Abstract
Soybean is one of the world’s most widely cultivated food crops, and soybean seeds are supplied from national seed resources in Korea. However, the transmission of seed-borne diseases through infected soybean seeds is problematic. Among these diseases, soybean seed decay is caused by Diaporthe spp. Infecting the pods, and the infected seeds show rotting symptoms. Most diseased seeds are removed during the selection process; however, it is difficult to distinguish infected seeds that do not display symptoms. Hence, a sequence-based method was devised to screen Diaporthe-infected seeds. Based on the nuclear ribosomal internal transcribe spacer (ITS) region of the pathogen, a primer was designed to distinguish the infection from other soybean seed pathogens. As a result of the comparison between healthy and Diaporthe-diseased seeds by using the primers, Diaporthe was detected only in the diseased seeds. Therefore, it is possible to distribute healthy soybean seeds by detecting Diaporthe-diseased seeds at the genetic level using the Diaporthe-specific primers.
Keywords:
Soybean (Glycine max(L.) Merr) is a major crop worldwide and a source of protein and oil [Citation1]. As the area under soybean cultivation gradually increases, the damage caused by diseases also increases. Soybean seeds are severely infected by Diaporthe species, showing symptoms including stem cancer and seed decay [Citation2,Citation3]. The diseased seeds are smaller and lighter than healthy seeds, and the germination rate also decreases, thus, it severely decrease soybean yield, quality, and stability [Citation4]. It is important to remove the diseased seeds before sowing; however, it is difficult to discern the Diaporthe-infected seeds in the early-infection stage, and the diseased seeds are also cultivated. Therefore, in this study, we developed specific primers to detect Diaporthe spp. and Diaporthe eres-infected seeds before sowing, and separate it from healthy seeds.
Diseased soybean seeds were obtained from Agricultural Research and Extension Services, Agricultural business establishment, and Agricultural Technology Center in various locations in South Korea (). Fungal pathogens were isolated using the method described by Nguyen (2014) with modifications [Citation5]. Briefly, diseased-seeds were surface-sterilized using 70% ethanol for 30 s and 1% NaClO for 30 s, washed with sterile distilled water three times, and air-dried on sterile filter paper (Whatman, No.2). The seeds were incubated on water agar at 25 °C for 7 days. The hyphal tips were transferred to acidified potato dextrose agar (APDA, pH 4.5) and incubated for 30 days for conidia production. The single fungal spores were isolated using a microscope, incubated on APDA at 25 °C for 7 days and then transferred to potato dextrose agar (PDA) at 25 °C for 7 days. The gDNA of isolated fungi was extracted using the Qiagen DNeasy mini plant kit standard protocol. The internal transcribed spacer (ITS) region was amplified via the polymer chain reaction (PCR) using universal primers ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [Citation6]. The PCR cycling conditions were: initial denaturation at 94 °C for 30 s, 30 cycles of 55 °C for 30 s and 72 °C for 1 min, and a final extension at 72 °C for 10 min. The amplified sequences were analyzed by Macrogene Inc (Seoul, South Korea). The sequence results were firstly aligned with reference species obtained from a BLAST search in the NCBI database (http://www.ncbi.nim.nih.gov/RefSeq/) using DNA-Star, and phylogenetic trees were generated using MEGA X. In total, 22 fungi were isolated from decayed soybean seeds and identified as Cercospora spp., Fusarium spp., Alternaria alternata, Corynespora cassiicola, and Diaporthe spp., including D. eres and D. longicola ().
Table 1. List of fungal isolates obtained from soybean seeds with decay symptom.
To determine the molecular detection region for Diaporthe spp., ITS, elongation factor-α (EF-α), calmodulin (CAL), and b-tubulin (TUB) gene were tested [Citation7]. The nucleotide sequences of amplified EF-α, CAL, and TUB were highly variable in length based on each fungal isolate, it was difficult to find conserve nucleotides of genus Diaporthe. Therefore, in this study, ITS region was used for design specific primers to distinguish Diaporthe spp., from Cercospora spp., Fusarium spp., A. alternate, and C. cassiicola.
The consensus sequences flanking the 18S rRNA and 28S rRNA sequences of D. eres, D. angelicae (KJ590735.1), D. cuppatea (KC343057.1), D. ganjae (KC343112.1), D. longicola (KJ590729.1), D. lusitanicae (KC343136.1), D. melonis (KC343142.1), D. phaseolorum (KJ590738.1), D. sojae (KJ590715.1), D. subordinaria (KC343213.1), Diaporthe sp. (KC343203.1), Alternaria alternata (KU254607.1), Fusarium solani (NR_163531.1), Botrytis cinerea (MW820601.1), Cercosproa kikuchii (NR_119616.1), and Sclerotinia sclerotiurum (MG818952.1) were compared to design ITS-based detection primers for Diaporthe spp. (). Three candidate primer sets, i.e. D_sp1, D_sp2, and D_sp3, were designed (). The specificity was assayed using ten soybean seed-borne pathogens, i.e. four Diaporthe spp., Fusarium spp., F. oxysporum, F. solani, R. solani, A. alternata, and S. sclerotiurum. The culturing of the ten pathogens, as well as the gDNA extraction and PCR amplification, were conducted as described above. The PCR products were electrophoresed on a 1.5% agarose gel stained with GelRed nucleic acid in TAE and then visualized under a UV-light using a Bio-Rad Gel document system (Bio-Rad, Hercules, CA, USA). The PCR amplicon (386 bp) was only observed in Diaporthe species but not in the tested Alternaria, Fusarium, Butrytis, Cercospora, and Sclerotinia species. In addition, cross-reactivity of the primer sets was not observed (). Among the candidate primer sets, the D_sp3 (forward/reverse) had the highest sensitivity, and it was used for further testing.
Figure 1. Maximum likelihood phylogenetic analysis of the Diaporthe species using ITS primers to develop a specific marker to detect Diaporthe spp. The bootstrap numbers represent the percent of 1000 replicates (only values greater or equal to 70% are shown) (A); specific detection of Diaprothe spp. from seven isolates, including Cercospora kikuchii, B. cienrea, F. solani, Sclerotinia sclerotiorum, and Alternaria alternata (B); lane M, 1000 bp DNA ladder; lanes dsp, Diaporthe sp.; lane De, D. eres; lane fsp, Fusarium sp.; lane foxy, F. oxysporum; lane fsol, F. solani; lane, R. solani; and lane aalt, A. alternata (C); Conventional PCR amplification of different amounts of Diaporthe eres DNA with the primers D_sp3F3R. M, 100 bp ladder marker; 10 ng; 1 ng; 100 pg; 10 pg; 1 pg; 100 fg; 10 fg. (D); detection of Diaporthe spp. using D_sp3F3R from Cercospora sojina, C. kikuchii, D. eres, and soybean seeds with decay and discoloration; lane Cs, C. sojina C01; lane Ck, C. kikuchii; lane De, D. eres D01; lane Hs, healthy seed; lanes Ds, seeds with decay symptoms; lanes Ps, brown and purple seeds. (E).
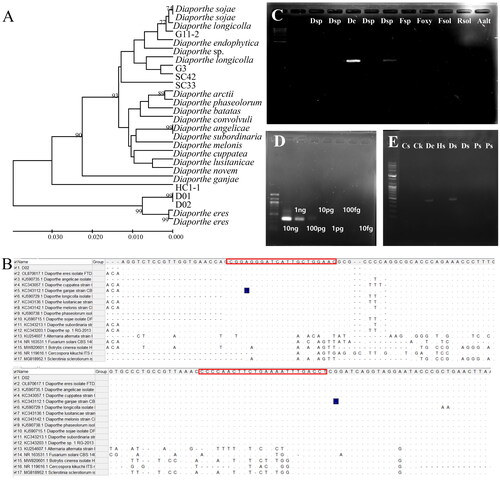
Table 2. Information on primers used in this study.
The designed D_sp3 primer for molecular detection of Diaporthe species was applied to separate Diaporthe-diseased seeds from healthy seeds. For the preparation of the artificial diseased seeds, the surface was disinfected with 70% ethanol for 30 s and 1% NaClO for 30 s, washed with sterile distilled water three times, and air-dried on sterile filter paper. Seeds were inoculated with the mycelia and spores of Diaporthe spp. (SC42, HC1-1, G3), D. eres (D02), Alternaria alternata, Fusarium spp., F. oxysporum, F. solani, and Rhizoctonia solani and incubated. The gDNA of diseased and healthy seeds were prepared as described above. The PCR was conducted by using ten-fold diluted gDNA (10 ng, 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, and 10 fg). The D_sp3 product was detected in Diaporthe-inoculated seeds, and the primer sensitivity was up to 10 pmol (). For the development of a molecular marker for D. eres, a phylogenetic tree was constructed based on ITS regions sequence alignment analysis of Diaprothe spp. (). The specificity of the D_eres primers to detect D. eres was confirmed (). Using the D_eres 3F3R primer set with the 10-fold dilution series of D. eres D02 gDNA consistently displayed detection limits of 10 ng, 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, and 10 fg, respectively, after 30 cycles of amplification (), while the D_eres 3F3R primer set had a sensitivity of up to 100 pg of gDNA. In order to test the specificity of the D_eres 3F3R primer set, Diaporthe spp., Alternaria alternata, Fusarium spp., F. oxysporum, F. solani and Rhizoctonia solani, were tested. The primer set showed specificity only in the Diaporthe spp. The primer set D_eres 3F3R was only amplified in the gDNA of seeds with symptoms of seed decay by D. eres ().
Figure 2. Maximum likelihood phylogenetic analysis of the Diaporthe species using ITS primers to develop a specific marker to detect D. eres. The bootstrap numbers represent the percent of 1000 replicates (only values greater or equal to 70% are shown) (a); specific detection of D. eres from seven isolates, including Cercospora kikuchii, B. cienrea, F. solani, Sclerotinia sclerotiorum, and Alternaria alternata (B); lane M, 1000 bp DNA ladder; lanes 1 and 2, Diaporthe sp.; lane 3, D. eres; lanes 4 and 5, Diaporthe sp.; lane 6, Fusarium sp.; lane 7, F. oxysporum; lane 8, F. solani; lane 9, R. solani; and lane 10, A. alternata (C); Conventional PCR amplification of different amounts of D. eres DNA with the primers D_eres 3F3R. M, 100 bp ladder marker; 1, 10 ng; 2, 1 ng; 3, 100 pg; 4, 10 pg; 5, 1 pg; 6, 100 fg; 7, 10 fg. (D); detection of Diaporthe spp., D_sp3F3R from Cercospora sojina, C. kikuchii, D. eres, and soybean seeds with decay and discoloration; lane a, C. sojina C01; lane b, C. kikuchii; lane c, D. eres D01; lane d, healthy seed; lanes e and f, seeds with decay symptoms; lanes g and h, brown and purple seeds. (E).
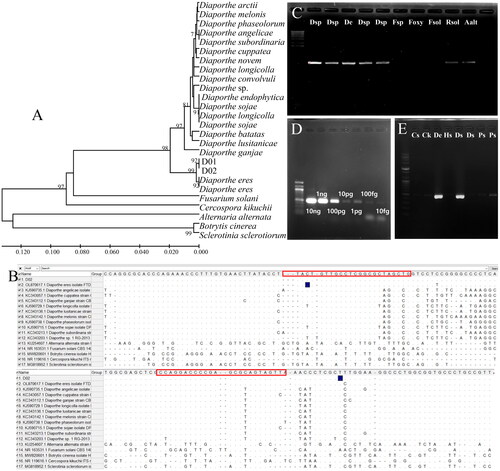
In this study, the primer set, D_sp3, was developed as a molecular detection marker for the soybean seed decay pathogen Diaporthe spp. The marker was specific to Diaporthe species in in silico assays and soybean seeds and did not detect Fusarium sp., F. oxysporum, F. solani, R. solani, A. alternate, or S. sclerotiurum. Soybean seed decay is caused by various Diaporthe species other than D. eres, and soybean disease caused by D. sojae, D. longicolla, D. caulivora, and D. aspalathi are continuously reported [Citation8]. Many diagnostic kits for the detection of plant pathogenic viruses and bacteria have been developed; however, sequence-based detection kits for plant fungal pathogens must be developed. It is especially important to detect seed-borne fungal pathogens during seed storage because the diseased seeds could spread in the fields, decreasing productivity. Therefore, the detection marker identified in this study could be used to verify the integrity of seeds, and infected seeds could be separated from healthy seeds in an early stage. Moreover, since Diaporthe spp. can spread to the pod through the vessels in the stem, the designed primer could be applied to detect it in various plant tissues during the growing season. In the future, studies developing markers for direct use in the field for other seed pathogens, such as Cercospora sojina, C. kikuchii, and Septoria glycine will be conducted. Additionally, multiplex PCR markers combined with detection markers for all of these will be studied.
Acknowledgments
I would like to thank Dr. M.K. Sang for her advice in conducting this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mena E, Stewart S, Montesano M, et al. Soybean stem canker caused by Diaporthe caulivora; pathogen diversity, colonization process, and plant defense activation. Front Plant Sci. 2019;10:1733. doi: 10.3389/fpls.2019.01733.
- Shan Z, Li S, Liu Y, et al. First report of Phomopsis seed decay of soybean caused by Phomopsis longicolla in South China. Plant Dis. 2012;96(11):1693–1693. doi: 10.1094/PDIS-04-12-0401-PDN.
- Hosseini B, El-Hasan A, Link T, et al. Analysis of the species spectrum of the Diaporthe/Phomopsis complex in European soybean seeds. Mycol Progress. 2020;19(5):455–469. doi: 10.1007/s11557-020-01570-y.
- Petrović K, Vidić M, Riccioni L, et al. First report of Diaporthe eres species complex causing seed decay of soybean in Serbia. Plant Disease. 2015;99(8):1186–1186. doi: 10.1094/PDIS-01-15-0056-PDN.
- Nguyen TH, Mathur SB, Neergaard P. Seed-borne species of Myrothecium and their pathogenic potential. Transact Br Mycol Soc. 1973;61(2):347–IN16. doi: 10.1016/S0007-1536(73)80156-1.
- White TJ, Bruns TD, Lee SB, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315–322.
- Udayanga D, Castlebury LA, Rossman AY, et al. Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia. 2014;32(1):83–101. doi: 10.3767/003158514X679984.
- Ghimire K, Petrović K, Kontz BJ, et al. Inoculation method impacts symptom development associated with Diaporthe aspalathi, D. caulivora, and D. longicolla on soybean (Glycine max). Plant Dis. 2019;103(4):677–684. doi: 10.1094/PDIS-06-18-1078-RE.
