Abstract
Auricularia is one of the broadly cultivated edible mushrooms in Korea. Most of the Korean Auricularia strains used for cultivation and breeding are known as A. auricula-judae. Recently, this species has been reported to belong to a species complex. Therefore, this study was carried out to genetically clarify the bred and cultivated Korean A. auricula-judae strains. The internal transcribed spacer (ITS) and IGS1 rDNA region sequences were determined from 10 A. auricula-judae strains by PCR and sequencing. Variation in the nucleotide sequence and sequence length of the two rDNA regions were found among the seven A. auricula-judae strains. A maximum-likelihood (ML) phylogenetic tree based on the ITS sequences clearly placed all the 10 Korean A. auricula-judae strains in the A. heimuer clade of the A. auricula-judae complex. A. heimuer is diverged from A. auricula-judae. An ML phylogenetic tree based on the IGS1 sequences revealed the close relationship between Korean A. heimuer strains to Chinese A. heimuer strains. But each strain could be distinguishable by the IGS1 sequence. Furthermore, progeny strains in the seven Korean strains could be differentiated from their parental strains by the IGS1 sequence based phylogenetic tree. Our results are expected to be used to complement the distinction of domestic Auricularia cultivars.
Auricularia auricula-judae, a well-known wood ear mushroom, has emerged as a new impetus for researchers interested in developing functional foods [Citation1]. Recently, species clarification of A. auricula-judae complex was performed with the A. auricula-judae complex and related species from 33 wild collections and 10 cultivated samples worldwide using the ITS and nLSU ribosomal RNA gene [Citation2]. Importantly, it was found that all the wild and cultivated A. auricula-judae samples from China are different from those from Europe. Consequently, the Chinese A. auricula-judae samples were reclassified as a new species, A. heimuer. By analyzing ITS, nLSU, rpb1, and rpb2 sequences using methods of maximum-likelihood (ML) and Bayesian Inference analyses, Wu et al. further confirmed that A. heimuer is phylogenetically different from A. auricula-judae [Citation3]. A. heimuer is considered to have a wide geographic distribution in East Asia.
The production of wood ear mushroom in Korea is continuously increasing and the cultivation area is also increasing according to the statistical data of Korea Statistical Information Service (KOSIS). From 2007 to 2013, the mushroom’s production in Korea increased to 239,087 kg. To enhance mushroom production and to improve mushroom quality, new hybrid varieties are being produced through crossbreeding between wood ear mushroom varieties worldwide. In Korea, the hybrid A. auricula-judae strain of “Yong-A” variety has been developed through breeding [Citation4]. Recently, more hybrid strains have been developed in the Forest Mushroom Research Center, Yeoju, South Korea. However, so far, there is no research on the genetic clarification of bred and cultivated Korean A. auricula-judae strains. Thus, this study was performed to genetically clarify the Korean A. auricula-judae strains and to examine whether the IGS1 polymorphism is feasible in the differentiation of parental and progeny strains which were resulting from breeding processes.
Seven A. auricula-judae strains that are already developed and cultivated in Korea were used in this study (). All these diploid strains were obtained from the Forest Mushroom Research Center, Yeoju, South Korea. Sanjo900A and F7760 strains are cultivated and sold varieties in Korea. F7094 and F7557 strains were derived from specimens collected in Yanggu and Baekdusan, respectively. As a cross, Sanjo901A strain is from a cross between Sanjo900A and F7557 strains and A99 strain is from a cross between F7760 and F7557 strains. F7932 strain is a hybrid of F7760 and F7557 strains like A99 strain, but the color of the fruiting body is white due to mutation.
Table 1. Korean Auricularia heimuer strains (formerly A. auricula-judae) clarified in this study.
To analyze the ITS and IGS sequence of the Korean Auricularia strains, the mycelium core was inoculated on malt extract agar overlayered with cellophane sheet and cultured at 25 °C for one week. After scraping the cultured mycelium with a surgical scalpel blade no. 21, about 100 mg of mycelium was used for DNA extraction. Genomic DNA was extracted according to the protocol described by Kim et al. [Citation5]. The internal transcribed spacer (ITS) sequence was PCR amplified using the universal ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5'TCCTCCGCTTATTGATATGC-3′) primers [Citation6]. PCR amplification of the IGS1 was done using A. auricula-judae specific primers IGS1-5F (5′-TATGTCCCGCATGTGTTAGT-3′) and IGS1-3R (5′-GCGTCTATAAGGCGTAACTA-3′) [Citation7]. The PCR conditions were set and performed according to the methods described by Kim et al. and Li et al. [Citation5,Citation8]. The fidelity of the PCR amplicon was confirmed through an electrophoresis using an 1% agarose gel. As a result of PCR, single band of the expected size was amplified from each strain with both the ITS and IGS1 primers. Thus, the single DNA band in the gel was cut and gel extracted using QIAquick Gel Extraction Kit from Qiagen Corp. (Hilden, Germany). The gel extracted PCR amplicon was directly sequenced by Macrogen Corp. (Seoul, South Korea). The determined nucleotide sequences were searched through the BLAST program in the GenBank database in the online site of National Center for Biotechnology Information (NCBI), Bethesda, MD (https://www.ncbi.nlm.nih.gov/) to confirm whether the PCR amplicons are the ITS or IGS1 sequences of Auricularia species. Reference sequences of the ITS and IGS1 used for the phylogenetic analysis were downloaded from the GenBank database of NCBI. Multiple sequence alignment was performed using the Clustal Omega program and MAFFT [Citation7,Citation9]. An ML phylogenetic tree was constructed with MEGA X program and UNIGENE program [Citation10,Citation11].
The determined nucleotide sequences of ITS of the 10 A. auricula-judae strains are deposited in the GenBank with accession numbers MW830133, MW830137–MW830142 (). The sequence analysis of ITS reveals that all Korean A. auricula-judae strains are over 632 bp (). Interestingly, there is difference in the length of the ITS sequences among the seven strains. The ITS sequence lengths ranged from 632 bp to 645 bp. The longest length was found in A99 and F7932 strains and the shortest in Sanjo900A strain. However, as results of BLAST search, all the ITS sequences of seven Korean A. auricula-judae strains were found to be consistent with known Auricularia heimuer ITS sequences. Consequently, we further compared the ITS sequences of seven A. auricula-judae strains by phylogenetic analysis to clarify their taxonomic position. The known ITS sequences of 32 Auricularia species in the GenBank databases were included in this phylogenetic analysis. The ML phylogenetic tree constructed based on the ITS sequences shows that all the Korean strains are grouped into A. heimuer species (). The A. heimuer species clade including all Korean strains was clearly separated from A. auricula-judae clade. Phylogenetic study of Wu et al. illustrated that A. heimuer strains from China, Japan, Thailand grouped together and separated from A. auricula-judae [Citation2]. Wu et al. also demonstrated that A. heimuer is distantly related with A. auricula-judae clade which has strains of European origin [Citation3]. Together with these reports, the results of clarified that all of the seven Korean A. auricula-judae strains are A. heimuer.
Figure 1. Maximum-likelihood phylogenetic tree based on ITS sequence. New sequences of Korean Auricularia strains generated in this study are indicated in bold. Bootstrap values higher than 60% are shown in the branches. GenBank accession number is given in front of strain names.
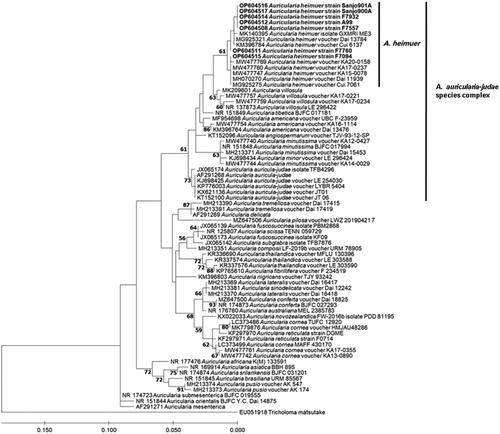
Recently, A. angiospermarum, A. americana, A. auricula-judae, A. heimuer, A. minor, A. minutissima, A. tibetica, and A. villosula, were treated as A. auricula-judae complex by the phylogenetic analysis [Citation3,Citation12]. Among these species, A. heimuer is the third most important cultivated mushroom worldwide [Citation13] and the most important Auricularia species in China [Citation14]. From the investigation of the specimen collection from wild sources in Korea, A. americana, A. heimuer, A. minutissima, and A. villosula were found as the species belonging to the A. auricula-judae complex [Citation15]. A. auricula-judae was not found in the wild specimen collection. In the present study, we further confirmed that A. auricula-judae is not found in the cultivated strains or bred strains for cultivation such as listed in .
IGS1 region sequences have been applied to design and develop IGS1-based specific primers (IGS1-5F and IGS1-3R) for A. auricula-judae detection by Li et al. [Citation8]. Thus, we tried to validate whether the A. auricula-judae specific primers could amplify IGS1 sequences from the seven A. heimuer strains listed in since these strains were previously known as A. auricula-judae. Interestingly, the A. auricula-judae specific IGS1 primers amplified single band from each of the seven Korean Auricularia strains. All the amplified single bands were confirmed as IGS1 sequences through sequencing and GenBank homology search. The determined nucleotide sequences of IGS1 of the seven A. heimuer strains were deposited in the GenBank with accession numbers OP471460–OP471466 (). Our results demonstrate that IGS1-5F and IGS1-3R primers are not A. auricula-judae specific. The reason for this nonspecific amplification of IGS1 sequences of A. heimuer could be explained by the fact that Li et al. did not include A. heimuer strains for their research on specific primer development because A. heimuer was not present at that time [Citation8]. Additionally, their study analyzed four A. auricula-judae strains only from Heilongjiang province. In fact, extension tests on A. auricula-judae strains from other provinces in China and foreign countries should have been performed to confirm the fidelity of the two primer specificities. Later, taxonomic studies showed that A. auricula-judae strains from various provinces in China were separated from strains from European counties, and A. auricula-judae strains in China were reclassified as A. heimuer [Citation2,Citation3]. That is why the A. auricula-judae specific IGS1 primers could amplify the seven Korean A. heimuer strains (formerly A. auricula-judae).
IGS1 nucleotide sequences of seven Korean strains of A. heimuer in were different in length. The IGS1 sequence length ranged from 1804 bp to 2042 bp. The longest length was found in F7557 strain and the shortest in F7760 strain. The IGS1 region of the seven Korean A. heimuer strains has no repeats as like Chinese Auricular strains [Citation14]. But sequence polymorphism clearly existed among the seven Auricularia strains. Phylogenetic analysis based on the IGS1 sequences showed that the seven Auricularia strains could be separated into several subclades (). These results indicate that each Korean A. heimuer strain could be distinguishable by the IGS1 sequence. Since A. auricula-judae is reclassified as A. heimuer strains, we regarded all A. auricula-judae strains in as A. heimuer strains in interpretation of the results of . All A. auricula-judae strains from various provinces in China were also formed several subclades, indicating genetic variation is present among the Chinese strains [Citation14].
Figure 2. Maximum-likelihood phylogenetic tree based on IGS1 sequences. Korean Auricularia strains generated in this study are indicated in bold. Bootstrap values higher than 60% are shown in the branches. All A. auricula-judae strains were from Chinese origin and genetically clarified as A. heimuer [Citation4,Citation5]. GenBank accession number is given in front of strain names.
![Figure 2. Maximum-likelihood phylogenetic tree based on IGS1 sequences. Korean Auricularia strains generated in this study are indicated in bold. Bootstrap values higher than 60% are shown in the branches. All A. auricula-judae strains were from Chinese origin and genetically clarified as A. heimuer [Citation4,Citation5]. GenBank accession number is given in front of strain names.](/cms/asset/e54d607a-732c-4187-a8c3-7f312410522d/tmyb_a_2182024_f0002_b.jpg)
The phylogram also shows that all Chinese A. auricula-judae and seven Korean are grouped together as A. heimuer (). Each Chinese strain could be distinguishable by the IGS sequences. In addition, compared to the ITS sequences which could not distinguish Korean A. heimuer strains from Chinese A. heimuer strains, the IGS1 sequences could do distinguish. Six out of seven Korean strains were distantly related with the A. heimuer strains of Chinese origin. Only one Korean A. heimuer strain F7760 was positioned more closely to Chinese strains. It is interesting result because the A99 strain is a progeny generated from the hybrid of parental F7760 x F7557 strains. These results support that all A. auricula-judae strains with Chinese origin in are A. heimuer. The progeny strains A76, F932, and A99 were diverged from their parental F7557 strain. These results indicate that the IGS1 sequence is useful for discrimination of not only species among species but also bred strains within a species. We could use IGS1 sequence as a useful genotype maker for a bred strain of A. heimuer.
In conclusion, we genetically clarified seven A. heimuer strains which are bred, cultivated and commercially sold in Korea based on the ITS and IGS1 sequence analysis. Duplication and deletion of IGS1 region may have occurred via unequal crossing over during mitotic or meiotic recombination resulting in difference in length. The genetic diversity information between A. heimuer strains is expected to be used to complement the distinction of domestic Auricularia cultivars and to be the reference for the crossing studies with other Auricularia species. The usefulness of the IGS1 region in the characterization of monokaryotic strains of button mushroom was demonstrated [Citation16]. Therefore, further studies of IGS1 in monokaryotic strains generated from basidiospores of the good-traited A. heimuer cultivars would be benefit to improve wood ear mushroom breeding program.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu E, Zhang Y, Liu F, et al. Review on Auricularia auricula-judae as a functional food: growth, chemical composition, and biological activities. J Agric Food Chem. 2021;69(6):1739–1750.
- Wu F, Yuan Y, Malysheva VF, et al. Species clarification of the most important and cultivated Auricularia mushroom "Heimuer": evidence from morphological and molecular data. Phytotaxa. 2014;186(5):241–253.
- Wu F, Tohtirjap A, Fan L-F, et al. Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J Fungi. 2021;7(11):933.
- Kim KJ, Kim D, Lee SJ, et al. Characterization of a new cultivar of Auricularia auricula-judae “Yong-A”. J Mushroom. 2018;16(3):198–202.
- Kim SH, Uzunovic A, Breuil C. Rapid detection of Ophiostoma piceae and O. quercus in stained wood by PCR. Appl Environ Microbiol. 1999;65(1):287–290.
- Badotti F, de Oliveira FS, Garcia CF, et al. Effectiveness of ITS and sub-regions as DNA barcode markers for the identification of Basidiomycota (Fungi). BMC Microbiol. 2017;17(1):1–12.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Li L, Wei L, Bian Y-B, et al. Development of species-specific primers for identifying Auricularia auricula-judae using intergenic spacer 1 (IGS1) sequences. Afr J Biotechnol. 2011;10(69):15494–15500.
- Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7(1):539.
- Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549.
- Okonechnikov K, Golosova O, Fursov M, et al. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166–1167.
- Malysheva VF, Bulakh EM. Contribution to the study of the genus Auricularia (Auriculariales, Basidiomycota) in Russia. Novosti Sistematiki Nizshikh Rastenii. 2014;48:164–180.
- Yuan Y, Wu F, Si J, et al. Whole genome sequence of Auricularia heimuer (Basidiomycota, Fungi), the third most important cultivated mushroom worldwide. Genomics. 2019;111(1):50–58.
- Li L, Zhong CH, Bian YB. The molecular diversity analysis of Auricularia auricula-judae in China by nuclear ribosomal DNA intergenic spacer. Electron J Biotechnol. 2014;17(1):27–33.
- Cho SE, Kwag YN, Lee DH, et al. Current taxonomical status of Korean Auricularia species. Kor J Mycol. 2021;49(1):21–31.
- Kwon HW, Choi MA, Yun YH, et al. Genetic and biochemical characterization of monokaryotic progeny strains of button mushroom (Agaricus bisporus). Mycobiology. 2015;43(1):81–86.
