Abstract
Potato (Solanum tuberosum L.) is one of the most important food crops in Korea. In July 2021 and 2022, dark black-rot symptoms with pink tinges were observed on field-grown potato tubers in Hongsung and Chuncheon, Korea, respectively. We obtained four isolates (HSv05 and HSv10 from Hongsung, and CCp03 and CCp05 from Chuncheon) from diseased tubers and identified these isolates as Pythium aphanidermatum by analyzing the sequences of internal transcribed spacer rDNA region and mitochondrial cytochrome c oxidase subunit II (COX2) mtDNA gene. Additionally, we compared the cultural and morphological characteristics of these four isolates with those of the reference isolate KACC 48066 of P. aphanidermatum and the literature. Further, we tested the pathogenicity of all these isolates against potato tubers. The cultural and morphological characteristics of the four test isolates were similar to those of the reference isolate and the literature; all four test isolates proved pathogenic to potato tubers. Therefore, we concluded that P. aphanidermatum is the causal agent of potato leak and this is the first report of the disease on potato in Korea.
1. Introduction
Potato (Solanum tuberosum L.) is one of the most important food crops that is widely cultivated worldwide. Potato is the fourth most produced crop, after maize, wheat, and rice, with an annual global production of 375 million tons in 2022 [Citation1]. Particulary, in Korea, 587,000 tons of potatoes were produced that same year [Citation1], out of which approximately 75% was consumed in the domestic market, while only 25% was exported [Citation2]. Because of such high dependence of the domestic market on national production, ensuring stable potato production is important to provide current demand.
In general, potatoes are exposed to various diseases during cultivation and post-harvest storage. In the cultivation, the crop can be affected by a range of soilborne diseases, including dry rot (Fusarium sambucinum), late blight (Phytophthora infestans), leak (mainly Pythium ultimum var. ultimum), pink rot (Phytophthora erythroseptica), and powdery scab (Spongospora subterranea) among many others [Citation3]. Furthermore, potatoes can be seriously affected by post-harvest diseases during storage conditions [Citation4, Citation5]. Therefore, as researched in other host-parasite systems [Citation6,Citation7], appropriate control measures should be considered at pre- and post-harvest stages in potato production.
In July 2021 and 2022, dark black-rot symptoms with pink tinges were observed on field-grown potato tubers in Hongsung and Chuncheon, Korea, respectively. When the diseased tubers were cut and exposed to air, their inner tissues gradually turned grey and eventually black with pink tinges. Such symptoms were similar to those described for leak, also called watery wound rot, which is mainly caused by P. ultimum var. ultimum [Citation8]. In addition, potato leak is sometimes induced by Pythium aphanidermatum and occasionally by Pythium delicense [Citation8]; hence, accurate identification of the pathogen causing the disease to potato tubers is paramount for effective control to maintain the quality of marketable potatoes. Therefore, the objectives of this study were (i) to identify this unknown causal agent by sequence analyses of internal transcribed spacer (ITS) region and the mitochondrial cytochrome c oxidase subunit II (COX2) gene as well as cultural and morphological comparisons with those of the reference isolate KACC 48066 of P. aphanidermatum and literature, and (ii) to test the pathogenicity of this species against potato tubers.
2. Materials and methods
2.1. Potato tuber sampling and pathogen isolation
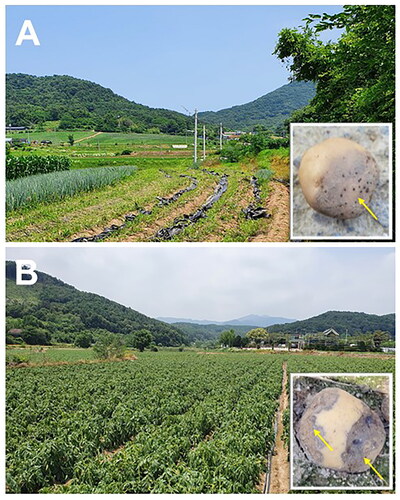
In July 2021, potato (cv. Dubaek) tubers showing dark black-rot symptoms with pink tinges were obtained from a field of the Global Agro-Consulting Company in Hongsung (36°34'02"N, 126°43'46"E), Korea (). Then, in July 2022, potato (unknown cultivar) tubers with similar symptoms as observed in Hongsung, Korea, were obtained from a field of Chuncheon (37°54'04"N, 127°41'42"E), Korea (). Pathogen isolation and culture preparation for experiments using the sampled potato tubers were conducted as described previously [Citation9]. Briefly, for pathogen isolation from diseased potato tubers, lesion pieces cut from the tubers were sterilized with 1% NaOCl for 1 min, washed twice with sterile distilled water (SDW), and blotted on sterile Whatman No.1 filter papers to remove excess water. Then, they were placed on acidified potato dextrose agar (PDA) (Difco, Becton, Dickinson & Co., Sparks, USA) and incubated in the dark at 28 °C for 1–2 d. Mycelia grown from these pieces were transferred to acidified PDA and cultured under the same conditions. Four hyphal-tip cultures (an isolate from a diseased tuber) from Hongsung (isolates HSv05 and HSv10) and Chuncheon (isolates CCp03 and CCp05) were obtained for further experiments.
2.2. Cultural and morphological characterization
The four isolates (HSv05, HSv10, CCp03, and CCp05) were subject to cultural and morphological comparisons with those of reference isolate KACC 48066 (originated from tomato) of P. aphanidermatum, obtained from the Korean Agricultural Culture Collection (KACC) in Wanju, Korea, and the literature [Citation10]. For cultural characterization, the test and reference isolates were cultured on PDA and V8 juice agar (Campbell’s, Camden, USA) at 28 °C for 24 h. Cultures on each medium were compared for mycelial growth. The colony diameter (mm) was determined 24 h after inoculation on the media. This experiment was conducted twice with three plates (replicates) each.
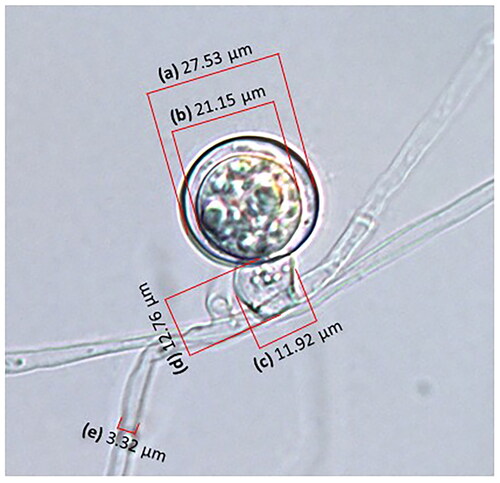
The morphological characteristics (shape and diameter of hyphae, shape, and/or size of sporangia, oogonia, antheridia, and oospores) of the test and reference isolates were examined using the cover slip (18 × 18 mm No.1, Paul Marienfeld GmbH & Co.KG, Lauda-Königshofen, Germany) culture technique developed in this study (Supplementary Figure 1). To prepare the sample cultures, sterile cover slips were dipped in liquid corn meal agar (CMA) (BBL, Becton, Dickinson & Co., Sparks, USA) and dried. Subsequently, these CMA-coated slips were placed in plates containing CMA (ca. 2-cm away from the inoculated points), and the centers of the media were inoculated with the isolates. After 2-d incubation of the plates at 28 °C, agars of upper surface of the cover slips were removed with sterile paper towel and mycelial agars of lower surface of the slips were mounted with lactic acid. Then, these slips were placed on glass slides and examined under a differential interference-contrast (DIC) light microscope (Zeiss AX10 equipped with AxioCam MRc5, Carl Zeiss, Oberkochen, Germany), as shown in . On the other hand, for zoospore observation, zoospores of the test and reference isolates were produced by the procedure described by Khan et al. [Citation11]. Briefly, the isolates were cultured on 10% strengthened-V8 juice agar at 25 °C under continuous fluorescent light. After 48 h, the cultures were cut into 1-cm-wide strips and half of the alternative strips were transferred to empty Petri dishes. Each Petri dish was then flooded with 20 ml of SDW and incubated for an additional 48 h under the same conditions. The water in the Petri dishes was replaced with 10 ml of SDW; these dishes were further incubated under fluorescent light at 20 °C for 4 h. Thereafter, zoospores were harvested from the plates and their morphology and size were determined using an Olympus BX50X-3 light microscope (Olympus Optical Co., Tokyo, Japan).
2.3. Molecular characterization
For molecular characterization of the test and reference isolates, genomic DNA was extracted from mycelia grown on PDA at 28 °C for 3 d using a LaboPass DNA Isolation Kit (Cosmogenetech, Seoul, Korea) according to manufacturer instructions. The ITS region of the test isolates was amplified using the universal primers ITS 1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS 4 (5′-TCCTCCGCTTATTGATATGC-3′) [Citation12]. In addition to ITS region amplification, the mitochondrial cytochrome c oxidase subunit II (COX2) gene sequence of the isolates was amplified using primers FM66 (5′-TAGGATTTCAAGATCCTGC-3′) and FM52 (5′-GTTGTCCTAATTCCATTCTAA-3′) [Citation13]. Amplification of the ITS region and COX2 gene by PCR, as well as DNA sequencing and phylogenetic (neighbor-joining and maximum-likelihood methods) analysis, were conducted according to the procedure described by Sang et al. [Citation14]. The experiments were conducted twice and similar results were obtained on both occasions.
2.4. Pathogenicity tests
For the pathogenicity tests of the test and reference isolates, all isolates were hole-inoculated on potato (cv. Dubaek) tubers as described by Oh et al. [Citation15]. Healthy tubers surface-sterilized with 1% NaOCl for 1 min were used for the inoculation tests. Mycelial plugs (5 mm in diameter) of the isolates grown on V8 juice agar at 28 °C for 3 d or medium plugs without mycelia (uninoculated controls) were inoculated in holes (5 mm in depth) made on the outer surface of tubers using a sterile 5-mm cork borer. These inoculated tubers were placed in plastic containers [(17 (W) × 13 (L) × 23 (H) cm] with two layers of wet paper towels and incubated at 28 °C. At 24 h after inoculation, the diameters of the lesions that appeared on the inner tissues of the inoculated tubers were determined as follows: length (mm) + width (mm)/2. After disease assessment of the inoculated tubers, the inoculated isolates were re-isolated from symptomatic tissues. Infected tuber tissues were cut, surface-sterilized with 1% NaOCl for 1, 1.5, 2, and 3 min, and washed twice with sterile distilled water. After blotting these pieces on sterile Whatman No. 1 filter papers, they were placed on acidified PDA. Mycelia from the pieces were then transferred to PDA, and the recovered mycelia were subjected to further identification using ITS region and COX2 gene sequence analyses, as described previously. These experiments were conducted twice with four replicates each for disease assessment. In addition, the molecular re-identification of the inoculated tubers were conducted twice, and produced similar results on both occasions.
On the other hand, for the observation of symptoms on the outer tissues of potatoes (unknown cultivar, originated in Youngam, Korea) tubers, tubers were pin-prick-inoculated with 10-μl aliquots of the mycelial suspension [3 ml SDW per plate (3-d-old culture)] of the test and reference isolates and SDW (uninoculated control), respectively, as described by Lee et al. [Citation16]. The inoculated tubers were incubated at 28 °C for 24 h, as described previously, and then further incubated in the containers without wet towels for additional 4 d. Then, any symptoms appearing on outer tissues of the inoculated tubers were photographed. This experiment was conducted twice and similar results were obtained on both occasions.
2.5. Statistical analysis
Data were analyzed using Statistical Analysis Systems software (SAS Institute, Cary, NC, USA). After the homogeneity of the variances between data from repeated experiments was confirmed using Levene’s test [Citation17], the data were pooled and further analyzed. General linear model procedures were used for variance analysis and means were separated using the least significant difference (LSD) test at p < 0.05.
3. Results
3.1. Pathogen isolation, and cultural and morphological characterization
In July 2021, symptoms observed on diseased potato tubers originated in a field of Hongsung, Korea were similar to those of leak caused by P. aphanidermatum (). Furthermore, diseased tubers with similar symptoms were observed in a field of Chuncheon, Korea, in July 2022 (). Potato tubers with dark black-rot symptoms were soft, mushy, and creamy-colored on the outer tissues and violet-colored on the inner tissues. In addition, diseased tubers produced a rotting smell and were subsequently covered with white mycelia.
Isolates HSv05 and HSv10 from Hongsung, and isolates CCp03 and CCp05 from Chuncheon showed similar cultural features (). Thus, the colonies of all of these isolates and those of the reference isolate KACC 48066 were white and formed large amounts of cottony aerial mycelia without distinctive growth patterns on PDA or V8 juice agar. Colony diameters of the test isolates ranged from 70.5 to 79.0 mm on PDA and from 64.5 to 77.5 mm on V8 juice agar at 24 h after inoculation, while those of the reference isolate were only 52.5 and 51.4 mm in diameter on PDA and V8 juice agar, respectively (). Likewise, the morphological features of the test isolates were similar to those of the reference isolate and the literature [Citation10] ( and ). Hyphae of the test isolates were branched, hyaline, aseptate, and 4.5–9.0 μm (average 6.5–7.3 μm) in diameter; thus, they were quite similar to those of the reference isolate and the literature. In general, other morphological features of the test isolates, including shape and diameter of the zoospores, sporangia, oogonia, antheridia, and oospores, were similar to those of the reference isolate and the literature ( and ). The cultures of two representative isolates, HSv05 (accession number: KACC 410270) and CCp03 (KACC 410269), among four isolates used in this study were deposited at KACC, Wanju, Korea.
Figure 3. Cultural characteristics of the test isolates, HSv05, HSv10, CCp03, and CCp05, compared with those of the reference isolate KACC 48066 of Pythium aphanidermatum, grown for 24 h on (A) potato dextrose agar and (B) V8 juice agar at 28 °C. Mycelial growth (mm) [mean (n = 6) ± standard deviation] is shown in the center of each plate at 24 h after inoculation. Different letters following values indicate significant (p < 0.05) differences between isolates on the medium.
![Figure 3. Cultural characteristics of the test isolates, HSv05, HSv10, CCp03, and CCp05, compared with those of the reference isolate KACC 48066 of Pythium aphanidermatum, grown for 24 h on (A) potato dextrose agar and (B) V8 juice agar at 28 °C. Mycelial growth (mm) [mean (n = 6) ± standard deviation] is shown in the center of each plate at 24 h after inoculation. Different letters following values indicate significant (p < 0.05) differences between isolates on the medium.](/cms/asset/9f47619b-1d76-4eca-936d-12a3b0727903/tmyb_a_2373506_f0003_c.jpg)
Figure 4. Morphological characteristics [(A), zoospores; (B), toruloid zoosporangia (indicated by white arrows); and (C), thick-walled oospores (indicated by yellow arrows) attached by antheridia (indicated by red arrows)] of the test isolates, HSv05, HSv10, CCp03, and CCp05, compared with those of the reference isolate KACC 48066 of Pythium aphanidermatum grown on (A) 10% V8 juice agar or (B and C) corn meal agar. Scale bar = 10 μm (A and C) or 20 μm (B).
![Figure 4. Morphological characteristics [(A), zoospores; (B), toruloid zoosporangia (indicated by white arrows); and (C), thick-walled oospores (indicated by yellow arrows) attached by antheridia (indicated by red arrows)] of the test isolates, HSv05, HSv10, CCp03, and CCp05, compared with those of the reference isolate KACC 48066 of Pythium aphanidermatum grown on (A) 10% V8 juice agar or (B and C) corn meal agar. Scale bar = 10 μm (A and C) or 20 μm (B).](/cms/asset/861fe16e-cbd3-4936-a13e-10685140e23c/tmyb_a_2373506_f0004_c.jpg)
Table 1. Morphological characteristics of isolates, HSv05, HSv10, CCp03, and CCp05, as compared with those of the reference isolate KACC 48066 of Pythium aphanidermatum, and fungal descriptions by Van der Plaats-Niterink [Citation10].
3.2. Molecular characterization of the test isolates
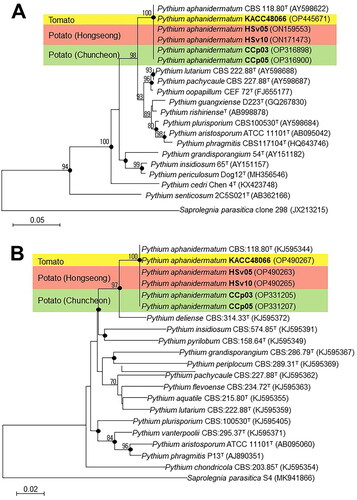
Partial sequences of the ITS (646, 758, 692, 653, and 758 bases) regions and COX2 (817, 815, 813, 864, and 929 bases) genes of the test isolates (HSv05, HSv10, CCp03, and CCp05) and the reference isolate (KACC 48066) were obtained for phylogenetic analysis. Neighbor-joining analysis using these ITS region sequences (alignment length = 608 bases) revealed that all test isolates clustered with P. aphanidermatum CBS 118.80T (accession number: AY598622) (100% similarity) (). In turn, analysis using the COX2 gene sequences (alignment length = 442 bases) revealed that the isolates clustered with P. aphanidermatum CBS 118.80T (accession number: KJ595344) (100% similarity) (). Similarly, application of the maximum-likelihood method using both gene sequences revealed that all test isolates also clustered with P. aphanidermatum CBS 118.80T (100% similarity) (). Therefore, based on all these results, all test and reference isolates (HSv05, HSv10, CCp03, CCp05, and KACC 48066) were identified as P. aphanidermatum. The sequences of the ITS regions and COX2 genes of the test isolates HSv05 (accession numbers: ON159553 and OP490263), HSv10 (ON171473 and OP490265), CCp03 (OP316898 and OP331205), CCp05 (OP316900 and OP331207), and KACC 48066 (OP445671 and OP490267) were deposited in GenBank.
Figure 5. Phylogenetic trees constructed using the neighbor-joining method showing the relationships between test isolates, HSv05, HSv10, CCp03, and CCp05, and the reference isolate KACC 48066 of Pythium aphanidermatum and other members of the genus Pythium based on sequence analyses of (A) the internal transcriptional space region and (B) the cytochrome c oxidase subunit II gene. The numbers at the branching points are bootstrap values (>70%) for 1,000 replicates. Black dots on the branching points indicate that the corresponding nodes were also recovered with bootstrap values (>70%) in trees constructed using the maximum-likelihood method. Scale bars indicate the number of nucleotide substitutions per 100 nucleotides of the sequences. Saprolegnia parasitica was used as the outgroup. GenBank accession numbers are shown in parentheses. T = type strain.
3.3. Pathogenicity tests of the test isolates
The test isolates HSv05, HSv10, CCp03, and CCp05 and the reference isolate KACC 48066 proved to be pathogenic to potato tubers (). Thus, dark black-rot symptoms, which are typical symptoms of leak caused by P. aphanidermatum, were observed on the outer tissues of the tubers 5 d after pin-prick-inoculation (). On the other hand, soft, watery, and rotted tissues with pink tinges were detected in the inner tissues of the tubers 24 h after hole-inoculation (). Further, rotting odor similar to that of diseased tubers sampled in the fields of Hongsung and Chuncheon was detected in the inoculated tubers. The reference isolate also caused similar symptoms in the inner and outer tissues of the inoculated bulbs (). The diameters of the inner tissue lesions in the tubers caused by the test isolates ranged from 20.7 to 27.3 mm 24 h after inoculation, but the reference isolate had a diameter of only 18.3 mm in the tubers ().
Figure 6. (A) Typical, dark black-rot symptoms on the outer tissues of tubers pin-prick-inoculated with 10-μl aliquots of the mycelial suspensions of the test isolates, HSv05, HSv10, CCp03, and CCp05, and the reference isolate KACC 48066 of Pythium aphanidermatum, as observed 5 d after inoculation at 28 °C. (B) Watery-rot symptoms with pink tinges in the inner tissues of potato tubers hole-inoculated with mycelial plugs (5 mm in diameter) of all test isolates, as observed 24 h after inoculation at 28 °C. Lesion length (mm) [mean (n = 8) ± standard deviation] are shown on the center of each plate. Different letters following values indicate significant (p < 0.05) differences between isolates. The V8 juice-agar plugs without mycelia (uninoculated control) did not produce any disease symptoms on the treated tubers. (C) Test isolates were re-isolated on acidified potato dextrose agar (PDA) from (B) symptomatic tissues (indicated by red arrows) of the inoculated tubers after surface-sterilization in 1% NaOCl solution for 1–3 min. The re-covered isolates are shown on acidified PDA 18 h after incubation at 28 °C.
![Figure 6. (A) Typical, dark black-rot symptoms on the outer tissues of tubers pin-prick-inoculated with 10-μl aliquots of the mycelial suspensions of the test isolates, HSv05, HSv10, CCp03, and CCp05, and the reference isolate KACC 48066 of Pythium aphanidermatum, as observed 5 d after inoculation at 28 °C. (B) Watery-rot symptoms with pink tinges in the inner tissues of potato tubers hole-inoculated with mycelial plugs (5 mm in diameter) of all test isolates, as observed 24 h after inoculation at 28 °C. Lesion length (mm) [mean (n = 8) ± standard deviation] are shown on the center of each plate. Different letters following values indicate significant (p < 0.05) differences between isolates. The V8 juice-agar plugs without mycelia (uninoculated control) did not produce any disease symptoms on the treated tubers. (C) Test isolates were re-isolated on acidified potato dextrose agar (PDA) from (B) symptomatic tissues (indicated by red arrows) of the inoculated tubers after surface-sterilization in 1% NaOCl solution for 1–3 min. The re-covered isolates are shown on acidified PDA 18 h after incubation at 28 °C.](/cms/asset/4c9b947e-2a97-40a9-afb6-9e1ff73c8e05/tmyb_a_2373506_f0006_c.jpg)
Subsequently, all isolates, including KACC 48066, were re-isolated from the symptomatic inner tissues of the inoculated tubers (). The re-covered isolates were re-confirmed as P. apahnidermatum after sequence analyses of the ITS region and COX2 gene. Conversely, V8 juice-agar plugs without mycelia (uninoculated control) did not produce any symptoms on the treated tubers, and mycelia were not observed on the medium.
4. Discussion
Using multiple approaches such as cultural, morphological, and molecular analyses, in this study, we identified four isolates, HSv05, HSv10, CCp03, and CCp05, obtained from field-grown diseased potato tubers from two different locations in Korea in July 2021 and 2022. The results allowed us to unequivocally identify these isolates as P. aphanidermatum. Moreover, our results revealed that they were pathogenic to potato tubers, in which pin-prick- or hole-inoculated tubers with mycelial suspensions or mycelial plugs produced dark black-rot and watery symptoms with pink tinges, similar to the symptoms of potato leak [Citation8].
Pythium aphanidermatum is widely distributed worldwide and has a broad host-spectrum infecting 261 plant species [Citation18]. Recently, this oomycete species has been reported as the causal agent of tomato (Solanum lycopersicum) crown and root rot [Citation19], bur cucumber (or bur gherkin) (Cucumis anguria) fruit rot [Citation20], common ice plant (Mesembryanthemum crystallinum) and mung bean (Vigna radiata) root rot [Citation21,Citation22], and Malvaceae okra (Abelmoschus manihot) stalk rot [Citation23]. In addition, although approximately 80% of the global potatoes in 2022 was produced in the Asia and Europe [Citation1], the oomycete species has been reported as the causal agent of potato leak only in Greece and India in the regions [Citation24]. Therefore, P. aphnidermatum described in this study is first identified as the causal agent of potato leak not only in Korea but in East Asia. Along with this species, P. ultimum var. ultimum has been also known to be the causal agent of the disease in other countries such as Canada, Kenya, Peru, and Scotland [Citation24]. In general, these two Pythium species may occur differentially on potato tubers, depending on temperature conditions; P. ultimum var. ultimum may aggressively cause potato leak at 25 °C, whereas P. aphanidermatum may do it at 30 °C [Citation25]. Further, the optimal growth temperature on culture media was 25 °C for P. ultimum var. ultimum and 30 °C for P. aphanidermatum [Citation25]. In fact, it is not surprising that P. aphanidermatum was recently reported to cause root and stem rot on stringy stonecrop (Sedum sarmentosum) during hot summers in Korea [Citation26]. This report indicates that this soilborne oomycete species is widely distributed in Korea; potato tuber infection by this pathogen is significantly affected by high temperatures during hot summers in Korea.
Potato leak is generally caused by Pythium species, mainly P. ultimum var. ultimum and, sometimes, P. aphanidermatum [Citation8]. This disease generally occurs in potato tubers only through wounds, resulting from planting, harvesting, or storage. Indeed, the disease is one of the most severe threats to potato tuber quality during pre- and post-harvest stages of potato cultivation, especially under high humidity conditions [Citation25]. For example, according to a two-year survey conducted in Tunisia, 30–39% of potato tubers were rotted by Pythium spp. infection [Citation27]. Therefore, with this new information on the causal agent, P. aphanidermatum, of potato leak in Korea, various disease management strategies, including cultural (e.g. crop rotation, soil solarization to reduce inoculum, or planting resistant cultivars), biological (e.g. using anti-oomycete antagonists), and chemical measures should be applied to achieve effective control of this destructive soilborne disease during potato cultivation and storage [Citation28–30].
Altogether, all the test isolates (HSv05, HSv10, CCp03, and CCp05) obtained from diseased tissues of potato tubers cultivated in Korea were identified as P. aphanidermatum based on ITS region and COX2 gene analyses, as well as cultural and morphological analyses. Moreover, it was evident that all the test isolates were pathogenic to potato tubers. To our knowledge, this is the first report of P. aphanidermatum causing leak on potato in Korea.
Supplemental Material
Download JPEG Image (87.9 KB)Acknowledgment
We thank the Korean Agricultural Culture Collection (KACC) for providing the isolate, KACC 48066, used as reference in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- FAO. Food and agriculture data; 2023. [accessed 2024 Jan 16]. Available from: https://www.fao.org/faostat/en/#home.
- Soley GT, Choi S. Potato and potato products. 2016. Potato update. Republic of Korea. USDA-FAS, GAIN Report number: KS1621, USDA Foreign Agricultural Service; 2016. [accessed 2024 Jan 16]. Available from: https://gain.fas.usda.gov/Recent%20GAIN%20Publications/2016%20Potato%20Update_Seoul_Korea%20-%20Republic%20of_7-22-2016.pdf.
- Lahkim LT. Chapter 9. Fungal, oomycete, and plasmodiophorid diseases of potato and their control. In: Caliskan ME, Bakhsh AB, Jabran K, editors. Potato production worldwide. Amsterdam: Elsevier Inc; 2023. p. 145–178.
- Gudmestad NC, Taylor RJ, Pasche JS. Management of soilborne diseases of potato. Austral Plant Pathol. 2007;36(2):109–115. doi: 10.1071/AP06091.
- Olsen N, Miller J, Nolte P. Diagnosis and management of potato storage diseases. Idaho: University of Idaho Extension; 2006. p. 1–6.
- Oh JY, Sajidah S, Volynchikova E, et al. Antifungal activity of thymol against Aspergillus awamori and Borytis aclada isolated from stored onion bulbs. Mycobiology. 2022;50(6):475–486. doi: 10.1080/12298093.2022.2158557.
- Volynchikova E, Kim KD. Anti-oomycete activity and pepper root colonization of Pseudomonas plecoglossicida YJR13 and Pseudomonas putida YJR92 against Phytophthora capsici. Plant Pathol J. 2023;39(1):123–135. doi: 10.5423/PPJ.OA.01.2023.0001.
- Salas B, Secor GA. Leak. In: Stevenson WR, Loria R, Franc GD, et al., editors. Compendium of potato diseases. Minnesota: The American Phytopathological Society; 2001. p. 30–31.
- Sang MK, Han GD, Oh JY, et al. Penicillium brasilianum as a novel pathogen of onion (Allium cepa L.) and other fungi predominant on market onion in Korea. Crop Prot. 2014;65:138–142. doi: 10.1016/j.cropro.2014.07.016.
- Van der Plaats-Niterink AJ. Monograph of the genus Pythium. Stud. Mycol. 1981;21:1–242.
- Khan A, Sutton JC, Grodzinski B. Effects of Pseudomonas chlororaphis on Pythium aphanidermatum and root rot in peppers grown in small-scale hydroponic troughs. Biocontrol Sci. Technol. 2003;13(6):615–630. doi: 10.1080/0958315031000151783.
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995.
- Martin FN. Phylogenetic relationships among some Pythium species inferred from sequence analysis of the mitochondrially encoded cytochrome oxidase II gene. Mycologia. 2000;92(4):711–727. doi: 10.1080/00275514.2000.12061211.
- Sang MK, Kim HS, Myung IS, et al. Chryseobacterium kwangjuense sp. nov., isolated from pepper (Capsicum annuum L.) root. Int J Syst Evol Microbiol. 2013;63(Pt 8):2835–2840. doi: 10.1099/ijs.0.048496-0.
- Oh JY, Han GD, Jeong JJ, et al. First report of Penicillium georgiense as a fungal pathogen of onion (Allium cepa L.). Crop Prot. 2015;72:83–89. doi: 10.1016/j.cropro.2015.02.009.
- Lee YJ, Mannaa M, Jeong JJ, et al. First report of dry rot of sweetpotato (Ipomoea batatas) caused by Diaporthe batatas in Korea. Plant Dis. 2016;100(8):1786–1786. doi: 10.1094/PDIS-02-16-0249-PDN.
- Levene H. Contributions to probability and statistics. In: Olkin I, editor. Essays in honor of harold hotelling. Standford: Stanford University Press; 1960. p. 278–292.
- Farr DF, Rossman AY. Fungal databases, U.S. national fungus collections; 2024. [cited 2024 Feb 8]. Available from: https://nt.ars-grin.gov/fungaldatabases.
- Gilardi G, Tabone G, Guarnaccia V, et al. First report of crown and root rot caused by Pythium aphanidermatum on tomato in Italy. Plant Dis. 2022;105(7):2022. doi: 10.1094/PDIS-10-20-2246-PDN.
- Nóbrega TF, Ferreira BW, Barreto RW. First report of Pythium aphanidermaturm causing fruit rot of Cucumis anguria. Australasian Plant Dis Notes. 2022;17(1):16. doi: 10.1007/s13314-022-00464-0.
- You XD, Park JE, Takase M, et al. First report of Pythium aphanidermatum causing root rot on common ice plant (Mesembryanthemum crystallinum). New Dis. Reports. 2015;32(1):36–36. doi: 10.5197/j.2044-0588.2015.032.036.
- Yan Q, Hu Y, Zhang Q, et al. Occurrence of root rot caused by Pythium aphanidermatum on mung bean (Vigna radiata) in China. Plant Dis. 2022;105(11):3764. doi: 10.1094/PDIS-02-21-0297-PDN.
- Qu Q, Liu S, Liu Y, et al. First report of Pythium aphanidermatum causing stalk rot on Abelmoschus manihot in China. Plant Dis. 2022;106(2):771. doi: 10.1094/PDIS-06-21-1133-PDN.
- USDA. Fungal databases – Fungus-Host by Country; 2024. [accessed 2024 Jun 5]. Available from: https://fungi.ars.usda.gov.
- Triki MA, Priou S, Mahjoub ME, et al. Leak syndrome of potato in Tunisia caused by Pythium aphanidermaturm and Pythium ultimum. Potato Res. 2001;44(3):221–231. doi: 10.1007/BF02357900.
- Kim Y, Lee Y, Chung J, et al. First report of root and stem rot caused by Pythium aphanidermatum on Sedum sarmentosum in Korea. Plant Dis. 2020;104(11):3072–3072. doi: 10.1094/PDIS-03-20-0532-PDN.
- Priou S, Mahjoub ME. Bacterial and fungal diseases in the major potato‐growing areas of Tunisia. Bull EPPO. 1999;29(1–2):167–171. doi: 10.1111/j.1365-2338.1999.tb00812.x.
- Halo BA, Al-Yahyai RA, Al-Sadi AM. Talaromyces omanensis and Aspergillus fumigatus endophytic fungi suppress Pythium aphanidermatum and its induced damping-off diseases of cucumber and radish. Archiv Phytopathol Plant Prot. 2023;56(9):665–685. doi: 10.1080/03235408.2023.2216350.
- Triki MA, Priou S, Mahjoub ME. Effect of soil solarization on soil-borne populations of Pythium aphanidermaturm and Fusarium solani and on the potato crop in Tunisia. Potato Res. 2001;44(3):271–279. doi: 10.1007/BF02357905.
- Tsror L, Erlich O, Hazanovsky M, et al. Fungicide field treatments to control potato leak caused by Pythium ultimum. Am J Potato Res. 2021;98(2):115–121. doi: 10.1007/s12230-021-09822-7.