Abstract
Truffles, belonging to the genus Tuber, are ectomycorrhizal (ECM) fungi that form underground ascocarps and primarily establish symbiosis with oaks and hazels. The cultivation of Tuber spp. involves transplanting inoculated seedlings that have formed ectomycorrhiza with Tuber species, with mulching being effective for truffle cultivation. In this study, we investigated the effects of mulching on the mycelial growth of four Tuber species (T. himalayense, T. koreanum, T. melanosporum, and T. borchii) in the Korean natural environment, highlighting the potential for Korea as a truffle cultivation site. We developed and tested species-specific primers for quantifying the soil mycelial biomass of Tuber spp. by qRT-PCR, determined the superior mulch color for mycelial growth, and identified the Tuber species exhibiting the highest growth rate in the Korean field environment. Our results demonstrated that white mulch significantly enhanced mycelial growth in Tuber species than black mulch, likely owing to its ability to maintain low soil temperatures, control weeds, and improve host plant growth. Among the Tuber species, T. himalayense showed the greatest growth potential in the Korean natural environment. Additionally, a significant and positive correlation was observed between the mycelial biomass of Tuber species and the growth of inoculated seedlings, as measured by the total stem length and the number of leaves, thereby indicating the importance of symbiosis between ECM fungi and host plants. This study provides valuable insights into truffle cultivation in Korea and highlights the potential of using white mulch to promote mycelial growth, thereby contributing essential data for understanding the appropriate environmental conditions for Tuber spp. cultivation in Korea. Further study is needed to assess the long-term impact of mulching and to explore the effectiveness of other mulching materials.
1. Introduction
Truffles, hypogeous ascocarps produced by the genus Tuber (Ascomycota, Pezizales), are highly valued for their unique flavor and aroma [Citation1–4]. This genus includes 180–230 species worldwide [Citation5,Citation6], primarily forming ectomycorrhizal (ECM) association with oak and hazel trees [Citation7]. Tuber species have been poorly studied. Only five species have been reported to date in Korea, including T. aestivum subsp. uncinatum, T. borchii, T. himalayense, T. huidongense, T. koreanum, and T. indicum [Citation8–12].
Tuber spp. is typically cultivated by transplanting seedlings inoculated with Tuber species in the field [Citation13]. However, regarding the formation of fruiting bodies, these fungi require specific environmental conditions, including calcareous soil with alkaline pH, stable and moderate soil temperatures, and a weed-free environment [Citation14,Citation15]. Mulching is a useful method for controlling these environmental conditions [Citation16] as it reduces extreme soil temperature fluctuations, maintains soil moisture, and inhibits weed growth [Citation15,Citation17–19]. Additionally, mulching affects the growth of ECM fungi [Citation20]. For example, different mulch colors and compositions can affect the mycelial growth of T. melanosporum, wherein a double-layer white woven polypropylene fabric was found to be the most suitable for growth [Citation18,Citation21].
In this study, we investigated the effects of black and white mulches on the mycelial growth of two Korean truffle species (Tuber himalayense and Tuber koreanum) and two European truffle species (Tuber borchii and Tuber melanosporum). The quantification of truffle mycelial growth in soil is primarily conducted using quantitative real-time PCR (qRT-PCR), which requires species-specific primers. Due to the lack of available primers for Tuber species, our study aimed to develop these primers. Therefore, the objectives of this study were to develop species-specific primers for quantifying the soil mycelial biomass of Tuber spp. via qRT-PCR, determine the superior mulch color for mycelial growth, and identify the Tuber species that exhibit the greatest growth in the Korean field environment.
2. Materials and methods
2.1. Experimental site and mulching design
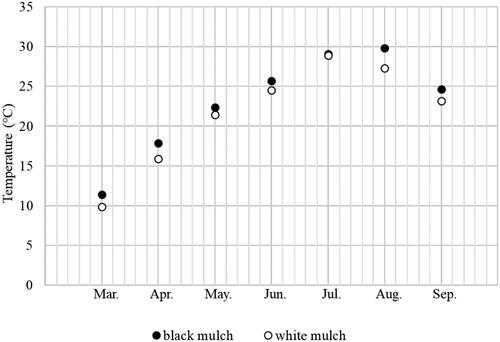
The study was conducted in a field at the Korea National University of Education in Cheongju, Korea (36°36′26.7″N, 127°21′44.9″E). Lime was applied to the experimental site to maintain a soil pH of approximately pH 8. Subsequently, 16 rows of ridges were created that were alternately mulched with a double layer of either black or white woven polypropylene fabric (Poolanna, Daegu, Korea). The soil characteristics of the experimental sites are analyzed by Cheillab (Seoul, Korea) and summarized in . To measure the atmospheric temperature and relative humidity, a data logger (RXW-THC-922, Onset Computer Corporation, Bourne, MA) was installed at the experimental site. The soil temperature was measured using an additional data logger (HT.w sensor, SensorPush, New York, NY) placed at a depth of 15 cm (). Both data loggers recorded data at 30-minute intervals throughout the study.
Table 1. Soil compositions in the experimental site.
2.2. Preparation of seedlings inoculated with truffles
Quercus acutissima seeds were collected from Cheongju, Korea, washed with distilled water, and surface-sterilized with 10% sodium hypochlorite for 10 min. To promote germination, the seeds were placed in a 280 mL pot filled with sterilized vermiculite and maintained in a greenhouse for six months under a 12-h photoperiod, with a relative humidity of 55 ± 5% and a temperature of 24 ± 1 °C. The ascomata of T. himalayense and T. koreanum were collected from Danyang and Gyeongju, respectively [Citation12,Citation22], whereas T. melanosporum and T. borchii were purchased from the Chiko Corporation in Korea. The ascomata were washed with distilled water and 70% ethanol for 10 min and then ground in sterile water to produce a spore suspension. The six-month-old Q. acutissima seedlings were inoculated with a 1.4 × 106 spore/mL suspension. The inoculated seedlings were then transplanted into a 280 mL pot filled with a sterilized mixture of vermiculite and perlite at a 1:1 ratio. They were maintained in a greenhouse for an additional six months under the same conditions as mentioned above. After 6 months, ECM formation was confirmed by observing roots under dissecting microscope. Genomic DNA from the ECM was extracted using the HiGene™ Genomic DNA Prep Kit (Biofact, Daejeon, Korea). The ribosomal DNA (rDNA) region containing the internal transcribed spacer (ITS) was amplified via PCR by using the ITS1F and ITS4 primers [Citation23]. PCR conditions were 95 °C (2 min), followed by 35 cycles of 95 °C (20 s), 50 °C (40 s), and 72 °C (1 min), and 72 °C (5 min). Electrophoresis was performed on 1.5% agarose gel for 20 min. Sequence analysis was conducted by Solgent (SolGent Co. Ltd., Daejeon, Korea), and sequence similarity was identified using BLAST.
2.3. Transplantation of inoculated seedlings
Twenty inoculated seedlings of each Tuber species were selected. In March 2022, five inoculated seedlings per ridge were transplanted, with a gap of 80 cm between seedlings (). For the experimental units, ten inoculated seedlings were transplanted into black and white mulch.
2.4. Soil sampling and growth measurement
Soil sampling and growth measurements of the inoculated seedlings were performed every three months following transplantation. Soil samples were collected at depths of 5 and 20 cm at three points, located 5 cm away from the host plant, using a soil corer with a diameter of 14 mm. Before processing, the soil samples were placed in polyethylene bags and stored at −35 °C. DNA from the soil samples was extracted using the DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany) after air-drying at room temperature and passing the soil through a 2-mm sieve. The growth of the inoculated seedlings was measured by recording the total stem length and the number of leaves every three months after transplantation.
2.5. Quantification of mycelial biomass via qRT-PCR
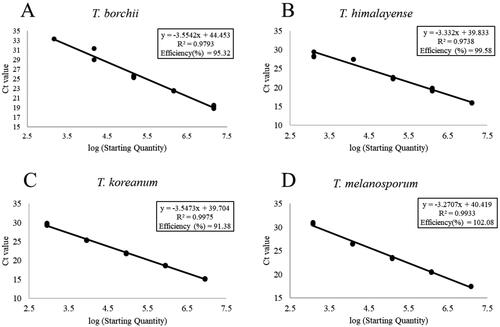
Specific primers for T. koreanum, T. borchii, and T. melanosporum were designed for the ITS2 region of rDNA using primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (). To confirm primer specificity, DNA was extracted from the soil samples at the experimental site before the transplantation of the inoculated seedlings and from the ascocarps of seven Tuber species (T. aestivum, T. borchii, T. himalayense, T. indicum, T. koreanum, T. magnatum, and T. melanosporum). Sequence analysis was conducted by Solgent (SolGent Co. Ltd., Daejeon, Korea), and the obtained sequences were identified using BLAST. DNA was extracted from the soil samples using PowerSoil Pro Kit (Qiagen, Hilden, Germany) and ascocarps of Tuber spp. standards using a HiGene™ Genomic DNA Prep Kit (Biofact, Daejeon, Korea) and amplified using qRT-PCR (LineGene Mini S, Bioer, Zhejiang, China). DNA for the standard curve was extracted from the ascocarps of Tuber spp. using HiGene™ Genomic DNA Prep Kit (Biofact, Daejeon, Korea). The concentration of the extracted DNA was measured using Nabi UV/Vis Nano Spectrophotometer (MicroDigital Co., Ltd., Seongnam, Korea). Four serial 10-fold dilution of first extraction was conducted. Serial 10-fold dilution from 10° to 10−4 was used for amplification (). The TK-f1/TK-r8 primers developed in this study were used to amplify the mycelia of T. koreanum and T. borchii in soil, whereas the TM-f1/TM-r1 primers were used to amplify the mycelium of T. melanosporum. Primers indi-fw/ITS4LNG [Citation24,Citation25] were used to amplify the mycelium of T. himalayense in the soil. The qRT-PCR had a total volume of 20 µL, including 10 µL of BioFACT™ Real-Time PCR master mix, 6 µL of nuclease-free water, 1 µL of each primer (10 pmol/µL), and 2 µL of template DNA. The PCR conditions were as follows: initial denaturation at 95 °C for 15 min and 40 cycles (95 °C for 20 s, annealing for 30 s, and 72 °C for 30 s); the annealing temperatures were 60 °C for indi-fw/ITS4LNG and 62 °C for TK-f1/TK-r8 and TM-f1/TM-r1. A melting curve analysis was performed by heating the samples to 95 °C for 15 s, followed by cooling to 60 °C for 1 min, and then heating at 95 °C for 15 s. All reactions were performed in triplicate.
Table 2. Primer name, sequences, size of PCR products, and annealing temperature (ta).
2.6. Statistical analysis
Statistical analysis was performed by Statistical Package for Social Sciences for Windows software, version 12.0 (IBM, Chicago, IL). A one-way ANOVA was conducted to confirm the effect of mulching on mycelial growth of Tuber spp. and difference in mycelial biomass among Tuber species. Mann–Whitney test and student’s t-test are conducted to confirm the effect of mulching on growth of inoculated seedlings. Linear regression analysis was conducted to confirm the relationship between mycelial biomass of Tuber spp. and the growth of inoculated seedlings. p Value below 0.05 is statistically significant.
3. Results
Negative controls were amplified with the ITS1F/ITS4 primers () but not with the TK-f1/TK-r8 primers (. The TK-f1/TK-r8 primers exclusively amplified the ascocarps of T. koreanum and T. borchii (), revealing an expected DNA band of 100–200 bp in size. BLAST analysis demonstrated a sequence similarity of 98.06% with T. borchii MF686459 and 98.23% with T. koreanum OM049190. Regarding the qRT-PCR, one peak appeared in the melting curve analysis (). Negative controls were amplified with the ITS1F/ITS4 primers () but not with the TM-f1/TM-r1 primers (). The TM-f1/TM-r1 primers specifically amplified the ascocarp of T. melanosporum, revealing an expected DNA band of approximately 100 bp in size (). BLAST analysis revealed a 98.67% similarity with T. melanosporum EU200420. Regarding the qRT-PCR, one peak appeared in the melting curve analysis (). The TK-f1/TK-r8 primers were specific for T. borchii and T. koreanum, whereas the TM-f1/TM-r1primers were specific for T. melanosporum.
Figure 4. PCR products amplified with ITS1F/ITS4 primers (A), TK-f1/TK-r8 primers (B), and TM-f1/TM-r1 primers (C). Lane 1, Tuber koreanum; lane 2, Tuber borchii; lane 3, Tuber melanosporum; lane 4, Tuber himalayense; lane 5, Tuber indicum; lane 6, Tuber aestivum; lane 7, Tuber magnatum; and lane 8; soil of the experimental site before transplantation; M, SolGent™ 100 bp Plus DNA Ladder.
Figure 5. Melting curve analysis via qRT-PCR using specific primers. (A) Tuber borchii; (B) Tuber koreanum; and (C) Tuber melanosporum.
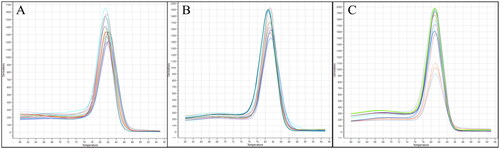
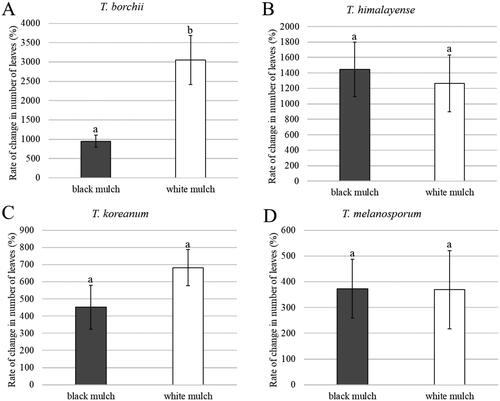
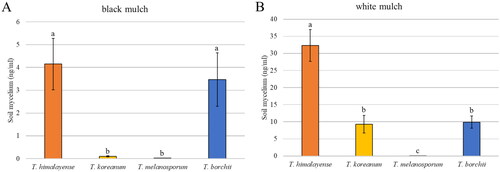
White mulching significantly enhanced the mycelial growth of T. himalayense, T. koreanum, and T. borchii, yielding a higher mycelial biomass than black mulching (). Although mycelial growth of T. melanosporum did not increase over time with either mulch type, it was greater under the white mulch than under the black mulch in both June and September. The growth rate was measured in terms of changes in the total stem length and number of leaves of seedlings and were compared between white and black mulches ( and ). For the inoculated seedlings of T. borchii, it was found that under white mulch, both rates of stem length and leaf numbers increased significantly more than under black mulch. However, no significant difference in the growth rate was observed between black and white mulch for the inoculated seedlings of T. himalayense, T. koreanum, and T. melanosporum.
Figure 6. Mycelial biomass of Tuber spp. under black mulch and white mulch. (A) Tuber borchii; (B) Tuber himalayense; (C) Tuber koreanum; and (D) Tuber melanosporum. Mean values ± standard error. Different letters above the bars show significant differences (LSD tests, p < 0.05).
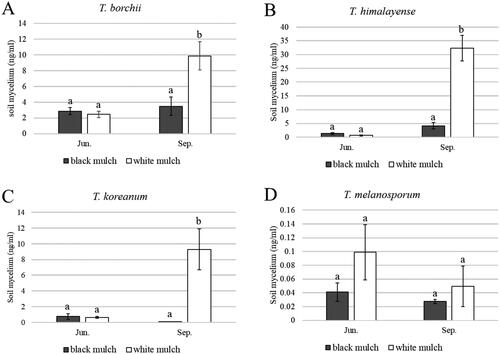
Figure 7. Rate of change in total stem length of inoculated seedlings by Mann–Whitney test. (A) Tuber borchii; (B) Tuber himalayense; (C) Tuber koreanum; and (D) Tuber melanosporum. Different letters above the bars show significant differences (p < 0.05).
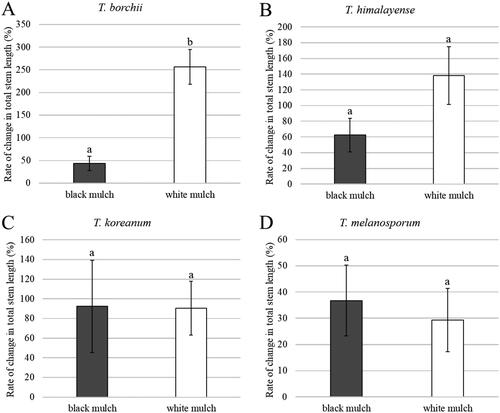
Figure 8. Rate of change in the number of leaves of inoculated seedlings by Student’s t-test. (A) Tuber borchii; (B) Tuber himalayense; (C) Tuber koreanum; and (D) Tuber melanosporum. Mean values ± standard error. Different letters above the bars show significant differences (p < 0.05).
The mycelial biomass was compared at six months post-transplantation. The order of abundance for the mycelial biomasses between both types of mulches is as follows: T. himalayense, T. borchii, T. koreanum, and T. melanosporum. A significant difference was observed in the mycelial biomass depending on the Tuber species (). T. himalayense had a significantly higher mycelial biomass than the other Tuber species under both types of mulches. Conversely, T. melanosporum showed a significantly lower mycelial biomass than the other Tuber species under both types of mulches. Among the native species, T. himalayense showed a significantly higher mycelial biomass than T. koreanum; however, among non-native species, T. borchii had a significantly higher mycelial biomass than T. melanosporum.
Figure 9. Mycelial biomass of Tuber spp. 6 months after transplantation under black mulch (A) and white mulch (B). Mean values ± standard error. Different letters above the bars show significant differences (LSD test, p < 0.05).
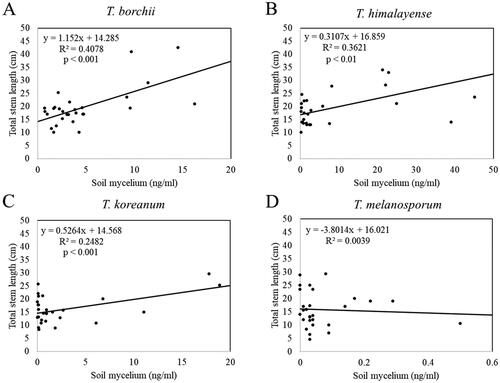
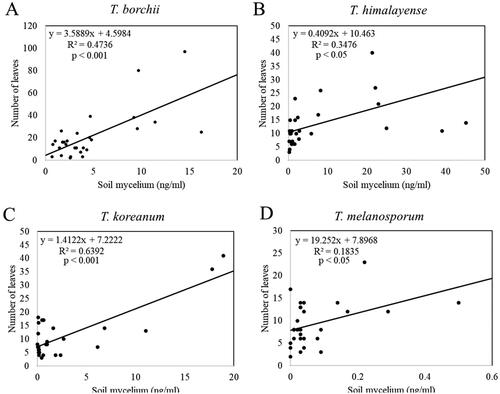
The total stem length of the inoculated seedlings was significantly and positively correlated with the mycelial biomasses of T. borchii, T. himalayense, and T. koreanum (). The number of leaves on the inoculated seedlings showed a significant and positive correlation with the mycelial biomasses of T. borchii, T. himalayense, T. koreanum, and T. melanosporum ().
4. Discussion
The observation of Tuber spp. mycelium growth in the Korean natural environment suggests that truffle cultivation is possible in Korea. In this study, we observed differences in Tuber spp. mycelium development depending on the mulching technique used. The mycelial biomass of Tuber spp. was significantly higher under white mulch than under black mulch, which is consistent with previous studies that found white mulch to be beneficial for Tuber spp. mycelial growth [Citation18,Citation21]. White mulches maintain lower soil temperatures than clear and black mulches [Citation26–28]; our study confirmed that the average soil temperature of white mulch was 1.4 °C lower than that of black mulch. Moreover, maintaining soil moisture under white mulch has proven to be more successful than overhead irrigation [Citation21]. Mulching is an effective way to control weeds [Citation29], with single-layered black, double-layered black, and double-layered white mulches having the lowest percentage of weeds over three years [Citation18]. This study confirmed the beneficial effects of white mulch on the mycelial growth of Tuber spp. compared to those of black mulch. To the best of our knowledge, this is the first study to confirm the effects of mulching on T. borchii, T. himalayense, and T. koreanum. The advantages of using white mulch in preventing extreme soil temperatures, maintaining soil moisture, controlling weeds, and improving the growth of host plants likely have a positive effect on the mycelial growth of these Tuber species.
Tuber spp. exhibit differences in mycelial biomass depending on their environmental preferences and adaptations, resulting in significant species-specific variations. This study revealed that T. himalayense, which is widely distributed in Asia [Citation30], has the greatest growth rate among other Tuber species in the Korean natural environment. However, T. melanosporum, a typical Mediterranean species [Citation16,Citation31], prefers the Mediterranean climate and has an environmental habitat opposite to that of Korea [Citation32], resulting in low mycelial biomass in this study. The pH preference for mycelium growth also varies depending on the species [Citation14,Citation33]; in this study, T. koreanum, a native species previously described to have optimal mycelium growth at a pH of 6.0 [Citation34], showed low mycelial biomass at the experimental site, which had a pH of 8. In contrast, T. borchii has a wide host range and adaptability to various environmental conditions [Citation35–37], as well as the potential for cultivation in areas where it has not previously been found. By simultaneously comparing multiple species, we found that T. himalayense and T. borchii have the greatest mycelial growth potential for cultivation in South Korea.
A significant and positive correlation was observed between the mycelial biomass of Tuber spp. and the growth of inoculated seedlings, likely resulting from the symbiosis between ECM fungi and host plants. Previous studies have reported a relationship between host plant growth and Tuber spp.; the larger the root collar diameter, the richer the mycelial biomass of Tuber spp. and the higher the truffle production [Citation7,Citation21,Citation38–40]. In this study, we used the total stem length and the number of leaves as growth measures of host plants and found that they had a significant and positive correlation with the soil mycelial biomass of Tuber spp. Therefore, these growth measures can be used as the indicators of mycelial biomass of Tuber spp.
In conclusion, this study highlights the effect of mulching on the mycelial growth of Tuber spp. Specifically, white mulch had a greater positive effect on the mycelial biomass than black mulch. This finding supports those of previous studies suggesting that double-layered white mulch is beneficial for the growth of Tuber spp. Additionally, white mulch helps prevent extreme soil temperatures and maintain soil moisture, leading to an improved growth pattern of host plants and weed control. Mycelial biomass varied depending on the Tuber species and their preferred environmental conditions. T. himalayense showed the greatest growth potential among the other Tuber species in the Korean natural environment. A significant and positive correlation was observed between the mycelial biomass of Tuber spp. and the growth of inoculated seedlings, indicating the importance of symbiosis between ECM fungi and host plants. The total stem length and the number of leaves are useful indicators of the mycelial biomass of Tuber spp. However, further long-term studies are needed, as the study period of six months may not fully capture the impact of mulching on Tuber spp. growth. Further investigations are necessary to examine the impact of other environmental factors on the mycelial biomass of Tuber spp. and investigate the effectiveness of other mulching materials.
Disclosure statement
No potential competing interest was reported by the authors.
Additional information
Funding
References
- Le Tacon F, Zeller B, Plain C, et al. Carbon transfer from the host to Tuber melanosporum mycorrhizas and ascocarps followed using a 13C pulse-labeling technique. PLoS One. 2013;8(5):e64626. doi: 10.1371/journal.pone.0064626.
- Payen T, Murat C, Bonito G. Truffle phylogenomics: new insights into truffle evolution and truffle life cycle. Advances in botanical research. Amsterdam, the Netherlands: Elsevier; 2014. p. 211–234.
- Bonet J, Fischer C, Colinas C. Cultivation of black truffle to promote reforestation and land-use stability. Agron Sustain Dev. 2006;26:69–76.
- Mello A, Murat C, Bonfante P. Truffles: much more than a prized and local fungal delicacy. FEMS Microbiol Lett. 2006;260(1):1–8. doi: 10.1111/j.1574-6968.2006.00252.x.
- Bonito GM, Gryganskyi AP, Trappe JM, et al. A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol. 2010;19(22):4994–5008. doi: 10.1111/j.1365-294X.2010.04855.x.
- Bonito G, Smith ME, Brenneman T, et al. Assessing ectomycorrhizal fungal spore banks of truffle producing soils with pecan seedling trap-plants. Plant Soil. 2012;356(1–2):357–366. doi: 10.1007/s11104-012-1127-5.
- Suz LM, Martín MP, Oliach D, et al. Mycelial abundance and other factors related to truffle productivity in Tuber melanosporum–Quercus ilex orchards. FEMS Microbiol Lett. 2008;285(1):72–78. doi: 10.1111/j.1574-6968.2008.01213.x.
- Shin KS, Park JS, Yoshimi S. Note on Tuber aestivum subsp. uncinatum newly recorded in Korea. Kor J Mycol. 1995;23:10–13.
- Park H, Gwon JH, Lee JC, et al. Report on Tuber huidongense, a truffle species previously unrecorded in Korea. Kor J Mycol. 2020;48:505–510.
- Park H, Gwon JH, Lee JC, et al. Morphological and phylogenetic characteristics of Tuber himalayense collected from rhizosphere of Quercus dentata in Korea. Kor J Mycol. 2021;49:101–108.
- Seok SJ, Lim YW, Kim CM, et al. List of mushrooms in Korea. Seoul: The Korean Society of Mycology; 2013.
- Park H, Gwon JH, Lee JC, et al. Report on a new truffle species, Tuber koreanum sp. nov., from Korea. Mycobiology. 2021;49(6):527–533. doi: 10.1080/12298093.2021.1992089.
- Murat C. Forty years of inoculating seedlings with truffle fungi: past and future perspectives. Mycorrhiza. 2015;25(1):77–81. doi: 10.1007/s00572-014-0593-4.
- Ge ZW, Brenneman T, Bonito G, et al. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil. 2017;418(1–2):493–505. doi: 10.1007/s11104-017-3312-z.
- Fischer C, Oliach D, Bonet Lledos JA, et al. Best practices for cultivation of truffles. Solsona: Centre Tecnològic Forestal de Catalunya; 2017.
- Tacon L. F. Influence of climate on natural distribution of Tuber species and truffle production. True truffle (Tuber spp.) in the world. Berling, Germany: Springer; 2016. p. 153–167.
- Mulumba LN, Lal R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008;98:106–111.
- Olivera A, Bonet JA, Palacio L, et al. Weed control modifies Tuber melanosporum mycelial expansion in young oak plantations. Ann Sci. 2014;71:495–504.
- Prosdocimi M, Tarolli P, Cerdà A. Mulching practices for reducing soil water erosion: a review. Earth Sci Rev. 2016;161:191–203.
- Goulart BL, Demchak K, Yang WQ. Effect of cultural practices on field grown ‘Bluecrop’ highbush blueberries, with emphasis on mycorrhizal infection levels. Acta Hort. 1997;446:271–278.
- Piñuela Y, Alday JG, Oliach D, et al. White mulch and irrigation increase black truffle soil mycelium when competing with summer truffle in young truffle orchards. Mycorrhiza. 2021;31(3):371–382. doi: 10.1007/s00572-020-01018-x.
- Park H, Gwon JH, Lee JC, et al. Morphological and phylogenetic characteristics of Tuber himalayense collected from rhizosphere of Quercus dentata in Korea. Korean J Mycol. 2021;49:101–108.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. London: Academic Press; 1990. p. 315–322.
- Paolocci F, Rubini A, Granetti B, et al. Typing Tuber melanosporum and Chinese black truffle species by molecular markers. FEMS Microbiol Lett. 1997;153(2):255–260. doi: 10.1111/j.1574-6968.1997.tb12582.x.
- Schelm S, Siemt M, Pfeiffer J, et al. Food authentication: identification and quantitation of different Tuber species via capillary gel electrophoresis and Real-Time PCR. Foods. 2020;9(4):501. doi: 10.3390/foods9040501.
- Lal Bhardwaj R. Effect of mulching on crop production under rainfed condition-a review. Agric Rev. 2013;34:188–197.
- Graham HAH, Decoteau DR, Linvill DE. Development of a polyethylene mulch system that changes color in the field. HortSci. 1995;30(2):265–269. doi: 10.21273/HORTSCI.30.2.265.
- Díaz-Pérez JC, Batal KD. Colored plastic film mulches affect tomato growth and yield via changes in root-zone temperature. J Am Soc Hortic Sci. 2002;127:127–135.
- Campiglia E, Mancinelli R, Radicetti E, et al. Effect of cover crops and mulches on weed control and nitrogen fertilization in tomato (Lycopersicon esculentum mill.). Crop Prot. 2010;29:354–363.
- Kinoshita A, Obase K, Yamanaka T. Ectomycorrhizae formed by three Japanese truffle species (Tuber japonicum, T. longispinosum, and T. himalayense) on indigenous oak and pine species. Mycorrhiza. 2018;28(7):679–690. doi: 10.1007/s00572-018-0860-x.
- Parladé J, HDl V, Pera J. Tools to trace truffles in soil. True truffle (Tuber spp.) in the world. Berlin, Germany: Springer; 2016. p. 249–266.
- Lionello P, Malanotte-Rizzoli P, Boscolo R, et al. The Mediterranean climate: an overview of the main characteristics and issues. Mediterranean climate variability. Amsterdam, the Netherlands: Elsevier; 2006. p. 1–26.
- Rosa-Gruszecka A, Hilszczańska D, Pacioni G. Virtual truffle hunting—a new method of burgundy truffle (Tuber aestivum vittad.) site typing. Forests. 2021;12(9):1239. doi: 10.3390/f12091239.
- Gwon JH, Park H, Eom AH. Effect of temperature, pH, and media on the mycelial growth of Tuber koreanum. Mycobiology. 2022;50(4):238–243. doi: 10.1080/12298093.2022.2112586.
- Zambonelli A, Lotti M, Giomaro G, Hall I, Stocchi V, editors. T. borchii cultivation: an interesting perspective. Edible mycorrhizal mushrooms and their cultivation. Proceedings of the Second International Conference on Edible Mycorrhizal Mushrooms; 3–6 July, 2001; Christchurch, New Zealand. Auckland, New Zealand: Crop & Food Research.
- Hall IR, Brown GT, Zambonelli A. Taming the truffle: the history lore and science of the ultimate mushroom. Portland: Timber; 2007.
- Iotti M, Lancellotti E, Hall I, et al. The ectomycorrhizal community in natural Tuber borchii grounds. FEMS Microbiol Ecol. 2010;72(2):250–260. doi: 10.1111/j.1574-6941.2010.00844.x.
- Büntgen U, Egli S, Camarero JJ, et al. Drought-induced decline in Mediterranean truffle harvest. Nat Clim Chang. 2012;2:827–829.
- Oliach D, Colinas C, Castaño C, et al. The influence of forest surroundings on the soil fungal community of black truffle (Tuber melanosporum) plantations. For Ecol Manag. 2020;470:118212.
- Şen İ, Piñuela Y, Alday JG, et al. Mulch removal time did not have significant effects on Tuber melanosporum mycelium biomass. For Syst. 2021; 30:eSC02.