds in medicinal mushrooms contributes to various biological activities which assist in the early inflammatory phase, keratinocyte proliferation, and its migration enhancement which are pertinent to wound rehabilitation. Lignosus rhinocerus (tiger milk mushroom) can reduce the inflammation phase in wound healing by fighting off bacterial infection and modulating pro-inflammatory cytokines expression in the early stage to avoid prolonged inflammation and tissue damage. The antibacterial, immunomodulating, and anti-inflammatory activities exhibited by most macrofungi play a key role in enhancing wound healing. Several antibacterial and antifungal compounds sourced from traditional botanicals/products may prevent further complications and reoccurrence of injury to a wounded site. Scientific studies are actively underway to ascertain the potential use of macrofungi as a wound healing agent.
1. Introduction
Wound treatment and its therapeutic impediments have become a fundamental healthcare concern, presenting a significant economically challenging burden worldwide [Citation1–5]. Based on the report published by Fior Markets [Citation6], the global market for skin and wound care is expected to reach USD 25.98 billion by 2025. The growing demand for skin and wound care products in the market is factored by the increasing number of diabetes cases, the rising geriatric population, and increasing awareness for wound care and management. Meanwhile, the global market for complementary and alternative medicine is expected to reach a whopping USD 296.3 billion by 2027; with a CAGR of 19.9%, considering the increasing demand for alternative medicine across developing countries and the expansion of traditional medicine applications in Asia [Citation7].
The practice of herbal medicine has begun in ancient times even when there was limited information on the disease(s), plants’ identity, and the prescribed regimens. Much of the knowledge regarding the usage of herbal medicaments was collected merely based on user experience [Citation8]. The usage of herbal medicine and its consumption is widely acceptable till this day and age across the world with many people seeking them as treatment and healthcare alternatives for a healthier life, higher efficiency with fewer side effects, and relatively low cost [Citation9–11]. Since 1999, the demand for alternative therapies showed an increasing trend in the United States, Australia, and Germany [Citation12] while herbal medicines are commonly utilized in Asian, African, and South American countries as part of their traditional medicaments [Citation13]. Herbal medicaments from plants; aka phytomedicine, have a seemingly good potential for wound healing. About 70% of pharmaceutical products in the market for wound healing purposes contain extract constituents of plant parts as their base material, whilst the remaining percentage is based upon mineral compounds (20%) and from animal sources (10%) [Citation14].
This review highlights the parameters for wound healing enhancement and details the scientific findings of macrofungi traditionally utilized for wound rehabilitation. It subsequently focuses on Lignosus rhinocerus, a prized medicinal mushroom from Southeast Asia; reporting on its phytochemicals and pharmacological properties which may be beneficial for wound rehabilitation. The prospects on the potential of L. rhinocerus as a wound healing agent are also deliberated as the conclusion to this review.
2. Parameters for wound healing enhancement
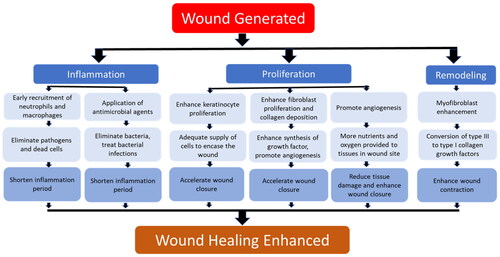
The wound healing mechanism is divided into four phases: (i) hemostasis, (ii) inflammation, (iii) proliferation, and (iv) remodeling. A series of complicated intercellular interactions involving a plethora of soluble factors, intercellular matrix components, immune cells, and signaling molecules occurs during these phases [Citation15–17]. Depending on the wound severity, it may lead to significant morbidity and mortality rates. Therefore, continuous efforts have been carried out to enhance wound healing through a multiphase mechanism. Key factors such as the accelerated inflammation phase, stimulation of new blood vessels, and enhancement of wound closure contraction are crucial for the treatment of cutaneous wounds.
depicts the key parameters for wound healing improvement. Enhancement of one or more of these parameters will be beneficial for wound rehabilitation. Despite the advancement of synthetic wound care products which were reported to be clinically effective, traditional therapies based on natural products such as plants, honey, and fungi, are considered good alternatives. Traditional therapies may help to overcome the increasing demand for new wound treatment possibilities at a much-reduced cost in the market.
2.1. Antibacterial treatment strategy for infected wounds
Bacterial infection causes prolonged inflammation which leads to delayed wound healing [Citation18]. Therefore, antibiotics are utilized to fight bacterial infections in the wound site and are commonly used as one of the standard treatments for wound care management [Citation19]. Finding an effective treatment is necessary to reduce bacterial colonization and infection, which will subsequently reduce the duration of inflammation and improve the wound healing process [Citation20].
2.2. Early induction of the inflammatory phase
Neutrophils and macrophages recruitment into the wound area is an essential event in the inflammatory phase for homeostasis maintenance, fighting pathogen invasion, and dead tissue removal. Previous studies have shown that the number of macrophages that migrate and infiltrate into the wound area is at maximum on day 3 post-wounding and remains until day 7. The number of neutrophils that migrated to the wound site was maximum at 12 h post-wounding and continuously declined until day 3. The inflammatory phase progression can be accelerated by early infiltration and elimination of neutrophils and macrophages, which prevent excessive inflammation that may lead to chronic wound(s) and scar formation [Citation21–25].
2.3. Keratinocyte proliferation and migration enhancement
The second phase of wound healing is re-epithelialization, a process that involves keratinocyte proliferation and migration to ensure an adequate supply of cells to encase the wound. The process of re-epithelialization which eventually leads to wound closure is one of the parameters for consideration of a healed wound. This re-epithelialization process is flawed in chronic wounds. Growth factors, cytokines, integrins, keratins, matrix metalloproteinases, chemokines, and extracellular matrix (ECM) play key roles in the regulation of keratinocyte proliferation and modulating wound closure [Citation26–28].
2.4. Fibroblast proliferation and collagen deposition enhancement
Another important process in the proliferative phase is collagen deposition, which plays an important role in wound healing. It involves fibroblast proliferation and migration [Citation29]. Fibroblasts play a key role in new ECM formation and collagen deposition in the wound area [Citation30,Citation31]. There have been reports that transforming growth factor-beta (TGF-β) could enhance fibroblast proliferation and collagen deposition which will enhance the synthesis of growth factor and angiogenesis to the newly formed tissue [Citation32,Citation33].
2.5. Angiogenesis promotion
Angiogenesis is a crucial process during the proliferation phase to provide nutrients and oxygen to the newly formed tissue in the wound site [Citation34]. Angiogenesis is flawed in chronic wounds. It leads to hypoxia, insufficient nutrient delivery, and causing further tissue damage [Citation35,Citation36]. Angiogenesis inhibition was reported in patients with diabetes as a high glucose condition leads to poor formation of new blood vessels [Citation35,Citation37]. It has been reported that induction and stabilization of hypoxia-induced factors will promote angiogenesis which can stimulate new vessels, enhance cell motility, and activate the transcription of vascular endothelial growth factor (VEGF) [Citation38,Citation39].
2.6. Collagen maturation and wound contraction
Both wound contraction and ECM maturation play important roles during the remodeling phase of wound healing [Citation29,Citation40,Citation41]. The expression and differentiation of myofibroblasts are crucial for wound contraction [Citation42]. Previous studies had reported that TGF-β stimulates fibroblast differentiation into myofibroblasts in wounds [Citation43,Citation44]. Therefore, wound healing progression can be accelerated by increasing TGF-β expression during the remodeling phase to increase the myofibroblast level and further enhance wound contraction [Citation45].
3. The utilization of macrofungi for wound healing
Fungi are members of a large, diverse group of heterotrophic organisms which are frequently found living on dead, decaying wood, and other organic matter. They are eukaryotic, with a scope of internal membrane systems, and membrane-bound organelles, and possess a distinct cell wall that is made largely from polysaccharides and chitin [Citation46]. The consumption of medicinal mushrooms has long been practiced and there is growing interest in the discovery of bioactive compounds with medicinal values from industries and the scientific communities alike [Citation47]. This section provides an overview of the capacities of fungi for wound healing enhancement, both in modern science and traditional knowledge.
3.1. Medicinal mushrooms for wound rehabilitation in the modern age
Recent research trend focuses on the identification of compound(s) responsible for wound healing processes outlined in the following section. This includes polysaccharides such as (1-3)-β-glucans, some secondary metabolites (terpenes, phenolics) as well as proteins and minerals (calcium, potassium, magnesium, iron, and zinc). The incorporation of their bioactive compound(s) into pharmaceutical/nutraceutical formulation allows the development of products for commercialization. For instance, Ganoderma lucidum (Lingzhi or Reishi), Sparassis crispa, and Tremella fuciformis were currently developed as skin care cream products and are commercially available in stores. shows the summary of the macrofungi studied for their wound healing activity.
Table 1. Summary of macrofungi studied for wound healing agents.
3.1.1. Agaricus spp.
The lectin(s) from Agaricus bisporus (common name: White button mushroom) was reported to exhibit dose-dependent inhibition of the proliferation of fibroblast and retinal pigment epithelium (RPE) cells, and collagen lattice contraction [Citation48–50]. These inhibition activities play an important role in controlling the scarring processes as a result of excessive myofibroblast expression and excessive collagen production. In a report by Lam et al. [Citation51], the incision wound model was used and performed on ICR mice, with 0.5 mg of A. bisporus extract mixed with 100 IU of vitamin E that was applied twice daily for 6 days. Immunochemistry, TUNEL assay, and Western blotting techniques were adopted to reveal an increased expression of epidermal growth factor (EGF) and proliferative cell nuclear antigen (PCNA), but the expression of TGF-β was not significant and few immune factor CD4 T cells were observed. It was suggested that A. bisporus extract may enhance wound healing activity by stimulating the expression of inflammatory and immune cells in the inflammation phase, enhancing keratinocyte proliferation, and promoting angiogenesis.
Agaricus blazei (Common name: Ji song rong) polysaccharides were shown to increase the recovery rate of wounded skin caused by burns on rats in a dose-dependent manner. Oral administration of 50 and 100 mg/kg of the A. blazei polysaccharides showed 45.7 and 63.2% recovery rates, respectively. The polysaccharides were isolated from the fruiting bodies of A. blazei by repeated extraction with hot water, cold NaOH, and then hot NaOH. Compositional analysis indicated that A. blazei polysaccharides are (1-6)-β-glucans, with the presence of glucose (93.87%), mannose (3.54%), and arabinose (2.25%). The level of pro-inflammatory IL-1β was further increased; indicating the potential of A. blazei polysaccharides as an effective way to promote wound healing by enhancing immunity activity. The rate of wound contraction was increased due to rapid wound contraction, epithelialization, and collagenization [Citation52]. In addition, IL-1β contributes to re-epithelialization, angiogenesis, and collagen synthesis [Citation53].
3.1.2. Auricularia auricula
The polysaccharides of A. auricula (Common name: Wood ear) showed significant wound healing effects in the porcine skin ex-vivo wound healing model. The extracted polysaccharides can promote keratinocyte migration up to 40% (140.43%) at 10 mg/ml 48 h post-exposure in comparison to the negative control containing PBS (100%). The reported effect is comparable to EGF (154.2%); a key player in stimulating keratinocyte proliferation during epithelialization [Citation54].
3.1.3. Ganoderma spp.
Water extract of G. lucidum (Common name: Lingzhi) mycelia was reported to shorten the recovery period of wound healing in an excision wound model. G. lucidum hot water extract stimulated early neutrophil recruitment into the wound area and on day 3 post-wounding, the wound was fully covered with a thin layer of epithelium, suggesting an enhancement in keratinocytes proliferation [Citation55]. On the other hand, rats treated with G. lucidum proteins exhibited increased wound closure rate (Day 5 − 18.8 mm2, Day 8 − 5.9 mm2) as compared to treatment by povidone-iodine (Day 5 − 22.4 mm2, Day 8 − 8.2 mm2) in an excision wound model study. Hematoxylin-eosin stained sections of granulation tissues at day 8 showed well-organized thick epithelium and deposition of collagen into the tissues, suggesting the proliferation of keratinocytes and fibroblasts [Citation56]. In another study, water extract of G. lucidum fruiting body was reported to possess an abundance of glutamic proteases of the peptidase G1 family. Glycoside hydrolases were also identified from G. lucidum, such as β-N-acetyl hexosaminidase, α-1,2-mannosidase, endo-β-1,3-glucanase, and β-1,3-glucanase [Citation57]. Several studies have reported that glycoside hydrolases were able to disrupt the formation of fungal and bacterial biofilm. Biofilm-related infections were one of the main factors leading to chronic wound healing [Citation58–60]. Protease, one of the key factors for tissue repair, plays a role in the influx of leukocytes, angiogenesis, and re-epithelialization in the wound healing process. It breaks down damaged extracellular matrix, especially matrix metalloproteinases, to enable the remodeling of the new tissue [Citation61].
The G. lucidum-derived LZ-8 protein was applied to the anterior lesion created on Sprague-Dawley rats’ liver. Histological analysis showed that wound size reduced and fibrosis increased in the LZ-8-treated liver tissues, compared to the untreated group [Citation62]. In a separate study, 10% (w/w) of polysaccharides from the fruiting body of G. lucidum hot water extract showed excision wound healing improvement in diabetic rats with higher wound closure rate (Day 8 - 60%, Day 12 - 97%) as compared to a positive control (Day 8 - 55%, Day 12 - 70%). Histopathological analysis showed more formation of capillary vessels on day 6 post-wounding with fewer inflammatory cells, good epithelialization, and well-formed granulation tissue compared to the negative control. G. lucidum may have shortened the inflammatory phase, enhanced collagen formation, and re-epithelialization as well as angiogenesis promotion [Citation63].
Ganoderma tsugae (Common name: Hemlock varnish shelf) has also been reported to enhance wound closure in an excision wound model. The treatment of G. tsugae chitin accelerated wound healing by stimulating the early recruitment of neutrophils and lymphocytes into the wound site within 24 h post-wounding and promoted early expression of PCNA and type I collagen. In addition, G. tsugae chitin promoted angiogenesis and the expression of trans-glutaminase (t-TGase), to the capillary vessels in the later phase of wound healing for sufficient blood supply and enhance of dermal matrix remodeling, respectively [Citation64–66].
3.1.4. Hericium erinaceus
H. erinaceus (common name: Lion’s mane) hot aqueous extract was topically applied on the wound in the dorsal of male Sprague-Dawley rats. The extract showed a higher rate of wound closure compared to negative control in an excision wound rat model. Histological analysis showed lower macrophage count, higher collagen content, and apparent angiogenesis in the treated group, leading to a conclusion that H. erinaceus aqueous extract promoted angiogenesis and collagen formation, and shortened the inflammation phase in the wound healing process [Citation67]. In another study, the fruiting bodies of H. erinaceus demonstrated a neurogenerative role in the peripheral nervous system. An in vivo study done using rats with traumatic nerve injury treated with polysaccharides from H. erinaceus (at a dosage of 30 mg/kg body weight) showed accelerated recovery of the sensory function of injured peripheral nerves [Citation68,Citation69].
3.1.5. Lentinus edodes
Polysaccharides from L. edodes (Common name: Shiitake) significantly enhanced oral ulcer healing rate by up to 73% as compared to the untreated group. ELISA demonstrated increased IL-1β and TNF-α expressions, which are important for re-epithelialization, angiogenesis, and collagen synthesis activities in wound healing enhancement [Citation70,Citation71].
3.1.6. Phellinus gilvus
Diabetic wounds are difficult to heal mainly due to the inhibition of angiogenesis caused by high glucose conditions. Impairment of angiogenesis leads to chronic hypoxia, decreased entrance of growth factors, and later further tissue damage [Citation35,Citation37]. Application of polysaccharides from P. gilvus (Common name: Mustard yellow) fruiting bodies aqueous extract on the dorsal side of the wound promoted dermal wound healing in both normal and streptozotocin-induced diabetic rats. The wounds of rats treated with P. gilvus showed a significant increase in re-epithelialization rate [Citation72,Citation73].
3.1.7. Sparassis crispa
Oral administration of S. crispa (Common name: Cauliflower mushroom) powder at 1000 mg/kg body weight was reported to accelerate wound closure in an excision wound diabetic rat model. Histology analyses revealed that S. crispa stimulated early recruitment of neutrophils and macrophages into the wound site, allowing early elimination of the cells and acceleration of the inflammation phase. S. crispa also enhanced re-epithelialization, fibroblast proliferation, and collagen deposition which promoted growth factor synthesis and angiogenesis to the newly formed tissue [Citation74,Citation75].
3.1.8. Tremella fuciformis
Wound treated with T. fuciformis (Common name: Silver ear fungus) polysaccharides (0.1 mg/kg) from fruiting bodies aqueous extract showed wound healing promotion effect by increasing keratinocyte migration rate up to 25% in an ex-vivo porcine skin wound healing model [Citation54]. T. fuciformis may enhance re-epithelialization and wound closure during the proliferation phase of the wound healing mechanism.
3.2. Ethnobotanical usage of mushroom preparations for wound rehabilitation
In addition to scientific research on the wound healing activity of fungi, there are a few mushrooms that are traditionally utilized for wound treatment. These macrofungi were reported in the literature for traditional wound treatment practices. summarizes the macrofungi utilized for traditional wound treatment.
Table 2. Summary of macrofungi utilized as traditional wound treatment.
3.2.1. Lignosus rhinocerus
The sclerotium of wild L. rhinocerus (Common name: Tiger Milk Mushroom) has been used by Aborigines as a traditional medicine to treat wounds, asthma, fever, breast cancer, stomach cancer, and food poisoning [Citation76,Citation77]. The L. rhinocerus powder also was mixed with Chinese rice wine and applied topically for treating lumps, sores, and boils [Citation78].
3.2.2. Handkea utriformis
H. utriformis (Common name: Puffball) has been used in traditional practices for the treatment of wounds but lacks scientific reports. The fruiting bodies of H. utriformis are used in traditional medicine for surgical and burn wound dressings [Citation79]. When the mature fruitbody of H. utriformis bursts or is impacted, clouds of brown dust-like spores are emitted and the spore powder is useful to stop bleeding. The practice can be found in the rural state of Europe, North America, and India. A review on “Puffball Usage among North American Indians” has reported that the Indian group from the Missouri River region utilized the puffball as a hemostat. The dried puffball was pulverized and applied to the wound to stop bleeding [Citation80]. An extensive survey among the Baiga and Bharia tribes in Madhya Pradesh, central India state, have been showing that the giant puffball was utilized to stop bleeding for healing wounds [Citation81]. An early report in 1860, reported on the usage of puffball as an anesthetic, like chloroform, for burnt treatment [Citation82].
3.2.3. Morchella esculenta
M. esculenta (common name: Yellow morel) has been used in Chinese medicine for thousands of years [Citation83]. It is also highly utilized by the various tribal group from Kupwara district (Kashmir, India) and Neelum Valley (Azad Jammu and Kashmir, Pakistan). The fruiting body of M. esculenta was pulverized to powder for wound application to speed up healing and acts as an antiseptic [Citation84,Citation85].
3.2.4. Fomes fomentarius
F. fomentarius (common name: Hoof fungus) was classified by The Greek physician Hippocrates circa 450 BCE as a potent anti-inflammatory agent for cauterizing wounds [Citation86,Citation87]. In European, West Siberian, and Indian folk medicine, F. fomentarius fruit body was made as part of the bandage material. It was pounded with water until it soften and externally applied to wounds to stop the bleeding [Citation88,Citation89]. In German and Austria, F. fomentarius was called a wound sponge or surgical sponge. It is widely used as a styptic by farmers, surgeons, and dentists up to the nineteenth century [Citation90,Citation91] ().
Figure 2. The isometric view of styptic bandage, invented in 1963 to facilitate the stanching of bleeding [Citation144].
![Figure 2. The isometric view of styptic bandage, invented in 1963 to facilitate the stanching of bleeding [Citation144].](/cms/asset/68564579-5e12-4d96-936a-97637fd093cc/tmyb_a_2164641_f0002_b.jpg)
3.2.5. Calvatia gigantea
C. gigantea (common name: giant puffball) has been reported to be traditionally used by American Indians in the Missouri River region and the Balkan peninsula in Europe. Due to its hemostatic properties, C. gigantea spores were traditionally powdered and applied to the wound as a dressing to stop bleeding, as well as in the treatment of inflammation. The Indians also reported applying the powder to the umbilicus of newborn infants [Citation92–94].
3.2.6. Fomitopsis betulina
F. betulina (common name: birch polypore) is a wood-rotting medicinal and edible mushroom when at a young stage. It has been practiced by the folk medicine of Russia, Poland, and other Baltic countries, primarily as an antiparasitic and antimicrobial agent and to stop wound bleeding [Citation90,Citation95]. Interestingly, F. betulina fruiting bodies were crushed to powder and used as snuff in Austria, Northern America, and Siberia as pain relievers [Citation93]. Snuffing the F. betulina powder may or may not contribute to wound healing, but it may aid in pain management for old folks during the wound healing process.
3.2.7. Lycoperdon pusillum
L. pusillum has been used traditionally by the tribal tribes in Northern and Central India (Local name: Anthua, Jatia Rutka) for controlling bleeding and wound care [Citation96]. In Nigeria, L. pusillum was used by the Aboriginals to treat abrasions, sores, deep cuts, hemorrhages, and urinary infections [Citation97]. The species of Lycoperdon were utilized as hemostatic agents by the American Indian, where the soft, central portion of dried, immature mushroom was powdered and covered into the wound to stop bleeding [Citation80]. This practice also can be found in Central India by the Baiga tribe [Citation96].
4. Tiger milk mushroom
Lignosus rhinocerus (Cooke) Ryvarden, locally known as tiger milk mushroom, “cendawan susu rimau,” “betes kismas,” “hurulingzhi,” and “hijiritake” inhabits the soil in the tropical region [Citation98–101]. It can only be found in the Asia-Pacific region encompassing South China, Southeast Asia, and Oceania [Citation102–104]. This mushroom is difficult to find in the wild as it grows in solitary. The collection of tiger milk mushrooms is rare and expensive due to the limited number of harvests from the wild. The decline of this mushroom caused by deforestation, overharvesting, and lack of know-how to cultivate has led this species to the brink of extinction [Citation105]. An enormous effort to domesticate the mushroom was performed circa 2009 where successful cultivation of L. rhinocerus (TM02®) using a specially formulated culture medium consisting of rice, water, and other food-based materials was reported by LiGNO™ Biotech Sdn. Bhd., a Malaysian SME using their in-house proprietary method. In brief, the inoculated medium is incubated in a controlled culture room for up to six months for sclerotia formation before harvesting. Commercial production of L. rhinocerus TM02® led to an increased opportunity for research on the various purported medicinal properties of the mushroom [Citation106–109].
The sclerotium is the part of the mushroom with medicinal value and it has been used as a traditional medicine to treat wounds, asthma, fever, breast cancer, stomach cancer, and food poisoning [Citation76,Citation77,Citation110]. To date, L. rhinocerus has been reported to show therapeutic activity such as immunomodulatory, neurite growth promotion, antiproliferative, antiviral, anti-inflammatory, antimicrobial, and antioxidant activities [Citation102,Citation111–118]. Although L. rhinocerus had been reported as a traditional medicine for wound treatment, there is a lack of scientific evidence that supported such usage. In the next subsection, the biological activities of L. rhinocerus and how they may help in enhancing wound healing activity are discussed.
4.1. Pharmacological relevance of L. rhinocerus to wound healing
Due to the declining numbers of wild type L. rhinocerus, some institutes or companies were making the initiative on developing techniques for the cultivation of L. rhinocerus, such as LiGNO™ Biotech Sdn. Bhd. (cultivar TM02®), Hong Kong Polytechnic University, and Sanming Mycological Institute [Citation108,Citation115,Citation119] to avoid this valuable mushroom from being extinct. Various in-depth scientific studies were then called for to elucidate the bioactivities of these cultivars. summarizes the pharmacological relevance of the wild and cultivated types of L. rhinocerus to wounds.
Table 3. Summary of pharmacological relevance of Lignosus rhinocerus to wound healing.
L. rhinocerus was reported to immunomodulate by increasing cytokines (IL-5, IL-6, and MIP-2) expression in RAW 264.7 cells conceivably through the NF-κB/MAPK signaling pathways [Citation115,Citation116,Citation119]. Both signaling pathways have been implicated in corneal epithelial wound healing, scratch injury, and cutaneous wound healing [Citation120–125]. MIP-2 dominates the early part of the inflammation phase by regulating the migration of granulocytes including neutrophils and stem cells [Citation126,Citation127]. Meanwhile, pro-inflammatory IL-5 and IL-6 cytokines are released to promote inflammation by inducing immune cells to the wound site and concurrently activating growth factors that contribute to angiogenesis and collagen synthesis [Citation128–131]. Increased release of IL-5, IL-6, and MIP-2 caused by L. rhinocerus will accelerate the inflammatory phase by early infiltration and elimination of neutrophils and macrophages.
Previous studies also demonstrated the anti-inflammatory activity of L. rhinocerus by its inhibition of TNF-α production in LPS-induced macrophages and reduced levels of IgE, Th2 cytokines, and eosinophil count [Citation117,Citation118]. Eosinophils play a beneficial role as pro-inflammatory cells in fighting pathogens through the release of cytokines and chemokines [Citation132]. However, overregulation of pro-inflammatory cytokines caused by infections, allergic reactions, and autoimmune disorder(s) may lead to prolonged inflammation and tissue damage [Citation133–135]. By regulating the expression of the pro-inflammatory cytokines, L. rhinocerus may assist in reducing the period of the inflammatory phase during wound healing.
Without proper treatment, generated wounds have a high possibility to be colonized by bacteria. The proliferation of colonized bacteria may lead to infection and delay wound healing [Citation18,Citation136]. Treatment of the wound site with an antimicrobial agent(s) may reduce the prolonged inflammation phase in wound healing. An antimicrobial assay was conducted on petroleum ether, chloroform, methanol, and water extracts of L. rhinocerus. The extracts showed active antimicrobial activity at 30 mg/ml [Citation114]. L. rhinocerus may be utilized for wound care management by curbing bacterial infections, however, further study is warranted.
Although lacking, scientific research has reported the potential usage of L. rhinocerus for wound rehabilitation. Ahmad et al. [Citation137] demonstrated the presence of protease and fibrinolytic activities in LR-1 of wild type L. rhinocerus sclerotium in which a clear zone on skim milk agar and fibrin plates was observed. The findings from this study may indicate the beneficial role of L. rhinocerus in new tissue formation and wound closure enhancement during the breakdown of damaged ECM proteins and foreign material. Administration of L. rhinocerus β-glucans at 100 µg/ml was also reported to hasten mucosal wound healing by accelerating IEC-6 cell proliferation and migration. The expression of Cdc-42, Rac-1, and Rho-A was found to be enhanced in the study [Citation138]. All in all, L. rhinocerus β-glucans may augment the epithelial restitution process during the wound healing mechanism [Citation139].
4.2. Future perspective of L. rhinocerus as wound healing promoting agent
L. rhinocerus has been utilized for many years due to its medicinal benefits. With its successful domestication and research advances, numerous bio-pharmacological activities and bioactive compounds have been reported. Yet, although L. rhinocerus had been reported as a traditional medicine for wound treatment, there is a lack of scientific basis to support the claim. L. rhinocerus is hypothesized to reduce the inflammation phase during wound healing by inhibiting microbial infection and increasing cytokines expression at the early stage of inflammation whereas its immunomodulating activity helps in regulating the pro-inflammatory cytokines to avoid prolonged inflammation and tissue damage. However, scientific studies are much needed to prove the hypothesis.
5. Conclusions
Various synthetic products have been described as wound care treatments, but some authors suggest that these products can cause toxic side effects, even at doses required to achieve good results. In recent years, various research has been conducted on natural products; including macrofungi, to develop novel pharmaceutics that target wounds and much effort has been invested to investigate its associated healing biology. The presence of these bioactive compound(s) may contribute to various biological activities which facilitate the early inflammatory phase, keratinocyte proliferation, as well as cell migration enhancement which are important mechanisms for wound healing. There are also a few fungi that are traditionally utilized for wound treatment but lack scientific reports. Interestingly, the utilization of macrofungi in traditional wound care was mostly for hemostatic purposes, which is to stop the bleeding. Now, the advancement of current wound care research and development enables researchers to develop products that was specific to enhance the wound healing process, especially on antimicrobial action and promote growth factor. As such, more research is required in this area to divulge supplementary scientific data to support their ethnobotanical uses. For instance, the mechanism(s) by which macrofungi may influence wound healing in bacterial and fungal infections can be investigated via microbial internalization (via bacterial challenge assay) and immunocytochemistry. This enables a deeper clinical understanding of wounds and their pathophysiology which will be significant to biomedical innovations and clinical trials.
Author contributions
Hui-Yeng Y. Yap: Conceptualization, Writing—Review & Editing, Supervision, Funding acquisition. Mohammad Farhan Ariffeen Rosli: Writing—Original Draft. Soon-Hao Tan: Writing—Review & Editing, Supervision. Boon-Hong Kong: Writing—Review & Editing, Supervision. Shin-Yee Fung: Writing—Review & Editing, Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grainger DW. Wound healing: enzymatically crosslinked scaffolds. Nat Mater. 2015;14(7):662–663.
- Griffin DR, Weaver WM, Scumpia PO, et al. Scaffolds assembled from annealed building blocks. Nat Mater. 2015;14(7):737–744.
- Kang J, Hu J, Karra R, et al. Modulation of tissue repair by regeneration enhancer elements. Nature. 2016;532(7598):201–206.
- Lee EJ, Huh BK, Kim SN, et al. Application of materials as medical devices with localized drug delivery capabilities for enhancing wound repair. Prog Mater Sci. 2017;89:392–410.
- Rani S, Ritter T. The Exosome - A naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2016;28(27):5542–5552.
- Avinash D. Global skin and wound care market is expected to reach USD 25.98 billion by 2025: Fior Markets. Los Angeles (CA): GlobeNewswire; 2020.
- James S. Complementary & alternative medicine market worth $296.3 billion by 2027: Grand View Research, Inc. New York (NY): Cision PR Newswire; 2020.
- Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6(11):1–5.
- World Health Organization. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva: World Health Organization; 2004.
- Abd Jalil MA, Shuid AN, Muhammad N. Role of medicinal plants and natural products on osteoporotic fracture healing. Evid Based Complement Altern Med. 2012;2012:714512.
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Neurol. 2014;4:1–10.
- Ernst E, Cassileth BR. How useful are unconventional cancer treatments? Eur J Cancer. 1999;35(11):1608–1613.
- Kaneno R, Fontanari LM, Santos SA, et al. Effects of extracts from Brazilian Sun-Mushroom (Agaricus blazei) on the NK activity and lymphoproliferative responsiveness of Ehrlich tumor-bearing mice. Food Chem Toxicol. 2004;42(6):909–916.
- Kumarasamyraja D, Jeganathan NS, Manavalan R. A review on medicinal plants with potential hypolipidemic activity. Int J Pharm Sci. 2012;2(4):101–107.
- Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58(3):298–312.
- Larouche J, Sheoran S, Maruyama K, et al. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209–231.
- Otterço AN, Andrade AL, Brassolatti P, et al. Photobiomodulation mechanisms in the kinetics of the wound healing process in rats. J Photochem Photobiol B. 2018;183:22–29.
- Sarheed O, Ahmed A, Shouqair D, et al. Antimicrobial dressings for improving wound healing. In: Alexandrescu V, editor. Wound healing - new insights into ancient challenges. London: IntechOpen; 2016. p. 298–378.
- Drucker CR. Update on topical antibiotics in dermatology. Dermatol Ther. 2012;25(1):6–11.
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392.
- Maeda S, Fujimoto M, Matsushita T, et al. Inducible costimulator (ICOS) and ICOS ligand signaling has pivotal roles in skin wound healing via cytokine production. Am J Pathol. 2011;179(5):2360–2369.
- Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205(1):43–51.
- Wang L-L, Zhao R, Li J-Y, et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128–136.
- Makino K, Jinnin M, Aoi J, et al. Knockout of endothelial cell-derived endothelin-1 attenuates skin fibrosis but accelerates cutaneous wound healing. PLoS One. 2014;9(5):e97972.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525.
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464.
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97(3):173–183.
- Mann A, Breuhahn K, Schirmacher P, et al. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol. 2001;117(6):1382–1390.
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674–686.
- Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5(3):119–136.
- McDougall S, Dallon J, Sherratt J, et al. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos Trans A Math Phys Eng Sci. 2006;364(1843):1385–1405.
- Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering. 2021;8(5):63.
- Leask A, Abraham DJ. TGF-B signaling and the fibrotic response. FASEB J. 2004;18(7):816–827.
- Tonnesen MG, Feng X, Clark RAF. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–46.
- Brem H, Sheehan P, Boulton AJM. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187(5):S1–S10.
- Junger M, Steins A, Hahn M, et al. Microcirculatory dysfunction in chronic venous insufficiency (CVI). Microcirculation. 2000;7:3–12.
- Stavrou D. Neovascularisation in wound healing. J Wound Care. 2008;17(7):298–300, 302.
- Ghosh G, Subramanian IV, Adhikari N, et al. Hypoxia-induced MicroRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest. 2010;120(11):4141–4154.
- Tchanque-Fussuo CN, Dahle SE, Buchman SR, et al. Deferoxamine: potential novel topical therapeutic for chronic wounds. Br J Dermatol. 2017;176:1056–1059.
- Clark RAF. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306(1):42–48.
- Li B, Wang JHC. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20(4):108–120.
- Marangoni RG, Korman BD, Wei J, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–1073.
- Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-B1 induces a-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111.
- Montesano R, Orci L. Transforming growth factor B stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci U S A. 1988;85(13):4894–4897.
- Yeh C-J, Chen C-C, Leu Y-L, et al. The effects of artocarpin on wound healing: in vitro and in vivo studies. Sci Rep. 2017;7(1):15513–15599.
- Warnock DW. Superficial, subcutaneous and systemic mycoses. Greenwood D, et al., editors. Elsevier Ltd: London; 2012.
- Machado MP, Filho ER, Terezan AP, et al. Cytotoxicity, genotoxicity and antimutagenicity of hexane extracts of Agaricus blazei determined in vitro by the comet assay and CHO/HGPRT gene mutation assay. Toxicol In Vitro. 2005;19(4):533–539.
- Batterbury M, Tebbs CA, Rhodes JM, et al. Agaricus bisporus (edible mushroom lectin) inhibits ocular fibroblast proliferation and collagen lattice contraction. Exp Eye Res. 2002;74(3):361–370.
- Kent D, Sheridan CM, Tomkinson HA, et al. Edible mushroom (Agaricus bisporus) lectin inhibits human retinal pigment epithelial cell proliferation in vitro. Wound Repair Regen. 2003;11(4):285–291.
- Wenkel H, Kent D, Hiscott P, et al. Modulation of retinal pigment epithelial cell behavior by Agaricus bisporus lectin. Investigat Ophthalmol Visual Sci. 1999;40(12):3058–3062.
- Lam WP, Wang CM, Tsui TY, et al. Extract of white button mushroom affects skin healing and angiogenesis. Microsc Res Tech. 2012;75(10):1334–1340.
- Sui Z, Yang R, Liu B, et al. Chemical analysis of Agaricus blazei polysaccharides and effect of the polysaccharides on IL-1β mRNA expression in skin of burn wound-treated rats. Int J Biol Macromol. 2010;47(2):155–157.
- Ambrozova N, Ulrichova J, Galandakova A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomedical papers of the medical faculty of. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(1):1–13.
- Khamlue R, Naksupan N, Ounaroon A, et al. Skin wound healing promoting effect of polysaccharides extracts from Tremella fuciformis and Auricularia auricula on ex-vivo porcine skin wound healing model. Singapore: IACSIT Press; 2012.
- Krupodorova TA, Klymenko PP, Barshteyn VY, et al. Effects of Ganoderma lucidum (curtis) P. Karst and Crinipellis schevczenkovi Buchalo aqueous extracts on skin wound healing. J Phytopharmacol. 2015;4(4):197–201.
- Gupta A, Kirar V, Keshri GK, et al. Wound healing activity of an aqueous extract of the Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher basidiomycetes). Int J Med Mushrooms. 2014;16(4):345–354.
- Kumakura K, Hori C, Matsuoka H, et al. Protein components of water extracts from fruiting bodies of the Reishi mushroom Ganoderma lucidum contribute to the production of functional molecules. J Sci Food Agric. 2019;99(2):529–535.
- Fleming D, Chahin L, Rumbaugh KP. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob Agents Chemother. 2017;61(2):1–9.
- Redman WK, Welch GS, Rumbaugh KP. Differential efficacy of glycoside hydrolases to disperse biofilms. Front Cell Infect Microbiol. 2020;10:377–379.
- Snarr BD, Baker P, Bamford NC, et al. Microbial glycoside hydrolases as antibiofilm agents with Cross-Kingdom activity. Proc Natl Acad Sci USA. 2017;114(27):7124–7129.
- McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care. 2013;2(8):438–447.
- Lin HJ, Chang YS, Lin LH, et al. An immunomodulatory protein (ling zhi-8) from a Ganoderma lucidum induced acceleration of wound healing in rat liver tissues after monopolar electrosurgery. Evid Based Complement Altern Med. 2014;2014:916531.
- Cheng PG, Phan CW, Sabaratnam V, et al. Polysaccharides-rich extract of Ganoderma lucidum (M.A. Curtis: fr.) P. Karst accelerates wound healing in Streptozotocin-induced diabetic rats. Evid Based Complement Altern Med. 2013;2013:671252.
- Hung W-S, Fang C-L, Su C-H, et al. Cytotoxicity and immunogenicity of SACCHACHITIN and its mechanism of action on skin wound healing. J Biomed Mater Res. 2001;56(1):93–100.
- Hung W-S, Lai W-FT, Leu B, et al. Effect of SACCHACHITIN on keratinocyte proliferation and the expressions of type I collagen and tissue-transglutaminase during skin wound healing. J Biomed Mater Res B Appl Biomater. 2004;70(1):122–129.
- Jiang H, Zheng M, Liu X, et al. Feasibility study of tissue transglutaminase for self-catalytic cross-linking of self-assembled collagen fibril hydrogel and its promising application in wound healing promotion. ACS Omega. 2019;4(7):12606–12615.
- Abdulla MA, Fard AA, Sabaratnam V, et al. Potential activity of aqueous extract of culinary-medicinal Lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) in accelerating wound healing in rats. Int J Med Mushrooms. 2011;13(1):33–39.
- Wong K-H, Naidu M, David P, et al. Peripheral nerve regeneration following crush injury to rat peroneal nerve by aqueous extract of medicinal mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae). Evid Based Complement Altern Med. 2011;2011:1–10.
- Wong K-H, Kanagasabapathy G, Bakar R, et al. Restoration of sensory dysfunction following peripheral nerve injury by the polysaccharide from culinary and medicinal mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Through its neuroregenerative action. Food Sci Technol. 2015;35(4):712–721.
- Yu Z, LiHua Y, Qian Y, et al. Effect of Lentinus edodes polysaccharide on oxidative stress, immunity activity and oral ulceration of rats stimulated by phenol. Carbohydr Polym. 2009;75(1):115–118.
- Chen X, HaiYan Z, JianHong Z, et al. The pharmacological effect of polysaccharides from Lentinus edodes on the oxidative status and expression of VCAM-1mRNA of thoracic aorta endothelial cell in high-fat-diet rats. Carbohydr Polym. 2008;74:445–450.
- Bae J-S, Jang K-h, Park S-C, et al. Promotion of dermal wound healing by polysaccharides isolated from Phellinus gilvus in rats. J Vet Med Sci. 2005;67(1):111–114.
- Bae JS, Jang KH, Jin HK. Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats. J Vet Sci. 2005;6(2):161–164.
- Kwon A-H, Qiu Z, Hashimoto M, et al. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am J Surg. 2009;197(4):503–509.
- Yamamoto K, Kimura T. Orally and topically administered Sparassis crispa (Hanabiratake) improved healing of skin wounds in mice with streptozotocin-induced diabetes. Biosci Biotechnol Biochem. 2013;77(6):1303–1305.
- Lai WH, Siti Murni MJ, Fauzi D, et al. Optimal culture conditions for mycelial growth of Lignosus rhinocerus. Mycobiology. 2011;39(2):92–95.
- Tan CS, Ng ST, Vikineswary S, et al. Genetic markers for identification of a Malaysian medicinal mushroom, Lignosus rhinocerus (cendawan susu rimau). Acta Hortic. 2010;859(859):161–167.
- Wong LZ. Health benefits of wild tiger’s milk mushroom. Selangor: TheStar; 2011.
- Petrović P, Vunduk J, Klaus A, et al. From mycelium to spores: a whole circle of biological potency of mosaic puffball. S Afr J Bot. 2019;123:152–160.
- Burk WR. Puffball usages among North American Indians. J Ethnobiol. 1983;3(1):55–62.
- Rai BK, Ayachi SS, Rai A. A note on ethno-myco-medicines from Central India. Mycologist. 1993;7(4):192–193.
- Berkeley MJ. Outlines of British fungology. London: Lovell Reeve; 1860.
- Wei W, Luo X, Zheng L, et al. Isolation of a wild Morchella spp. strain and the effects of its extract on Ethanol-Induced gastric mucosal lesions in rats. Zeitschrift Fur Naturforschung C-J Biosci. 2011;66(1-2):55–62.
- Lone FA, Lone S, Aziz MA, et al. Ethnobotanical studies in the tribal areas of district Kupwara, Kashmir, India. Int J Pharma Bio Sci. 2012;3(4):399–411.
- Mahmood A, Rifat NM, Zabta KS, et al. Ethnobotanical survey of plants from Neelum, Azad Jammu & Kashmir, Pakistan. Pak J Bot. 2011;43:105–110.
- Stamets P, Zwickey H. Medicinal mushrooms: ancient remedies meet modern science. Integr Med. 2014;13(1):46–47.
- Buller AHR. The fungus lore of the Greeks and Romans. Worcester: Baylis; 1914.
- Saar M. Fungi in Khanty folk medicine. J Ethnopharmacol. 1991;31(2):175–179.
- Vaidya JG, Rabba AS. Fungi in folk medicine. Mycologist. 1993;7(3):131–133.
- Grienke U, Zöll M, Peintner U, et al. European medicinal polypores – a modern view on traditional uses. J Ethnopharmacol. 2014;154(3):564–583.
- Kutalek R. Ethomykologie - eine ubersicht. Osterr Z. Pilzk. 2002;11:79.
- Gilmore MR. Uses of plants by the Indians of the Missouri River region. In: Thirty-third annual report of the Bureau of American Ethnology, 1911-1912. Bureau of American Ethnology; 1919. p. 43–154.
- Grundemann C, Reinhardt JK, Lindequist U. European medicinal mushrooms: do they have potential for modern medicine? - an update. Phytomedicine. 2019;66:1–33.
- Zivkovic J, et al. Ethnomycological investigation in Serbia: astonishing realm of mycomedicines and mycofood. J Fungi. 2021;7(349):1–21.
- Hobbs C. Medicinal mushrooms – an exploration of tradition, healing and culture. Canada: Botanica Press; 1995.
- Rai M, Tidke G, Wasser SP. Therapeutic potential of mushrooms. Nat Prod Radiance. 2005;4(4):246–257.
- Buswell JA, Chang ST. Edible mushrooms: attributes and applications. In: Chang AC, editor. Genetics and breeding of edible mushrooms. 1st ed. London: CRC Press; 1993.
- Haji Taha A. Orang Asli: the hidden treasure. Malaysia: Jabatan Muzium Negara; 2006.
- Huang N. Identification of the scientific name of Hurulingzhi. Acta Edulis Fungi. 1999;6:32–34.
- Yokota A. Tropical forests and people’s livelihood: stalls of traditional medicine vendors in Kota Kinabalu. JIRCAS Newsletter. 2011;62:9–10.
- Burkill IH, Birtwistle W, Foxworthy FW, et al. A dictionary of the economic products of the Malay peninsula. 2nd ed. Vol. 1. Kuala Lumpur: ministry of Agriculture; 1966.
- Eik L-F, Naidu M, David P, et al. Lignosus rhinocerus (Cooke) Ryvarden: a medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid Based Complement Altern Med. 2012;2012:320308.
- Nurraihana H, Norfarizan-Hanoon NA, Hasmah A, et al. Ethnomedical survey of aborigines medicinal plants in Gua Musang, Kelantan, Malaysia. Health Environ J. 2016;7(1):59–76.
- Katas H, Lim CS, Nor Azlan AYH, et al. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm J. 2019;27(2):283–292.
- Vikineswary S, Chang ST. Edible and medicinal mushrooms for sub-health intervention and prevention of lifestyle diseases. Tech Monitor. 2013;3:33–43.
- Abdullah N, Haimi MZD, Lau BF, et al. Domestication of a wild medicinal sclerotial mushroom, Lignosus rhinocerotis (Cooke) Ryvarden. Ind Crops Prod. 2013;47:256–261.
- Lau BF, Abdullah N, Aminudin N, et al. Ethnomedicinal uses, pharmacological activities, and cultivation of Lignosus spp. (tigeŕs milk mushrooms) in Malaysia - a review. J Ethnopharmacol. 2015;169:441–458.
- Tan CS. Setting-up pilot-plant for up-scaling production of ‘TigerMilk’-mushroom as dietary functional food. Technical Report MOA TF0109M004, Government of Malaysia; 2009.
- Yap HY, Kong BH, Fung SY. Bioactive properties of Malaysian medicinal mushrooms Lignosus spp. In: Deshmukh SK, Sridhar KR, Badalyan SM, editors. Fungal biotechnology: prospects and avenues. Boca Raton (FL): 2022; CRC Press. p. 18.
- Chang YS, Lee SS. Utilisation of macrofungi species in Malaysia. Fungal Divers. 2004;15:15–22.
- Yap YHY, Tan N, Fung S, et al. Nutrient composition, antioxidant properties, and anti-Proliferative activity of Lignosus rhinocerus Cooke sclerotium. J Sci Food Agric. 2013;93(12):2945–2952.
- Lau BF, Abdullah N, Aminudin N. Chemical composition of the tiger’s milk mushroom, Lignosus rhinocerotis (Cooke) Ryvarden, from different developmental stages. J Agric Food Chem. 2013;61(20):4890–4897.
- Ellan K, Thayan R, Raman J, et al. Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: an in-vitro study. BMC Complement Altern Med. 2019;19(1):212–260.
- Mohanarji S, Dharmalingam S, Kalusalingam A, et al. Screening of Lignosus rhinocerus extracts as antimicrobial agents against selected human pathogens. J Pharm Biomed Sci. 2012;18(11):1–4.
- Hu T, Huang Q, Wong K, et al. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int J Biol Macromol. 2017;94(Pt A):423–430.
- Sum AYC, Li X, Yeng YYH, et al. The immunomodulating properties of tiger milk medicinal mushroom, Lignosus rhinocerus TM02® cultivar (Agaricomycetes) and its associated carbohydrate composition. Int J Med Mushrooms. 2020;22(8):803–814.
- Johnathan M, Gan SH, Ezumi MFW, et al. Phytochemical profiles and inhibitory effects of tiger milk mushroom (Lignosus rhinocerus) extract on ovalbumin-induced airway inflammation in a rodent model of asthma. BMC Complement Altern Med. 2016;16:113–167.
- Lee SS, Tan NH, Fung SY, et al. Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the tiger milk mushroom. BMC Complement Altern Med. 2014;14(359):358–359.
- Guo C, Wong K-H, Cheung PCK. Hot water extract of the sclerotium of Polyporus rhinocerus Cooke enhances the immune functions of murine macrophages. Int J Med Mushrooms. 2011;13(3):237–244.
- Thuraisingam T, Xu YZ, Eadie K, et al. MAPKAPK-2 signaling is critical for cutaneous wound healing. J Invest Dermatol. 2010;130(1):278–286.
- Shreiber J, Efron PA, Park JE, et al. Adenoviral gene transfer of an NF-κB super-repressor increases collagen deposition in rodent cutaneous wound healing. Surgery. 2005;138(5):940–946.
- Wang L, Wu X, Shi T, et al. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem. 2013;288(34):24363–24371.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314–321.
- Chen J, Chen Y, Chen Y, et al. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell Physiol Biochem. 2016;39(6):2262–2274.
- Heo SC, Jeon ES, Lee IH, et al. Tumor necrosis factor-a-Activated human adipose tissue–derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131(7):1559–1567.
- Wang J, Mukaida N, Zhang Y, et al. Enhanced mobilization of hematopoietic progenitor cells by mouse MIP-2 and granulocyte colony-stimulating factor in mice. J Leukoc Biol. 1997;62(4):503–509.
- Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43.
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229.
- Childs DR, Murthy AS. Overview of wound healing management. Surg Clin North Am. 2017;97(1):189–207.
- Vagesjo E, Öhnstedt E, Mortier A, et al. Accelerated wound healing in mice by on-Site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 2018;115(8):1895–1900.
- Hayta SB, Durmuş K, Altuntaş EE, et al. The reduction in inflammation and impairment in wound healing by using strontium chloride hexahydrate. Cutan Ocul Toxicol. 2018;37(1):24–28.
- Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266.
- Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129.
- Barrel A. What to know about tumor necrosis factor. In: Chun C, editor. Medical news today. San Francisco (CA): Healthline Media; 2019.
- Gieseck RL III, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76.
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches wound management. Clin Microbiol Rev. 2001;14(2):244–269.
- Ahmad MS, Noor ZM, Ariffin ZZ. New thrombolytic agent from endophytic fungi and Lignosus rhinocerus. Open Conf Proc J. 2014;4:95–98.
- Veeraperumal S, Qiu H-M, Tan C-S, et al. Restitution of epithelial cells during intestinal mucosal wound healing: the effect of a polysaccharide from the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden. J Ethnopharmacol. 2021;274:114024.
- Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):348–353.
- Badalyan SM. Antiprotozoal activity and mitogenic effect of mycelium of culinary-medicinal shiitake mushroom Lentinus edodes (Berk.) singer (Agaricomycetideae). Int J Med Mushr. 2004;6(2):131–138.
- Hong LW, et al. Vitrification of dikaryotic mycelial cells from Lignosus rhinocerus. Pertanika J Trop Agric Sci. 2013;36(3):249–260.
- Læssøe T, Spooner B. The uses of ‘Gasteromycetes’. Mycologist. 1994;8(4):154–159.
- Anusiya G, Gowthama Prabu U, Yamini NV, et al. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered. 2021;12(2):11239–11268.
- Robins JS. Styptic bandage. Lowe WV, editor. 1963. Available from: https://patents.google.com/patent/US3113568A/en