Abstract
Wood-decaying fungi are essential decomposers in forest ecosystems. They decompose wood substrates by producing various lignocellulolytic enzymes, which have significant industrial and medical applications. A survey was conducted at the Juwangsan National Park from 2018 to 2019 to determine the diversity of macrofungi in Korea. Five previously unrecorded wood-decaying polyporoid and corticioid fungi were identified among the collected specimens: Eichleriella sinensis, Hymenochaete anomala, Hyphoderma subsetigerum, Lyomyces orientalis, and Pseudowrightoporia crassihypha. These species were identified based on morphological, molecular, and phylogenetic analyses of the internal transcribed spacer (ITS) and nuclear large subunit rDNA (nLSU) region. In this study, we provide detailed macro- and micro-morphological figures with phylogenetic trees to support the discovery of five new species in Korea.
1. Introduction
Wood-decaying fungi play a crucial role in forest ecosystems by degrading wood-based materials and contributing to nutrient cycling [Citation1]. They produce lignocellulolytic enzymes such as lignin peroxidase, manganese peroxidase, laccase, chitinase, cellulase, and hemicellulase, which breakdown lignin, cellulose, and hemicellulose [Citation2,Citation3]. These enzymes are applied to processes, such as pulping, wastewater treatment, and bioremediation of aromatic compounds [Citation4,Citation5]. In addition, wood-decaying fungi produce biologically active compounds that can be used for medicinal purposes [Citation6].
Juwangsan National Park is located in the Gyeongsangbuk-do Province, Eastern Korea. Juwangsan was designated as a national park in 1976 due to its unique geological structures, such as welding facies and columnar joints [Citation7]. Additionally, Juwangsan National Park has a representative vegetation of the mid-temperate zone dominated by pine and oak trees, which provides a suitable habitat for macrofungi [Citation8,Citation9]. A previous study reported 503 species in Juwangsan National Park [Citation10]. However, these species were identified based on morphological traits, suggesting the necessity of DNA-based molecular analysis for accurate identification. Morphological characteristics are used to identify species and determine their morphological evolution [Citation11]. However, highly speciose groups or complex clades of fungi can be misidentified [Citation12]. Thus, a DNA-based molecular analysis of the internal transcribed spacer (ITS) region is necessary. The ITS and nuclear large subunit rDNA (nLSU) regions in fungi are hypervariable because of the rapid evolution in its rRNA gene, a significant barcode gap, and easy amplification [Citation13]. Therefore, these regions have become the most popular region for fungal identification at the species level and was also applied in this study. We investigated the diversity of macrofungi in Juwangsan National Park from 2018 to 2019 and collected 406 macrofungal specimens during the survey. Consequently, we reported five previously unrecorded species [Citation14]. Furthermore, we discovered five other species new to Korea. The identification results are supported by morphological and molecular analyses.
2. Materials and methods
2.1. Sampling
Juwangsan National Park (36°19′–36°27′ N, 129°04′–129°14′ E) is located across Cheongsong-gun and Yeongdeok-gun of Gyeongsangbuk-do province, South Korea. Fungal specimens were collected from 2018 to 2019 along the trail near Dalgi Falls, Geumeungwangi, Jeolgol, and Jusan Reservoir. Collected specimens were dried at 60 °C for 24 h and stored with silica gel to prevent contamination. The specimens were deposited in the Korea University Collection (KUC) and National Institute of Biological Resources (NIBR).
2.2. Molecular approach
DNA was extracted from the dried specimens using an AccuPrep® Genomic DNA Extraction Kit (Bioneer, Daejeon, South Korea). The ITS region was amplified with ITS1F/ITS4B, ITS5/ITS4, or ITS5/LR3 primer sets [Citation15,Citation16]. For Eichleriella sinensis, nLSU region was amplified with LR0R/LR5 primer set [Citation17,Citation18]. PCR product purification was conducted using the AccuPrep® PCR Purification Kit and AccuPrep® Gel Purification Kit (Bioneer, Daejeon, South Korea) following the manufacturer’s instructions. Macrogen Inc. (Seoul, South Korea) performed DNA sequencing of amplified PCR products. Each sequencing result was edited using the SeqMan Lasergene package version 7.0.0 (DNAStar Inc., Madison, WI). The edited sequences were compared with sequences in GenBank using BLASTn v. 2.13.0. Reference sequences were obtained from the NCBI GenBank database (www.ncbi.nlm.nih.gov/genbank/) for phylogenetic analysis. Sequence alignment was conducted using MAFFT v. 7.453 [Citation19]. Phylogenetic analysis was performed with ITS or ITS + nLSU combined datasets employing the maximum-likelihood (ML) method, which used RAxML-HPC2 on XSEDE v. 8.2.12 with GTR + G model and 1000 bootstrap replicates for tree inference [Citation20]. The above phylogenetic analysis was performed using the CIPRES web portal [Citation21]. Phylogenetic trees were edited using FigTree v. 1.4.3 and Adobe Illustrator CS6 (Adobe Systems Inc., San Jose, CA). Bootstrap support values >70% are indicated in the tree. The sequences of the five species are deposited in GenBank.
2.3. Morphological observation
Microscopic structures were observed using an Olympus BX51 light microscope (Olympus, Tokyo, Japan) and images were captured using a DP20 microscope camera (Olympus, Tokyo, Japan). Macroscopic images of each specimen were captured using Sony α 6500 camera (Sony, Tokyo, Japan). At least 20 basidia and basidiospores were detected. The specific color terms conform to those in Munsell Soil Color Book [Citation22]. The following abbreviations are used: L = mean spore length; W = mean spore width; Q = L/W ratio.
3. Results
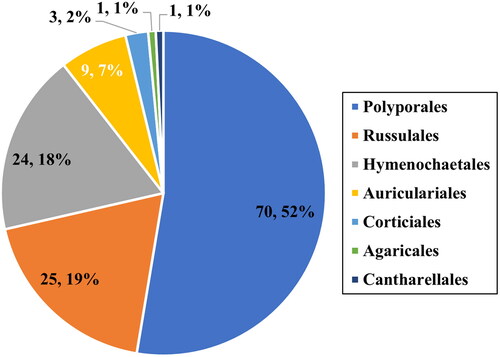
A total of 406 macrofungal specimens were collected from Juwangsan National Park between 2018 and 2019. Among them, 252 specimens were identified as wood-decaying polyporoid or corticioid fungal species. These specimens were classified into seven orders, 27 families, 67 genera, and 133 species, including new species candidates. The order Polyporales (70 species, 52%) was the most abundant, followed by Russulales (25 species, 19%), and Hymenochaetales (24 species, 18%) (). The most abundant species was Irpex lacteus (18 specimens), followed by Trametes versicolor (11 specimens), Xylobolus peculiare (nine specimens), and Stereum hirsutum (eight specimens). Six specimens (KUC20181101-43, KUC20180712-40, KUC20181101-24, KUC20190620-29, KUC20191011-08B, and KUC20191011-17B) were assigned to five unrecorded species candidates in Korea based on the ITS sequence homology. They were highly supported by high percentage of homology at 99–100% with reference sequences (). Meanwhile, KUC20181101-43 needed additional gene region (nLSU) for accurate identification. As a result, KUC20181101-43 (GenBank accession number: OR857463) showed 99% (729/730 bp) homology with E. sinensis He4196 and 100% (730/730 bp) homology with E. sinensis He5057. The phylogenetic analysis revealed that KUC20181101-43, KUC20180712-40, and KUC20190620-29 were clustered with E. sinensis, Hym. anomala, and L. orientalis, respectively, with 100% bootstrap values (). KUC20191011-08B and KUC20191011-17B were identified to P. crassihypha (). KUC20181101-24 was clustered in the Hyp. subsetigerum group (). However, three specimens had low resolution ( and ).
Figure 2. Maximum-likelihood tree of Eichleriella sinensis KUC20181101-43. The tree was constructed based on ITS+nLSU combined datasets of the genus Eichleriella. Amphistereum leveilleanum was used as an outgroup. The newly generated sequence is shown in blue and bold. Bootstrap support values more than 70% are shown. The numbers after scientific name indicate specimen ID and GenBank accession numbers (ITS and LSU regions).
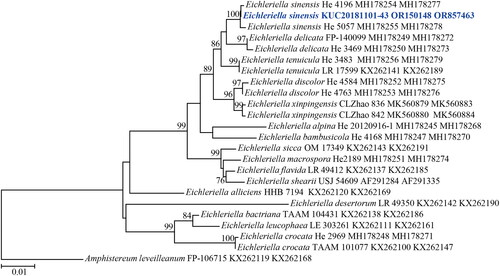
Figure 3. Maximum-likelihood tree of Hymenochaete anomala KUC20180712-40. The tree was constructed based on ITS sequence datasets of the genus Hymenochaete. Hydnoporia lamellata was used as an outgroup. The newly generated sequence is shown in blue and bold. Bootstrap support values more than 70% are shown. The numbers after scientific name indicate specimen ID and GenBank accession number (ITS region).
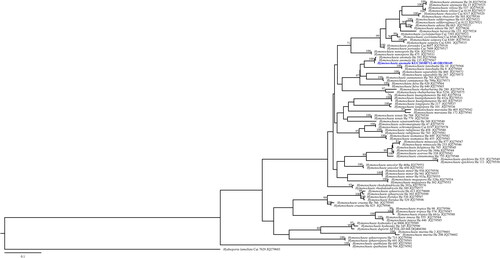
Figure 4. Maximum-likelihood tree of Lyomyces orientalis KUC20190620-29. The tree was constructed based on ITS sequence datasets of the genus Lyomyces. Xylodon asperus was used as an outgroup. The newly generated sequence is shown in blue and bold. Bootstrap support values more than 70% are shown. The numbers after scientific name indicate specimen ID and GenBank accession number (ITS region).
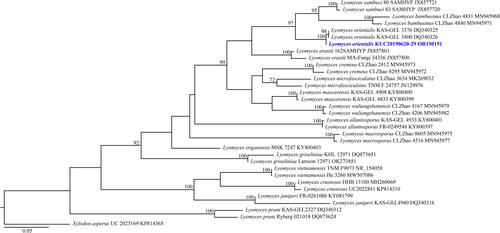
Figure 5. Maximum-likelihood tree of Pseudowrightoporia crassihypha KUC20191011-08B and KUC20191011-17B. The tree was constructed based on ITS sequence datasets of the genus Pseudowrightoporia. Dentipellis fragilis was used as an outgroup. The newly generated sequence is shown in blue and bold. Bootstrap support values more than 70% are shown. The numbers after scientific name indicate specimen ID and GenBank accession number (ITS region).
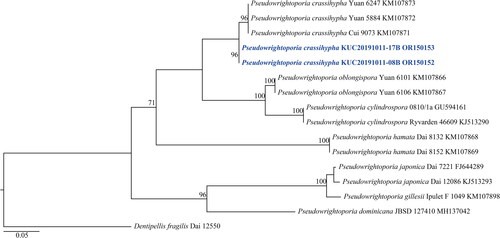
Figure 6. Maximum-likelihood tree of Hyphoderma subsetigerum KUC20181101-24. The tree was constructed based on ITS sequence datasets of the genus Hyphoderma. Physisporinus tibeticus was used as an outgroup. The newly generated sequence is shown in blue and bold. Bootstrap support values more than 70% are shown. The numbers after scientific name indicate specimen ID and GenBank accession number (ITS region).
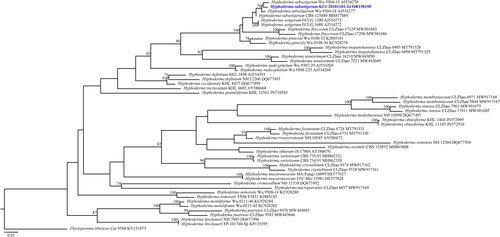
Figure 7. Morphological features of Eichleriella sinensis KUC20181101-43. (A) Basidiome; (B) Microscopic features, a. basidia; b. cystidia; c. skeletal hyphae; d. generative hyphae; e. basidiospores (Scale bars: A = 1 cm, B = 10 µm).
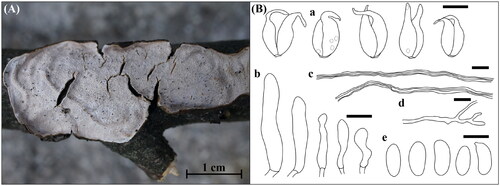
Figure 8. Morphological features of Hymenochaete anomala KUC20180712-40. (A) Basidiome; (B) Microscopic features, a. basidia; b. basidioles; c. setae; d. hyphae; e. basidiospores (Scale bars: A = 1 cm, B = 10 µm).
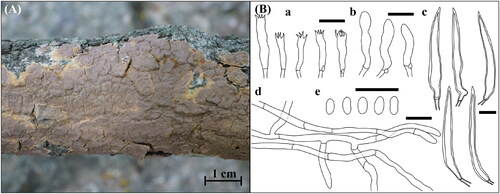
Figure 9. Morphological features of Hyphoderma subsetigerum KUC20181101-24. (A) Basidiome; (B) Microscopic features, a. basidia; b. basidiospores; c. septocystidia; d. hyphae (Scale bars: A = 1 cm, B = 10 μm).
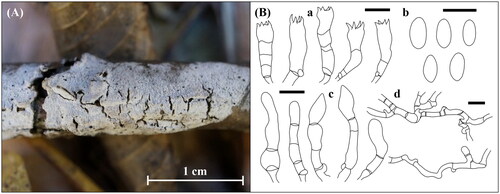
Figure 10. Morphological features of Lyomyces orientalis KUC20190620-29. (A) Basidiome; (B) Microscopic features, a. basidia; b. basidiospores; c. cystidia; d. hyphae (Scale bars: A = 1 cm, B = 10 μm).
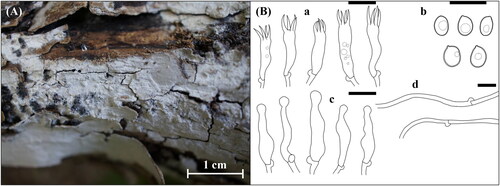
Figure 11. Morphological features of Pseudowrightoporia crassihypha KUC20191011-08Band KUC20191011-17B. (A) Basidiome; (B) Microscopic features, a. basidia; b. cystidia; c. basidiospores; d. hyphae (Scale bars: A = 1 cm for basidiome, 1 mm for pores, B= 10 μm).
Table 1. Molecular identification of five unrecorded fungal species in Juwangsan National Park.
4. Taxonomy
4.1. Eichleriella sinensis (Teng) S.H. He & Nakasone, J. Fungi 9 (3, no. 318): 16 (2023) [MB#847448] ()
Korean name: Junggukgajukbeoseot.
Basidiome annual, resupinate, effused, covering up to 2 mm thick. Hymenial surface crustaceous, waxy, granulose, with some cracks, white (7.5YR, 8.5/1). Hyphal system dimitic. Generative hyphae frequently branched, thin-walled, hyaline, 2.1–3.2 µm in diam. Skeletal hyphae septate, unbranched, thick-walled, brownish, 2.6–4.5 µm. Basidia two-celled, ovoid, smooth, hyaline, with narrowly extended tips, containing few oil droplets, 16.8–27.1 × 5.1–8.7 µm. Basidiospores narrowly cylindrical, smooth, thin-walled, hyaline, 8.7–14.5 × 3.9–6.4 µm (Q = 2.23–2.26). Cystidia rare, subcylindrical to clavate, smooth, thin-walled, hyaline, varying in length, 17.9–32.3 × 4.5–6.6 µm.
4.1.1. Specimen examined
Korea, Gyeongsangbuk-do, Cheongsong-gun, Juwangsan National Park, 36°22′19.0″ N, 129°11′06.2″ E, 388 m, mixed hardwood forest, occurring on hardwood branches near Jeolgol, November 1 2018, S. L. Kwon, KUC20181101-43 (NIBRFG0000514359).
4.1.2. Remarks
Eichleriella sinensis is characterized by a ceraceous and odontoid hymenial surface, cylindrical to narrowly cylindrical basidiospores, and a dimitic hyphal system with brownish, thick-walled skeletal hyphae [Citation23], which matches KUC20181101-43. However, numerous and larger cystidia were observed in the original description (15–50(–70) × 7.5–9(–12.5) µm) [Citation23]. In addition, four-celled basidia was described in the original description [Citation23], which differs from our specimen. But two-celled basidia was observed in E. aculeobasidiata [Citation24], which is currently treated as synonym of E. sinensis [Citation23]. This indicates that E. sinensis has two types of basidia: two-celled and four-celled. E. sinensis is morphologically similar with E. tenuicula, but E. tenuicula has larger allantoid basidiospores 16–21 × 5.5–6.0 µm (Q = 2.91–3.50) [Citation25].
4.2. Hymenochaete anomala Burt, Annals of the Missouri Botanical Garden 5: 358 (1918) [MB#101217] ()
Korean name: Damgalsaeksonamubineulbeoseot.
Basidiome annual, resupinate, effused, adherent, covering up to 1 mm thick. Hymenial surface crustaceous, smooth, with many cracks, reddish brown (5YR, 5/4), with yellow (10YR, 7/6) margin when fresh, brown (7.5YR, 4/2) when dry. Hyphal system monomitic, generative hyphae frequently septate, moderately branched, without clamp connections, interwoven, thin-walled, hyaline, 1.8–3.7 µm. Setae fusiform, subcylindrical, with an acute apex, smooth, bearing a wide lumen, thick-walled, projecting up to 25 µm above the hymenium, yellowish red (5YR, 5/8), 29.0–65.4 × 4.3–10.6 µm. Basidia subcylindrical to clavate, four-spored, smooth, thin-walled, hyaline, with a basal clamp connection, 12.2–19.0(–21.0) × 3.1–4.7 µm; basidioles similar to basidia in shape. Basidiospores cylindrical to narrowly cylindrical, smooth, thin-walled, hyaline, 3.0–4.5(–4.8) × 1.5–2.0(–2.3) µm (Q = 2–2.25). Cystidia absent.
4.2.1. Specimen examined
Korea, Gyeongsangbuk-do, Cheongsong-gun, Juwangsan National Park, 36°21′46.3″ N, 129°11′18.5″ E, 380 m, mixed hardwood forest, occurring on conifer branches near Jusan Reservoir, July 12 2018, S. L. Kwon, KUC20180712-40 (NIBRFG0000514362).
4.2.2. Remarks
Hymenochaete anomala is characterized by a crustaceous reddish-brown hymenial surface and thick-walled setae. Phylogenetically, Hym. luteobadia were the most closely related species. However, Hym. luteobadia has pileate basidiome, and numerous hyphidia with yellowish-brown granules [Citation26,Citation27]. Other closely related species (Hym. separabilis, Hym. yunnanensis, and Hym. fulva) had effused basidiomes. This indicates that morphological characteristics do not always correspond to phylogenetic relationships. Hym. minor exhibited micromorphological characteristics similar to Hym. anomala, but Hym. minor setae had encrustation at its apex [Citation27].
4.3. Hyphoderma subsetigerum Sheng H. Wu, Mycologia 89 (1): 136 (1997) [MB#436877] ()
Korean name: Dolgimokjaegoyakbeoseot.
Basidiome annual, resupinate, effused, thin. Hymenial surface membranaceous, chalky, papillate, with some cracks, white (2.5Y, 9.5/1), margin concolorous with main hymenial surface. Hyphal system monomitic, generative hyphae frequently septate, moderately branched, with frequent clamp connections, diverge in multiple orientations, thin-walled, hyaline, 3.0–4.7 µm. Basidia fusiform, subcylindrical, four-spored, smooth, thin-walled, hyaline, with a basal clamp connection, 21.6–28.0 × 4.4–7.1 µm. Basidiospores cylindrical to narrowly cylindrical, smooth, thin-walled, hyaline, (7.8)8.0–11.0(–11.4) × (3.7–)4.0–5.5(–5.6) µm (Q = 2–2.03). Septocystidia cylindrical, sometimes with narrow apex, 2–4 septa with clamp connections, thin-walled, hyaline, 22.4–62.0 × 4.6–6.3 µm.
4.3.1. Specimen examined
Korea, Gyeongsangbuk-do, Cheongsong-gun, Juwangsan National Park, 36°26′26.0″ N, 129°08′17.6″ E, 405 m, mixed hardwood forest, occurring on hardwood branches near Dalgi Falls, November 1 2018, S. L. Kwon, KUC20181101-24 (NIBRFG0000514360).
4.3.2. Remarks
This species is characterized by a papillate-to-grandinioid hymenial surface with cracks and smooth septocystidia. KUC20181101-24 has larger basidiospores than the original description (6.0–8.0 × 2.8–3.2 µm). KUC20130718-46, and KUC20160721B-02 were identified as Hyp. subsetigerum in previous studies [Citation28,Citation29]; however, they were not reported owing to the absence of morphological analyses. Therefore, we classified KUC20181101-24 as an unknown species. Hyp. subsetigerum were grouped under Hyp. setigerum complex [Citation30,Citation31]. Morphologically, Hyp. setigerum have longer and wider basidiospores (7.5–12.5 × 3.5–5.0 µm) than Hyp. subsetigerum [Citation32]. Our specimens exhibited micromorphological characteristics similar to those of Hyp. setigerum. Hyphoderma setigerum was previously considered a species group because of the morphological variations among specimens from distinct regions [Citation33,Citation34]. According to an advanced phylogenetic study, Hyp. subsetigerum formed a separate group from Hyp. setigerum [Citation30,Citation31]. These two species have different geological distributions: Hyp. subsetigerum was observed to occur in Asia and Hyp. setigerum s.s. in Northern Europe [Citation30]. This indicates that geological factors caused speciation within the Hyp. setigerum complex, leading to morphological and molecular variations [Citation30].
4.4. Lyomyces orientalis Riebesehl, Yurchenko & Langer, Mycological Progress 16 (9): 874 (2017) [MB#819975] ()
Korean name: Dongyangbaeksaekgoyakbeoseot.
Basidiome resupinate, effused, pellicular, covering up to 0.6 mm thick. Hymenial surface membranaceous, smooth, few projections, with frequent cracks, white (2.5Y, 9.5/1). Hyphal system monomitic, generative hyphae simple septate, unbranched, with clamp connections at all primary septa, interwoven, thin-walled, hyaline, 1.8–4.8 µm. Basidia subcylindrical to clavate, four-spored, smooth, thin-walled, hyaline, sometimes bearing oil droplets, with a basal clamp connection, 16.2–20.5 × 4.0–5.5 µm. Basidiospores broadly ellipsoid, smooth, thick-walled (0.3–0.4 µm), hyaline, containing an oil droplet, (5.0–)5.2–6.0(–6.4) × (3.7–)4.0–5.0(–5.1) µm (Q = 1.2–1.3). Cystidia capitate, smooth, thin-walled, hyaline, 13.2–28.6 × 2.1–6.6 µm.
4.4.1. Specimen examined
Korea, Gyeongsangbuk-do, Cheongsong-gun, Juwangsan National Park, 36°26′26.0″ N, 129°08′17.6″ E, 405 m, mixed hardwood forest, occurring on Pinus branches near Dalgi Falls, June 20 2019, S. L. Kwon, KUC20190620-29 (NIBRFG0000506081).
4.4.2. Remarks
Lyomyces orientalis is characterized by capitate cystidia and thick-walled basidiospores [Citation35]. The morphological characteristics of KUC20190620-29 were similar to those in the original description [Citation35]. In the phylogenetic tree, L. orientalis is closely related to L. bambusinus and L. sambuci. Similar to L. orientalis, L. bambusinus have slightly thick-walled (0.3–0.4 µm) basidiospores; however, L. bambusinus has two forms of cystidia; capitate and tapering, and smaller basidiospores 4.5–5.6(–5.8) × 3.3–4.3(–4.5) µm [Citation36]. Lyomyces sambuci differs from L. orientalis by having thin-walled and narrowly ellipsoid basidiospores of 4.5–6 × 3–3.5 µm, Q = 1.55 [Citation35].
4.5. Pseudowrightoporia crassihypha Y.C. Dai, Jia J. Chen & B.K. Cui, Persoonia 37: 28 (2015) [MB#812227] ()
Korean name: Neodomureunbaechakgumeongbeoseot.
Basidiome annual, resupinate, effused, orbicular, corky, poroid, up to 3 mm thick. Hymenial surface buff, ceraceous, yellow (10YR, 8/6) to very pale brown (10YR, 8/3). Pores circular to angular, 8–10 per mm, dissepiments thin, entire. Hyphal system dimitic, generative hyphae septate, sometimes branched, with few clamp connections, thin-walled, hyaline, 2.1–4.3 µm. Skeletal hyphae dominant, aseptate, unbranched, without clamp connections, thick-walled, hyaline, 4.0–6.5 µm. Basidia clavate to subclavate, four-spored, smooth, thin-walled, hyaline, with a basal clamp connection, 10.4–14.7 × 4.1–5.7 µm. Basidiospores broadly ellipsoid to ellipsoid, asperulate, thick-walled, hyaline, bearing an oil droplet, (3.1–)3.3–4.1(–4.3) × (2.3–)2.5–3.1(–3.3) µm (Q = 1.30–1.34). Cystidia clavate, smooth, thin-walled, hyaline, 15.5–23.3 × 3.0–5.3 µm.
4.5.1. Specimen examined
Korea, Gyeongsangbuk-do, Cheongsong-gun, Juwangsan National Park, 36°24′44.5″ N, 129°10′17.2″ E, 519 m, mixed hardwood forest, occurring on Pinus branches along Geumeungwangi trail, June 20 2019, S. L. Kwon, KUC20191011-08B (NIBRFG0000506161), occurring on dead branches along Geumeungwangi trail, June 20 2019, S. L. Kwon, KUC20191011-17B (NIBRFG0000506175).
4.5.2. Remarks
Pseudowrightoporia crassihypha is characterized by corky and yellowish basidiomes with pores, a dimitic hyphal system, and asperulate basidiospores [Citation37]. Our specimens had morphological characteristics similar to those in the original description [Citation37] and diverged well in the phylogenetic tree. Most of the Pseudowrightoporia specimens are distributed in the subtropical and tropical regions [Citation37,Citation38]. In addition, the holotype and paratypes of P. crassihypha (Yuan 6247, Yuan 5884, and Cui 9073) were collected from a subtropical region [Citation37]. Therefore, KUC20191011-08B and KUC20191011-17B are the first reports of P. crassihypha in temperate regions and a rare case of the genus Pseudowrightoporia. Morphologically, P. japonica has corky basidiome with pores (6–8 per mm) [Citation39,Citation40], resembling P. crassihypha. However, P. japonica has fewer cystidia and narrower skeletal hyphae (2.4–5.3 µm) than P. crassihypha [Citation39,Citation40].
5. Discussion
In this study, we discovered five unrecorded wood-decaying polyporoid and corticioid species in Juwangsan National Park using morphological and molecular analyses. Among the five species, the polyporoid species was P. crassihypha, and the other four were E. sinensis, Hym. anomala, Hyp. subsetigerum, and L. orientalis, which were corticioid species. A previous study conducted in Juwangsan focused on unrecorded cap-forming saprobic fungi [Citation14]; therefore, this is the first study on wood-decaying polyporoid or corticioid fungi in Juwangsan. Studies on polyporoid and corticioid fungi in Korea are relatively scarce, although globally, wood-decaying fungi have been studied intensively for a decade [Citation41–47]. Therefore, further research is required to determine the diversity of macrofungi in Juwangsan National Park, as well as the diversity of polyporoid and corticioid fungi in Korea.
Eichleriella Bres. is characterized by a leathery-to-ceraceous basidiome, two- or four-celled basidia, and cylindrical to narrow cylindrical basidiospores [Citation48,Citation49]. Currently, 17 species are listed in the genus Eichleriella [Citation24,Citation48,Citation50], and this is the first report of Eichleriella at the species level in Korea. According to a previous phylogenetic analysis, Eichleriella clusters with the genera Auricularia, Exidia, Exidiopsis, and Heterochaete in the nLSU region [Citation51]. However, these genera possess different basidia forms [Citation51]. Therefore, molecular analysis using the ITS region was performed, and consequently, Eichleriella was grouped with Amphistereum and Auricularia [Citation48]. In addition, four species of Exidiopsis and Heterochaete were reclassified as Eichleriella [Citation48]. Eichleriella is distinguished from Amphistereum by its hyphal structure, whereas the other morphological characteristics are similar [Citation48]. Therefore, accurate morphological observations and molecular approaches are required to clearly identify Eichleriella species.
Hymenochaete Lév is a white-rot fungi, and is characterized by brown effused basidiome, abundant yellowish to brownish hymenial setae, and simple septate hyphae [Citation27,Citation52–55]. Previously, species with poroid and smooth hymenial surfaces were classified as Hymenochaete [Citation53–55] but currently, species with effused-reflexed and pileate basidiomes (H. odontoides, H. rubiginosa, H. subporioides, and H. xerantica) are also classified as Hymenochaete. Owing to their morphological variability, many Hymenochaete species were included in other genera, including Cerrenella, Cyclomyces, Cycloporellus, Dichochaete, Hydnochaete, Hymenochaetella, and Stiptochaete are currently considered illegitimate names [Citation52]. More than 120 species were considered Hymenochaete in 2012 [Citation27] and 13 of them were listed in Korea according to the NIBR database. This must be revised following the phylogenetic reclassification and delimitation studies of Hymenochaete.
Hyphoderma Wallr. is characterized by resupinate to effused-reflexed basidiome with membranaceous, smooth to tuberculate hymenial surface, and monomitic hyphal system with clamp connections [Citation41,Citation56]. Although approximately 100 species are accepted as Hyphoderma worldwide [Citation57,Citation58], only 11 species have been reported in Korea. Previously, six Hyphoderma species were clustered with Hypochnicium, Physisporinus, and Rigidoporus species [Citation59,Citation60]. These species are currently classified as Hyphodermataceae and not as Meripilaceae (Physisporinus and Rigidoporus) and Podoscyphaceae (Hypochnicium) [Citation58]. Hyphoderma species have long been studied in Europe [Citation41,Citation56,Citation61] and recently in China [Citation31,Citation57,Citation58,Citation62]. Thus, most Hyphoderma species are distributed in Europe, and some have been reported in southern China and Japan [Citation58]. In addition, most Hyphoderma species occur in hardwood, whereas a few occur in softwood [Citation58]. Therefore, additional studies are required to determine the diversity of Asian Hyphoderma species and identify the relationships between Hyphoderma and its host trees.
Lyomyces P. Karst. has 23 species worldwide and is new to Korea at the species level [Citation35,Citation36,Citation63,Citation64]. Lyomyces is a genus in the family Schizoporaceae, which is characterized by an effused basidiome with a smooth to odontoid hymenial surface, a monomitic hyphal system with abundant clamp connections, and thin- to thick-walled basidiospores [Citation35,Citation36,Citation65,Citation66]. They usually occur on dead hardwood branches and stems and sometimes on softwood [Citation35,Citation36]. The genus Lyomyces was first introduced by Karsten as Lyomices [Citation65] and was previously recognized as Hyphodontia owing to a lack of molecular data [Citation67]. Based on the molecular and phylogenetic analyses, Lyomyces was reintroduced by dividing Hyphodontia s.l. to Hyphodontia, Kneiffiella, Lagarobasidium, Lyomyces, Palifer, Rogersella, and Xylodon [Citation63,Citation64]. Currently, Rogersella is considered synonymous to Lyomyces [Citation35]. In addition, Xylodon encompasses Lagarobasidium, Palifer, and other genera (Odontiopsis and Schizopora) along with some mislabeled species of Hyphodontia (family Hyphodontiaceae) and Kneiffiella (family Chaetoporellaceae) [Citation64,Citation68–71]. Lyomyces is now considered the second largest genus in the family Schizoporaceae, followed by Xylodon. Therefore, accurate identification is required, following the recent taxonomic revision studies [Citation70,Citation71].
Pseudowrightoporia Y.C. Dai, Jia J. Chen, and B.K. Cui is a small genus with 11 species that is new to Korea [Citation37,Citation38]. Pseudowrightoporia are characterized by a corky basidiome, pores on the hymenial surface, a dimitic hyphal system, and asperulate basidiospores [Citation37,Citation38]. Pseudowrightoporia is a newly introduced genus that diverged from Wrightoporia s.l. due to the polyphyly of Wrightoporia [Citation37]. In addition to Pseudowrightoporia, some Wrightoporia species have been reclassified as Amylonotus, Amylosporus, and two new genera, Larssoniporia and Wrightoporiopsis [Citation37]. Thus, W. africana, W. aurantipora, W. cylindrospora (generic type), W. gillesii, W. japonica, W. solomonensis, and W. straminea were transferred to Pseudowrightoporia based on morphological and phylogenetic analyses [Citation37,Citation38]. Most Pseudowrightoporia species have been reported in tropical and subtropical regions [Citation38], and few have been reported in temperate regions. Further studies are required to determine the global diversity of Pseudowrightoporia species.
Wood-decaying fungi are diverse and taxonomically complex. Many of its species have been misclassified because of morphological similarities between distinct groups. Currently, species delimitation and reclassification of wood-decaying fungi are being conducted owing to the development of DNA-based identification [Citation37,Citation52,Citation60,Citation70,Citation72,Citation73]. Consequently, many species have been reclassified into other genera and families. However, their scientific names have not yet been completely updated in online databases, such as GenBank, which confuses mycologists and the public. In conclusion, further studies are needed to resolve the confusion regarding accepted species names and for the species delimitation and reclassification of phylogenetically complex groups.
Acknowledgements
The authors thank Editage (www.editage.co.kr) for English language editing.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Lonsdale D, Pautasso M, Holdenrieder O. Wood-decaying fungi in the forest: conservation needs and management options. Eur J Forest Res. 2008;127(1):1–22. doi: 10.1007/s10342-007-0182-6.
- Blanchette RA. Delignification by wood-decay fungi. Annu Rev Phytopathol. 1991;29(1):381–403. doi: 10.1146/annurev.py.29.090191.002121.
- Tuor U, Winterhalter K, Fiechter A. Enzymes of white-rot fungi involved in lignin degradation and ecological determinants for wood decay. J Biotechnol. 1995;41(1):1–17. doi: 10.1016/0168-1656(95)00042-O.
- Gao D, Du L, Yang J, et al. A critical review of the application of white rot fungus to environmental pollution control. Crit Rev Biotechnol. 2010;30(1):70–77. doi: 10.3109/07388550903427272.
- Hakala TK, Maijala P, Konn J, et al. Evaluation of novel wood-rotting polypores and corticioid fungi for the decay and biopulping of Norway spruce (Picea abies) wood. Enzyme Microb Technol. 2004;34(3–4):255–263. doi: 10.1016/j.enzmictec.2003.10.014.
- Wasser SP, Weis AL. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspectives. Int J Med Mushrooms. 1999;1(1):31–62. doi: 10.1615/IntJMedMushrooms.v1.i1.30.
- Hwang S-K, Kim J-H. Topographical landscapes and their controlling geological factors in the Juwangsan National Park: welding facies and columnar joints. J Petrol Soc Korea. 2009;18:195–209.
- Hur T, Jang S. Distribution of higher fungi in Juwangsan National Park. J Korean Inst Forest Recreat. 2011;15:15–20.
- Kim Y, Kang G. Floristic study on Juwangsan National Park. Korean J Environ Ecol. 1995;8:81–92.
- Ko P-Y, Hong K-S, Choe S-Y, et al. Distribution of spontaneously growing mushrooms in the Juwangsan National Park. J Mushroom. 2018;16:65–69.
- Hyde KD, Abd-Elsalam K, Cai L. Morphology: still essential in a molecular world. Mycotaxon. 2011;114(1):439–451. doi: 10.5248/114.439.
- Lutzoni F, Kauff F, Cox CJ, et al. Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot. 2004;91(10):1446–1480. doi: 10.3732/ajb.91.10.1446.
- Raja HA, Miller AN, Pearce CJ, et al. Fungal identification using molecular tools: a primer for the Natural Products Research Community. J Nat Prod. 2017;80(3):756–770. doi: 10.1021/acs.jnatprod.6b01085.
- Kwon SL, Jang S, Kim C, et al. Note of five unrecorded mushrooms including three rare species on Mount Juwang in Korea. Mycobiology. 2020;48(3):157–168. doi: 10.1080/12298093.2020.1759348.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. editors PCR protocols: a guide to methods and applications. New York (NY): Academic Press; 1990. p. 315–322.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x.
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172(8):4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990.
- Hopple JSJr., Vilgalys R. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia. 1994;86(1):96–107. doi: 10.1080/00275514.1994.12026378.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010.
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033.
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE) New Orleans (Lousiana): Institute of Electrical and Electronics Engineers (IEEE); 2010. p. 1–8.
- Color BM. Munsell soil-color charts with genuine Munsell® color chips. Grand Rapids (MI): Munsell Color; 2009.
- Li Y, Nie T, Nakasone KK, et al. Taxonomy and phylogeny of corticioid fungi in Auriculariaceae (Auriculariales, Basidiomycota): a new genus, five new species and four new combinations. J Fungi. 2023;9(3):318. doi: 10.3390/jof9030318.
- Wang H, Wang D-Q, Zhao C-L. Eichleriella aculeobasidiata sp. nov. (Auriculariales, Basidiomycota) evidenced by morphological characters and phylogenetic analyses in China. Kew Bull. 2022;77(1):325–332. doi: 10.1007/s12225-022-10001-y.
- Roberts P. Caribbean heterobasidiomycetes: 3. British Virgin Islands. Mycotaxon. 2008;105:137–147.
- Defigio DA. A taxonomic analysis of the corticate species of the genus Hymenochaete [Ph.D. dissertation]. Illinois State University; 1970.
- He S-H, Dai Y-C. Taxonomy and phylogeny of Hymenochaete and allied genera of Hymenochaetaceae (Basidiomycota) in China. Fung Divers. 2012;56(1):77–93. doi: 10.1007/s13225-012-0174-9.
- Jang S, Kim C, Kim G, et al. Diversity of basidiomycetous fungi in Mt. Yeonin Provincial Park. Korean J Nat Conserv. 2016;15(1):41–53. doi: 10.30960/kjnc.2016.15.1.41.
- Jang Y, Jang S, Lee J, et al. Diversity of wood-inhabiting polyporoid and corticioid fungi in Odaesan National Park, Korea. Mycobiology. 2016;44(4):217–236. doi: 10.5941/MYCO.2016.44.4.217.
- Nilsson RH, Hallenberg N, Nordén B, et al. Phylogeography of Hyphoderma setigerum (Basidiomycota) in the Northern hemisphere. Mycol Res. 2003;107(Pt 6):645–652. doi: 10.1017/s0953756203007925.
- Yurchenko E, Wu S-H. Hyphoderma pinicola sp. nov. of H. setigerum complex (Basidiomycota) from Yunnan, China. Bot Stud. 2014;55(1):71. doi: 10.1186/s40529-014-0071-5.
- Wu S-H. New species of Hyphoderma from Taiwan. Mycologia. 1997;89(1):132–140. doi: 10.1080/00275514.1997.12026764.
- Eriksson J, Ryvarden L. The Corticiaceae of North Europe volume 3, Coronicium-Hyphoderma. Fungiflora Oslo. 1975;3:287–546.
- Yurchenko EO, Zmitrovich IV. Variability of Hyphoderma setigerum (Corticiaceae s.l., Basidiomycetes) in Belarus and Northwest Russia. Mycotaxon. 2001;78:423–434.
- Yurchenko E, Riebesehl J, Langer E. Clarification of Lyomyces sambuci complex with the descriptions of four new species. Mycol Progress. 2017;16(9):865–876. doi: 10.1007/s11557-017-1321-1.
- Chen J-Z, Zhao C-L. Morphological and molecular identification of four new resupinate species of Lyomyces (Hymenochaetales) from Southern China. MycoKeys. 2020;65:101–118. doi: 10.3897/mycokeys.65.48660.
- Chen J, Cui B, Dai Y. Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia. 2016;37(1):21–36. doi: 10.3767/003158516X689666.
- Vizzini A, Angelini C, Losi C, et al. Diversity of polypores in the Dominican Republic: Pseudowrightoporia dominicana sp. nov. (Hericiaceae, Russulales). MycoKeys. 2018;34(34):35–45. doi: 10.3897/mycokeys.34.25371.
- Nunez M, Ryvarden L. New and interesting polypores from Japan. Fung Divers. 1999;3:107–121.
- Cui B-K, Dai Y-C. Wrightoporia (Basidiomycota, Aphyllophorales) in China. Nova Hedwigia. 2006;83(1–2):159–166. doi: 10.1127/0029-5035/2006/0083-0159.
- Bernicchia A, Gorjón S. Fungi Europaei 12: Corticiaceae s.l. Lomazzo: Edizioni Candusso; 2010. p. 1–1007.
- Dai Y-C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience. 2011;52(1):69–79. doi: 10.1007/S10267-010-0068-1.
- Cui B-K, Li H-J, Ji X, et al. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fung Divers. 2019;97(1):137–392. doi: 10.1007/s13225-019-00427-4.
- Westphalen MC, Motato-Vásquez V, Tomšovský M, et al. Additions to the knowledge of hydnoid Steccherinaceae: Cabalodontia, Etheirodon, Metuloidea, and Steccherinum. Mycologia. 2021;113(4):791–806. doi: 10.1080/00275514.2021.1894536.
- Wu F, Man X, Tohtirjap A, et al. A comparison of polypore funga and species composition in Forest ecosystems of China, North America, and Europe. Forest Ecosyst. 2022;9:100051. doi: 10.1016/j.fecs.2022.100051.
- Wu F, Zhou L-W, Vlasák J, et al. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fung Divers. 2022;113(1):1–192. doi: 10.1007/s13225-021-00496-4.
- Luo K-Y, Zhao C-L. Morphology and multigene phylogeny reveal a new order and a new species of wood-inhabiting basidiomycete fungi (Agaricomycetes). Front Microbiol. 2022;13:970731. doi: 10.3389/fmicb.2022.970731.
- Malysheva V, Spirin V. Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps. Fungal Biol. 2017;121(8):689–715. doi: 10.1016/j.funbio.2017.05.001.
- Bresadola G. Fungi polonici a cl. Viro B. Eichler lecti: Hayn. Ann Mycol. 1903;1:65–96.
- Liu X, Shen S, Zhao C. Morphological and molecular identification of a new species of Eichleriella (Auriculariales, Basidiomycota) in China. Phytotaxa. 2019;404(6):245–254. doi: 10.11646/phytotaxa.404.6.3.
- Wei M, Oberwinkler F. Phylogenetic relationships in Auriculariales and related groups – hypotheses derived from nuclear ribosomal DNA sequences. Mycol Res. 2001;105(4):403–415. doi: 10.1017/S095375620100363X.
- Miettinen O, Larsson K-H, Spirin V. Hydnoporia, an older name for Pseudochaete and Hymenochaetopsis, and typification of the genus Hymenochaete (Hymenochaetales, Basidiomycota). Fungal Syst Evol. 2019;4:77–96. doi: 10.3114/fuse.2019.04.07.
- Dai Y-C. Hymenochaetaceae (Basidiomycota) in China. Fung Divers. 2010;45(1):131–343. doi: 10.1007/s13225-010-0066-9.
- Parmasto E. The genus Hymenochaete (Hymenomycetes): infrageneric classification and satellite genera. Doc Mycol. 1995;25:305–315.
- Parmasto E. Hymenochaetoid fungi (Basidiomycota) of North America. Mycotaxon. 2001;79:107–176.
- Wallroth KFW. Flora cryptogamica Germaniae: Algas et fungos. 2. Norimbergae: Schragius; 1833.
- Guan Q-X, Li Y-F, Zhao C-L. Morphological and phylogenetic evidence for recognition of two new species of Hyphoderma (Basidiomycota) from Southern China, with a key to all Chinese Hyphoderma. MycoKeys. 2021;83:145–160. doi: 10.3897/mycokeys.83.69909.
- Guan Q-X, Zhao C-L. Taxonomy and phylogeny of the wood-inhabiting fungal genus Hyphoderma with descriptions of three new species from East Asia. J Fungi. 2021;7(4):308. doi: 10.3390/jof7040308.
- Larsson K-H. Re-thinking the classification of corticioid fungi. Mycol Res. 2007;111(Pt 9):1040–1063. doi: 10.1016/j.mycres.2007.08.001.
- Justo A, Miettinen O, Floudas D, et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017;121(9):798–824. doi: 10.1016/j.funbio.2017.05.010.
- Larsson KH. Two new species in Hyphoderma. Nord J Bot. 1998;18(1):121–127. doi: 10.1111/j.1756-1051.1998.tb01106.x.
- Ma X, Huang R-X, Zhang Y, et al. Hyphoderma fissuratum and H. mopanshanense spp. nov. (Polyporales) from Southern China. Mycoscience. 2021;62(1):36–41. doi: 10.47371/mycosci.2020.08.004.
- Hjortstam K, Ryvarden L. A checklist of names in Hyphodontia sensu stricto-sensu lato and Schizopora with new combinations in Lagarobasidium, Lyomyces, Kneiffiella, Schizopora, and Xylodon. Synopsis fungorum. 2009;26:33–55.
- Riebesehl J, Langer E. Hyphodontia s.l. (Hymenochaetales, Basidiomycota): 35 new combinations and new keys to all 120 current species. Mycol Progress. 2017;16(6):637–666. doi: 10.1007/s11557-017-1299-8.
- Karsten P. Enumeratio Thelephorearum Fr. et Clavariearum Fr. Fennicarum, systemate novo dispositarum. Rev Mycol Toulouse. 1881;3:21–23.
- Bernicchia A, Gorjón S. Fungi Europaei 12: Corticiaceae s.l. Alassio: Edizioni Candusso; 2010. p. 731–744.
- Eriksson J. Studies in the heterobasidiomycetes and homobasidiomycetes—Aphyllophorales of Muddus National Park in North Sweden. Uppsala: Acta Universitatis Upsaliensis; 1958.
- Viner I, Spirin V, Zíbarová L, et al. Additions to the taxonomy of Lagarobasidium and Xylodon (Hymenochaetales, Basidiomycota). MycoKeys. 2018;41(41):65–90. doi: 10.3897/mycokeys.41.28987.
- Riebesehl J, Yurchenko E, Nakasone KK, et al. Phylogenetic and morphological studies in Xylodon (Hymenochaetales, Basidiomycota) with the addition of four new species. MycoKeys. 2019;47:97–137. doi: 10.3897/mycokeys.47.31130.
- Cho Y, Kim JS, Dai Y-C, et al. Taxonomic evaluation of Xylodon (Hymenochaetales, Basidiomycota) in Korea and sequence verification of the corresponding species in GenBank. PeerJ. 2021;9:e12625. doi: 10.7717/peerj.12625.
- Luo K-Y, Chen Z-Y, Zhao C-L. Phylogenetic and taxonomic analyses of three new wood-inhabiting fungi of Xylodon (Basidiomycota) in a Forest ecological system. J Fungi. 2022;8(4):405. doi: 10.3390/jof8040405.
- Dong J-H, Zhang X-C, Chen J-J, et al. A phylogenetic and taxonomic study on Steccherinum (Polyporales, Basidiomycota): focusing on three new Steccherinum species from Southern China. Front Cell Infect Microbiol. 2022;12:1103579. doi: 10.3389/fcimb.2022.1103579.
- Westphalen M, Rajchenberg M, Tomšovský M, et al. A re-evaluation of Neotropical Junghuhnia s.lat. (Polyporales, Basidiomycota) based on morphological and multigene analyses. Persoonia. 2018;41(1):130–141. doi: 10.3767/persoonia.2018.41.07.