ABSTRACT
This paper explores the potential of connected health solutions to solve the problems currently facing healthcare systems around the world with a particular interest in their decision support capabilities. Leveraging three selected projects in which we have been involved in the area of maternal and child health, the paper proposes a blueprint for connected health decisions in a variety of settings, namely: home-based, community-based, ward-based scenarios as well as the specific scenario of low-income countries. This blueprint can be used to frame discussions on connected health solutions and discuss their decision support potential.
KEYWORDS:
1. Introduction
Whilst DSS researchers have often focused on business settings, the ambition to understand decision-making and to improve it with decision support has been identified as applicable to a very broad range of disciplines. All the way back to 1967 – contemporary of Ackoff’s (Citation1967) seminal paper – Rabson (Citation1967) proposed that clinical decision-making could be augmented by DSS. Closer to current times, medical experts have sought to implement evidence-based decision-making in medical practice and this has boosted the importance of Clinical Decision Support (Browson et al, Citation1999; Crowley et al., Citation2020). Thus, the science of decision support is not rooted in business settings and is inherently multi-disciplinary when it is applied to problems that arise within diverse field of inquiry, from spatial exploration to climate change.
In this paper, we systematically review 3 selected examples of high potential connected health projects in the area of maternal and child health to seek to abstract core principles that can frame discussions pertaining to the development of connected health applications. Leveraging the lessons learnt in the development and implementation of these applications, we propose a model blueprint for the architecture and operation of connected health solutions that have a substantial decision support dimension. Such decision support exploits the large volume of data that can be collected by connected health sensors by structuring them and directing their decision support outputs at either or both patients and clinicians.
The rest of this paper is structured as follows: first, we review the business and medical cases, which underpins the development of connected health solutions in modern healthcare systems in the context of the ‘perfect storm’ they are faced with. Then, we explore three case of applications we developed in maternal health (DSS for the early identification of pre-eclampsia) and child health (DSS for the first 15 minutes of life in the delivery room and DSS for child and adolescent mental health – CAMHS). The paper closes on the presentation of our proposed blueprint for DSS oriented connected health solutions.
2. Smarter medical decision support with connected health
The area of connected health illustrates how the emergence of relevant technologies, coupled with new ideas on how to apply them to salient problems can lead to the development of decision support frameworks that deliver impacts on a large scale (O’Raghallaigh & Adam, Citation2017).
Connected health is a term used to describe a technology-driven model of healthcare service provision. While a standard definition has yet to be formally adopted, an appropriate definition of connected health is ‘a conceptual model for health management where devices, services or interventions are designed around the patient’s needs, and health related data is shared, in such a way that the patient can receive care in the most proactive and efficient manner possible’ (Caulfield & Donnelly, Citation2013, p. 704). One may therefore view connected health as an ecosystem that contains three pillars – interventions, services, and devices. These pillars are utilised together to improve overall patient outcomes, which each of them being closely integrated to ensure the seamless transfer of information between services providers and services recipients.
Devices play a critical part in the Connected Health ecosystem. Mobile Health (m-Health) is a term used to describe the suite of mobile devices, such as sensors, mobile phones, and related software applications; used to remotely monitor patient health and wellbeing and enabling patients to play a more active role in their own care. While Connected Health refers to the entire ecosystem, m-Health refers to technology that enables the transfer of information between patients and healthcare providers, acting as an intermediary between both (Payton et al., Citation2011). This m-Health, or device, intermediary acts in combination with the other two pillars to ensure patients and relevant clinicians receive the most up-to-date and relevant information, very often focused on vital signs or other key measurements of patient health or patient outcomes.
2.1 Understanding the healthcare crisis: why is connected health becoming crucial?
While life expectancy has continued to increase in most places in the world over recent decades, there is evidence that the related concept of healthy life expectancy is not keeping up (Balanda et al., Citation2010). As life expectancy lengthens, so does the prevalence of a number of chronic conditions such as obesity, diabetes and elevated blood pressure (BP) which dramatically affect the life of portions of the population.
As well as being responsible for a significant number of early deaths, chronic conditions reduce the quality of life of many adults and represent a substantial financial cost to patients, insurers, and the health and social care systems. In recent times, the number of people seeking care for such conditions is increasing and there is a growing shortage of healthcare professionals in both the primary and secondary care areas of many health systems (Harris, Citation2019; Nugent et al., Citation2011). In fact, the demand for services is growing at such a rate that our reliance on face-to-face interactions for the delivery of care is compromised and the healthcare systems of many countries are faced with the medical equivalent of a ‘perfect storm’ (Rozenblum & Bates, Citation2013).
One striking aspect of this evolution is that both developing and developed countries are facing the same crisis, although in different shapes. The medical area illustrates very well that developing countries should not try to go through the same slow stages of development as developed countries have. Where there is a new technological frontier, developing countries should strive to leapfrog past evolutionary steps and catch up with the latest advances. Because it is infrastructure light, connected health can be a very viable next generation in the development of healthcare systems the world over (Topol, Citation2015; Zackery et al., Citation2022). For instance, many African countries have mobile telecom networks on a par with the most advanced European countries, and this is a source of great opportunities for them to catch up in a number of domains including healthcare.
The current healthcare crisis is here to stay in the medium term and is resulting in lengthening waiting lists, less attention to individual patients, overworked medical staff, even closure of medical facilities as operating theatres cannot be staffed adequately, against the backdrop of significant budgetary constraints. Thus, the effectiveness of healthcare systems is reducing, which negatively impacts the experiences of patients, making it harder to maintain patient safety and adhere to quality standards (Kido & Tsukamoto, Citation2020).
Since 2020, the occurrence of the COVID pandemic has brought even more disruptions in already severely strained healthcare systems (Lal et al., Citation2021) and it has been suggested that digital health is the best way to address the inadequacies and limitations of healthcare systems during such global health events (Kapoor et al., Citation2020).
2.2 The burden of chronic conditions
One area which is a substantial contributory factor in the current evolution is the rise of chronic conditions (Ludwig, Citation2011) either genetic or arising from unhealthy sedentary lifestyles and other factors such as a poor diet and poor sleep hygiene. Diabetes and hypertension for instance, are reaching epidemic proportion. Insofar as these are partly behavioural, they cannot be adequately managed through medical intervention alone. They require sustained changes in the individual’s lifestyle and behaviour and connected health can help in such cases where it is used to provide real-time data to individuals to support their decision-making in relation to their lifestyle choices (e.g. exercising patterns).
Given the increasingly early age at which some of these conditions appear, obesity for instance, it is essential to educate individuals from an early age and develop their commitment to ‘looking after themselves’. This is a crisis, which is systemic – many factors in society conspired to bring it about and continue to propagate it. Consumerism, advertising, social media are other very hard to control factors are all contributors. Equally, the solution is also systemic – it involves many actors in society – the medical insurance systems: health practitioners, insurance providers; the education system: schools, teachers, sports coaches, as well as parents and the individuals themselves.
Yet, conventional fee-for-service reimbursement models are discouraging preventive technologies and services that would keep people healthy and out of the hospital. Against this backdrop, connected health appears a sustainable way to provide more effective healthcare whilst also reducing costs within the context of healthcare systems that are nearly bankrupt by double figure annual medical inflation. New approaches, enabled by emerging technologies, can allow individuals to become more aware of their health status, to take greater responsibility for their lifestyle, and to make knowledgeable decisions about their behaviours. In this context, knowledgeable means data-driven and evidence-based, in a landscape where too many people – notably teenagers – are exposed to low-quality advice about many aspects of their lives through the social media. The incentives they face are often conflicting and their attention is being pulled in many different directions, resulting in them facing complex lifestyle choices, many of them in competition with a healthy lifestyle.
2.3 The future is already here
The Internet of Things (IoT) is growing at rapid pace (Jindal et al., Citation2018) and component costs have plummeted, opening up the possibility of connecting just about anything, making it possible for healthcare providers and patients to work together to improve health in novel ways, for instance, when patients are not with their health professional, using mobile apps to engineer remote monitoring and remote advising (J. C. Kvedar et al., Citation2015). Sensors are one way in which healthcare can move beyond face-to-face engagements with specialists, providing a more complete picture of an individual’s health. Not only is this approach much cheaper than face-to-face care, it can also digitally empower individuals to conveniently and unobtrusively care about their health, for instance, using a blood pressure monitor app and a connected weighing scale that provide nurses with automatic alerts. Nurses can review this data each day and proactively call the highest risk patients and, when appropriate, alert their doctors.
The adoption of these technologies, however, requires patient engagement. Studies have shown that although activity trackers are very popular (Finkelstein et al., Citation2016), 32% of users give up on their devices after only three months and 50% abandon them after about a year (Jakicic et al., Citation2016). Many individuals are also ambivalent about their own behaviour even when they know it is damaging to their health (J. C. Kvedar et al., Citation2015). Many people want to do what is best for their health, but they struggle to jump from good intentions to good behaviour. Connected technologies must be rolled out on a wide scale to empower individuals to change their behaviours. The goal of connected health must go beyond merely collecting data towards providing decisive decision support for both patients and clinicians.
3. Decision support at the heart of connected health solutions
On the basis of these observations, we contend that connected health solutions, which leverage the data they collect to deliver decision support features, both for patients and for clinicians, have significant potential for addressing the challenges currently faced by healthcare systems around the world. To explore this potential in more detail, this paper explores three definitive scenarios of connected health solutions that illustrate their potential in delivering better healthcare to specific patient populations. On the one hand, these scenarios correspond to research projects we have conducted in recent years and there is an element of opportunism in their selection. On the other hand, these three scenarios are interesting because they present high intensity, acute cases of health issues, which have not received sustained attention from researchers in the medical world, due for instance, to limited funding. These three scenarios are related to women health and children health, areas which have traditionally not been a clear focus for the medical industry. Pre-eclampsia (scenario 1) still kills thousands of women and children every year, although the mechanics and identification of this pathology have been understood for years (English et al., Citation2015). The deployment of leading-edge technology in delivery rooms (scenario 2) has been extremely limited over the last 100 years (Adam & Dempsey, Citation2020). And the use of technology in pursuit of delivering better mental health (scenario 3) has also started to develop recently (Aguilera, Citation2015; Areán et al., Citation2022). Thus, these three scenarios present opportunities to use connected health technologies and DSS principles to radically transform the way care is delivered to the groups of patients targeted by these initiatives, against the backdrop of acute shortages and shortfalls in the corresponding area of our healthcare systems.
3.1 Scenario 1: The case of Pre-Eclampsia monitoring
Hypertension is a common medical disorder of pregnancy, estimated to occur in 6% to 8% of cases. In Ireland and other developed nations, hypertension in pregnancy remains the second cause of maternal mortality, accounting for 16% of maternal deaths (Berg et al., Citation2003). White Coat Hypertension (WCH) is a common phenomenon when a diagnosis of elevated blood pressure (BP) occurs in the clinical setting but normal BP persists, outside of medical visits (Brown et al., Citation2005). Clinical blood pressure measurements at scheduled antenatal visits taken by trained professionals are the gold standard for diagnosing and treating hypertension in pregnancy. However, there is increasing evidence to suggest that current BP monitoring is error-prone and that ambulatory BP monitoring in the patient’s own environment is more accurate (Brown et al., Citation2005; Perry et al., Citation1991). Patients with persistently elevated BP outside of the clinical setting are more likely to develop pre-eclampsia which, although relatively uncommon, carries great risks for mother and foetus (Sibai, Citation1996). Thus, accurate identification of individuals with WCH versus those with true hypertension is critical (Bellomo et al., Citation1999). Despite progress with ambulatory BP devices, they are still intrusive and interfere with normal activities of daily living (Carney, Citation1997). They also still require visits to the antenatal clinic for review and interpretation (Staessan et al., Citation1999). As maternity care in Ireland (Health Service Executive, Citation2010) and other developed countries is split between the family doctor, community or hospital midwife, and obstetrician this can further complicate matters as BP results should be shared within multidisciplinary teams.
An alternative for remote BP monitoring is Home Blood Pressure Monitoring, which commonly consists of patients using an automated device as often as they can – often several times per day. This patient centred approach means measurements can be taken at the patients’ discretion over a long period of time (O’Brien, Citation2008). This leaves the patient free to choose a convenient time and place to measure BP. There is evidence that long-term HBPM throughout pregnancy could be a much better predictor of pre-eclampsia (Waugh et al., Citation2001). Early stand-alone HBPM devices used self-reporting and allowed BP measurements to be manipulated by patients and thus automated solutions that upload measurement directly are needed. It is also important to take the opportunity to provide feedback or advice to expectant mothers if they have high BP readings. This can involve all healthcare providers including consultants, nurses and GPs.
The solution we have developed, called LEANBH (for Learning to Evaluate Blood Pressure at Home) improves the transfer, analysis, presentation, and access of BP data and additional risk factors. It allows multidisciplinary healthcare teams and expectant mothers to quickly identity variations in BP and take appropriate interventions to reduce the risk of pre-eclampsia thereby improving antenatal care, all this in a way, which is inherently evidence-based.
The LEANBH application provides an ambulatory automated self BP monitoring using our integrated platform, which comprises of:
A mobile phone application uploaded on patients’ mobile phones
A Connected Health Gateway (CHG) which uploads BP readings taken by patients
A dedicated Electronic Health Record database
A clinician Decision Support dashboard for remote monitoring
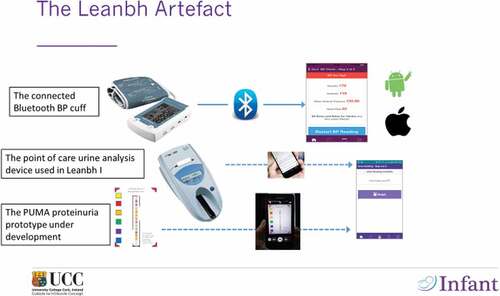
illustrates the Leanbh protoptype in its initial and current form. The complete diagnosis for pre-eclampsia involves BP measurement, backed up by an analysis of the level of protein recorded in urine (Proteinuria). In this initial version of Leanbh, we provided patients with a point of care urine analysis, which was quite expensive. This meant that the solution could not be scaled up easily. Since then, we have started developing the PUMA prototype, which uses a mobile phone camera to read a reactive strip and upload proteinuria results without patient intervention (see bottom of the diagram in ).
Our experiments with Leanbh have been successful with excellent patient feedback and closer medical scrutiny applied to all patients, resulting in faster and more accurate detection of BP variations. Whilst the implementation of Leanbh does require additional roles to be created within clinical services to monitor the online stream of results, it does make economic sense as it reduces greatly the number of false positives. From a decision support stand point, Leanbh is a game changer because it allows clinicians to scrutinise the evolution of a patient’s condition very efficiently, based on BP readings, information about any symptoms experienced by women and the added value of proteinuria readings. An alert system brings any elevated reading to their attention and allows them to push relevant advice back out to patients, including the instruction to report to an emergency room immediately if the need arises. The existence of ‘baseline’ BP data virtually eliminate the risk of white coat hypertension and other false positives.
3.2 Scenario 2: The case of the delivery room
The complex scenario of the care for a newborn in the delivery room is a critical care situation where decision support can play a decisive role. The decision-making of neonatologists involves reliance on both specialised formal medical knowledge and intuition rooted in years of experience of clinical practice. It follows the Recognition Primed Decision-Making (RPDM) model proposed by Klein (Citation1993), involving a recognition stage, leading to a diagnosis and then to rapid feedback loops requiring the neonatologist to revise their first impression and change the treatment applied to their patient until their health improved.
The delivery room is an extreme case of decision-making where vital decisions must be made in a compressed timeframe. Neonatologists in the delivery room are faced with a category of problems, which are concerned with the transition to extrauterine life of infants; which can either happen seamlessly or involve complex pathologies. Clinicians have a number of interventions at their disposal to treat these pathologies. This is an interesting decision-making scenario where the use of intuition is necessary, but within an identified bounded problem/solution set. Whereas in the business world, the number of moving parts is considerable, and the variability of many parameters is confounding, the delivery room scenario offers us the opportunity to study the role of intuition in decision-making in a real life, but controlled environment.
The delivery room is also a very specific decision-making arena, involving experts from at least two distinct domains of expertise: obstetrics (focusing on the mother) and neonatology (focusing on the newborn). The decision-making scenario explored in this paper is the unique moment where the two patients are separated from each other, and the infant transitions to life in the outside world. Specifically, we are interested in the first 15 minutes of life, before patients are transferred to either the nursery, special care unit or neonatal intensive care unit. In studying this situation, we have somewhat simplified it by focusing on the actions and decisions of the neonatologists, whereas in fact, the obstetrician is another key actor in the delivery room – focused on the health of the mother who may need special care herself.
Our focus on newborns arises from the significant evidence available that a substantial proportion of health care issues in preterm infants, some of which may have lifelong implications, occur in these first minutes of life. Most human beings are born in conditions such that they require little or no care in the process of transitioning from intrauterine life to independent life. Births are normal life events and not medical interventions. However, a small proportion of newborns, about 10% in the western world for instance, do require care, some of them intensive, to complete the transition into the world. There is evidence that a proportion of health issues developed by individuals throughout their lives results from incidents, which occurred in the first minutes of life. This is particularly the case for those born before 32 weeks in gestation, most of whom require substantial assistance at delivery, for instance, ventilation, intubation or even resuscitation.
All these interventions must be underpinned by a precise diagnosis, inasmuch as they can improve the prognosis in certain cases but can also be detrimental in other cases. Ventilation for instance, may be beneficial in terms oxygen saturation, but can also result in lung injury. Crucially, the current state of knowledge of known interventions only gives incomplete indications about when or in which case these should be applied. Thus, the diagnosis in the first minutes of life is critical and high impact, with the potential for significant injury, including long-term brain injury.
Also, whilst recent decades have witnessed a significant increase in the number of monitoring options for preterm infants in the neonatal intensive care unit setting, the monitoring of preterm infants in the delivery room has changed very little over the same period, other than the introduction of pulse oximetry into the delivery room approximately 10 years ago. Thus, little or no objective information is permanently recorded about events in these first minutes of life and tangible learning opportunities are purely and simply lost. In addition, a certain amount of subjectivity still characterises the initial assessment of at-risk newborns and the determination of which interventions should be undertaken. This evaluation rests on a significant proportion of intuition, tacit knowledge and accumulated experience by medical staff. We felt that this meant this scenario is a perfect candidate for investigating the potential for a DSS application.
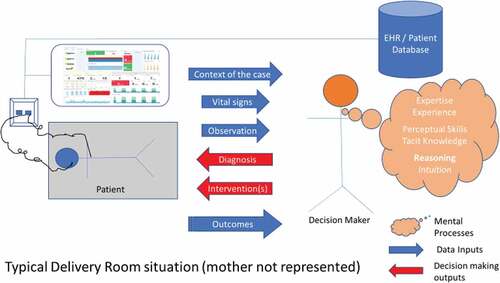
Our study is using in-depth retrospective cases (Koukouris, Citation1994) where the decision-making of the neonatologist was reviewed based on the evidence available to them and the manner in which a diagnosis was arrived at to understand how to best support their decision-making in the delivery room. shows an abstracted and simplified view of a delivery room (without mother) with some limited data collection and representation technologies, and the decision-maker and their patient.
The blue and red arrows in represent the inputs and outputs of the decision-making process, namely: (1) inputs (Context of the case; What do the vital signs say/how do they evolve; What are the important observations at birth – e.g.: the appearance of the baby) and (2) Outputs (What is the diagnosis; What intervention(s) is (are) therefore undertaken; What are the observed outcomes at 6 hours, 1 day, 3 days)
The figure also represents our research question, namely: the mental processes involved in the decision-maker turning the inputs into outputs based on:
Elements of formal expertise which underpin the diagnosis & choice of care
Tacit knowledge invoked in the diagnosis and selection of intervention
Tangible elements of experience which underpin the diagnosis
Explicit Reasoning
Intuitive elements of decision making
This project is at its very beginning, but we are seeking funding to pursue our research and conduct the clinical trial, which is required to prove the case for developing the solution on a large scale. Access to large volume of cases is required, and we are teaming up with other hospitals in Ireland to achieve this.
3.3 Scenario 3: The Case of CAMHS – can we fix the waiting lists?
Child and Adolescent Mental Health Services (CAMHS) are a critical resource to provide high-level mental health services to a population. The WHO (Citation2003) has stated that a lack of attention paid to CAMHS ‘may lead to mental disorders with lifelong consequences, undermines compliance with health regimens, and reduces the capacity of societies to be safe and productive’ (p. 1). Reports have found that at any point in time approximately 2% of children will require specialist mental health expertise (Kelly, Citation2015). The same report states that children under the age of 18 make up a quarter of the Irish population; this means that approximately 0.5% of the total population of Ireland will require intervention from the CAMHS. In other countries in the world, the proportion of under 18 is much higher and overall, nearly 30% of the world population is under 18 in 2022 according to the World Factbook (Central Intelligence Agency (Ed.), Citation2011).
With the number of adolescents requiring CAMHS intervention increasing, studies show that health services struggle to meet the demand (Dubicka & Bullock, Citation2017). This ultimately harms young people, with mental health incidents such as self-harm showing an upward trend (Morgan et al., Citation2017). Whilst the situation seems particularly acute for CAMHS, the trend towards increasing waiting list for medical treatment is a general one in many healthcare systems with the traditional model for healthcare delivery, overly reliant on one-to-one encounters between patients and carers, under severe pressure. As stated in section 2, healthcare systems around the world are facing a perfect storm, aggravated by the COVID pandemic. Faced with these seemingly uncontrollable factors, healthcare systems need a game-changing paradigm and specialists have turned towards technology for solutions to reduce transactions costs, increase the productivity and speed of services and reduce inequities in access to services (J. Kvedar et al., Citation2014).
The benefit of connected health for mental health services has been documented, with computerised cognitive behavioural therapy showings benefits in the treatment of depression and anxiety without drug treatment while yielding greater treatment satisfaction (Proudfoot et al., Citation2004).
With connected health offering healthcare organisations a way to increase their patient facing time while decreasing administration workloads, CAMHS providers can utilise connected health to tackle the problem of overwhelming demand for services. In many countries, just the existence of overly long waiting lists to access services is a threat to patients who have to wait up to a year to be assessed properly. The key issue with waiting lists is that they do not give specialists visibility on acute cases which, as a result, are seen when their turn comes rather than when they need it most. The proactive nature of connected health can help prevent the occurrence or recurrence self-harm or suicide if clinicians are able to identify patients at risk as soon as they are referred rather than having to wait until triage and assessments have been done. Indeed, those who have had multiple incidences of self-harm tend to have poorer outcomes (Byrne & Rosen, Citation2014, pp. 255–263) and using automated techniques to assess patients who are referred to CAHMS services would make a radical difference.
We are at the very beginning of the story with our work on connected health for CAMHS, and we are hitting some significant obstacles with ethical approval, due to the vulnerability of the patient groups involved. However, we have already identified that developing some patient-direct capabilities would help reduce the waiting lists that prevail in the sector by giving much more rapid visibility on acute cases to clinicians. Providing such visibility on the cohort of patients that has yet to be seen for the first time, but have already been referred to the service could help triage the different cases and really accelerate the first appointment and treatment for the most acute cases. Such decision support capabilities could transform the effectiveness of CAHMS services around the world by reducing the time spent by specialists computing assessment scores and performing what are essentially administrative tasks that can be automated, and allowing them to apply their time to direct interaction with patients who need it most. Given limited resources in many area of mental health services, connected health solutions must be experimented with, in pursuit of greater patient focus. Ethical approval, whilst needed, is sometimes an obstacle.
4. Towards a blueprint for connected health initiatives
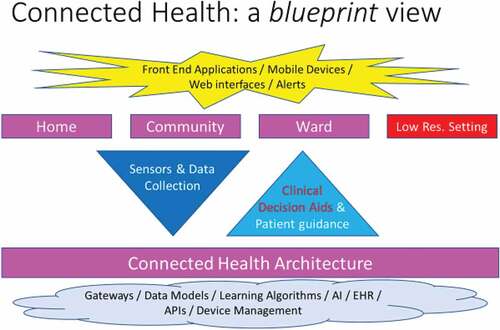
In this paper, we seek to propose a model blueprint for the architecture and operation of connected health solutions that have a substantial decision support dimension. Such decision support exploits the large volume of data collected by sensors and can be directed at either or both patients and clinicians. We have shown that Connected Health solutions can alleviate or eliminate problems across three different domains of healthcare, by providing technology-based solutions. The diagram in , which arises from our research, exposes this broad principle formally. It indicates the domains where connected health can be applied:
Home-based solutions which allow people to be monitored from their own home, without the need for travelling to hospitals or clinics. The opportunity afforded by home-based solutions arise because it offers the promise of round the clock interventions that are embedded in the places where people live.
Community-based solutions which allow patients to access services available in a hospital within the community: primary care centres, general practitioners’ practices, etc., … accelerating the integration of all the care, which patients must access. This integration is critical in the case of multiple illnesses present together. It can also greatly increase the speed at which patients can access key services.
Ward-based solutions where patients are monitored in real time using an array of wireless and wired sensors – their vitals being collected at regular intervals for instance, and stored on a dedicated platform. This offers the potential to increase the frequency of monitoring without any increase in workload, for staff who then can concentrate on treating patients rather than taking blood pressure readings and writing them down every hour or so. This can boost the implementation of electronic scorecards, particularly in intensive care wards where the frequency of monitoring is directly correlated with the speed with which patient deterioration is identified.
In low resource settings where conditions that can be normally treated in the western world are still potentially lethal. This is a special case, which has enormous potential in terms of improvements to health outcomes of patients. These improvements are both critical and broad ranging: from access to information and services, to diagnosis and treatment to disease surveillance and general population health, and with a transaction cost, which is far more affordable than that of a traditional system of care.
illustrates our proposed architecture for the delivery of connected health services. In part, the required architecture is already in place within most healthcare systems and in part it requires dedicated investment and development.
The bottom layer of the diagram shows the cloud-based part of the solution. It includes an Electronic Health Record (an industry standard in most healthcare systems). This is the basis for the systematic collection of all relevant patient data across time and across interventions. It also includes dedicated services for device management to be able to recognise the data uploaded by the different sensors used throughout the healthcare system and properly assign the data received to each patient. Any artificial intelligence system or set of algorithms would also be stored there where it could be invoked by users to analyse the data, either automatically in real time – raising alerts in case of health deterioration, or on demand, when a clinician is trying to diagnose a set of specific symptoms. At the top of the diagram, the top layer shows the front-end applications, running on the phones of the patients, on the tablets of the clinicians or the desktop of healthcare workers. These applications all contribute to various aspects of the solution: data collection, remote patient monitoring, note taking, provision of advice and instructions to patients, etc. The middle layer shows the flows of data – running from the front end to the back in the case of data collected and from the backend to the front end when it comes to the decision support provided to clinicians or the advice sent to the patient.
This architecture provides a template for a broad range of connected health applications. Connected health supports the acquisition of large volumes of health data and their analysis, underpinning the development of learning algorithms, which can consolidate doctors’ evidence-based decision-making, based on a faster and more accurate recognition of symptoms and the removal of remaining subjectivity. The area of hypertension in pregnancy and in particular the early diagnosis of pre-eclampsia is one example of a pathology where the collection of large-scale data and its systematic exploration using deep learning techniques can greatly consolidate diagnosis, accelerate the identification of at risk cases and reduce the number of false positives.
Given the scale of the challenges facing us and the high potential of connected health solutions, there is a clear opportunity to break the vicious circle of rising workloads, waiting lists and inadequate budgets. On the one hand, the scale of investment required will require that resources are set aside to fast track the development and implementation of digital health architectures. In many countries where the state does not have the financial capacity to front such investment, collaboration with industry will be required and some large players, notably in the high-tech industries, are getting ready to face up to the challenge. For such areas of the world as Africa, collaboration with these large players will be critical to leapfrog experimentation stages and move rapidly towards an efficient digital healthcare system.
On the other hand, there is also a technical challenge that must be met head on to assemble the multi-disciplinary teams that are required to properly specify and develop the applications that will realise the potential of connected health. Technologists, application developers, cloud services providers, medical experts, insurance providers, policymakers, parliamentarians and also of course, patients, will need to come together and undertake the vast Technology Assessment exercise that must underpin a rigorous and harmonious evolution from the current healthcare systems of the world to a global connected architecture to look after everyone equitably (McCarthy et al, Citation2016, Citation2019).
5. Conclusion
In this paper, we have presented three examples of high potential connected health projects in areas pertaining to maternal and child health. All three examples focus on health problems where care systems are either under-resourced and effectiveness is lacking as a result or little or no developments having taken place in many years. In our effort to abstract core principles that can guide the development of connected health systems, our first principle is that connected health solutions can unlock healthcare situations where step increases are needed.
These step improvements can certainly be delivered in terms of economies of scale – through reducing the time spent by specialists on mundane or administrative tasks or by making patient-to-specialist interaction more effective. We are particularly interested in the potential to apply core decision support concepts to connected health projects. The three examples we have studied all include a data intensive element whereby large volumes of data are collected and greater visibility on patients’ condition is given to carers. The exploitation of this new data resource is paramount in the development of game-changing connected health solutions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Richard Harris
Richard Harris is a PhD student within the Business Information Systems department at University College Cork (Ireland). He studied BIS at both undergraduate and postgraduate level and his Masters thesis involved the study of virtual machine recourse monitoring. He is currently researching for his PhD on the application of Connected Health and Decision Support Systems to the problems faced by Child and Adolescent Mental Health Services (CAHMS).
Eugene Dempsey
Eugene Dempsey is the inaugural Horgan Chair in Neonatology at University College Cork (Ireland), a Neonatologist at Cork University Maternity Hospital and is clinical lead at the INFANT Research Centre at University College Cork. He is a member of a number of international collaborations conducting randomised trials on different aspects of neonatal care. He leads a number of local clinical studies, supervising PhD students and junior doctors on many aspects of newborn medicine. He has been awarded a number of Higher Degrees, including a doctorate for work on Hypotension in the preterm infant, an MSc in Health Care Ethics and Law and an MA in Teaching and Learning, focused on simulation based procedural care addressing human factors in this area. He has over 200 publications in newborn care.
Deirdre Murray
Deirdre Murray is Professor and Head of Department in the Dept of Paediatrics and Child Health in University College Cork, Ireland. In 2019 she also became the Clinical Lead for Paediatrics in Cork University Hospital. She graduated from UCC in 1995 and trained in Paediatrics in Dublin, before specialising Paediatric Intensive Care in leading International Paediatric Hospitals in the UK and Australia. Her research interest is in early brain injury and neurodevelopmental outcome. In 2012 she was the first Irish Paediatrician to be awarded the prestigious HRB Clinician Scientist Award for her research on biomarkers in Hypoxic-Ischaemic Encephalopathy. She is a founding Principal Investigators in the Irish Centre for Fetal and Neonatal Translational Research (INFANT centre).
Simon Woodworth
Simon Woodworth is a Lecturer in Business Information Systems at University College Cork (Ireland), with research interests in Health informatics and Cyber Security. He holds a B.Sc. in Computer Science (1988), an M.Sc. in Management Information Systems (2005) and a Ph.D. in Business Information Systems (2013). He previously spent fifteen years working in the telecommunications sector. Dr Woodworth has worked on a number of health informatics research projects, in particular the tracking of hypertension in pregnant women in the home with a view to developing an early warning of pre-eclampsia, and a large scale project to provide mobile apps and decision support and learning tools to better manage large scale disasters (including COVID-19) in a European context. Current projects include the deployment of an electronic birth registry in Tanzania.
Paidi O’Raghallaigh
Paidi O’Reilly is Chair of the Digital Transformation Lab at Cork University Business School (CUBS) in University College Cork (Ireland). He is co-director of the 'Innovation through Design Thinking' postgraduate programme delivered jointly between CUBS and the School in Education at UCC. He was previously a Research Fellow in the SFI-funded INFANT Research Centre in UCC. Dr O’Reilly is on the global lecturing panel at the Irish Management Institute in Dublin. He has over twenty-five years of experience working on innovation and research projects in a range of industries, including finance, engineering and healthcare. In 2011, Dr O’Reilly was awarded a PhD for his research into the innovation strategies of organisations. He has published his work in leading academic and practitioner outlets.
Frédéric Adam
Frédéric Adam is Professor of MIS in the Department of Business Information Systems in Cork University Business School (University College Cork, Ireland). He also holds doctorates from the National University of Ireland and Université Paris VI (France). His research interests are focused on decision support systems, both in business and in the medical area, in which he has led a number of projects aimed at developing decision support artefacts for clinicians. He has also published extensively in the enterprise resource planning (ERP) area. His research has appeared in the Journal of Information Technology, the Journal of Strategic Information Systems, Decision Support Systems, the Journal of Medical Internet Research, and Information and Management. He is a principal investigator (PI) and a founding PI in the INFANT research centre, where he leads the Connected Health stream. An elected governor of University College Cork since 2011, Prof. Adam also sits on its finance committee.
References
- Ackoff, R.L. (1967). Management misinformation systems. Management Science, 14(4), 147–156. https://doi.org/10.1287/mnsc.14.4.B147
- Adam, F., & Dempsey, E. (2020). Intuition in decision making-Risk and opportunity. Journal of Decision Systems, 29(sup1), 98–116. https://doi.org/10.1080/12460125.2020.1848375
- Aguilera, A. (2015). Digital technology and mental health interventions: Opportunities and challenges. Arbor, 191(771), a210–a210. https://doi.org/10.3989/arbor.2015.771n1012
- Areán, P.A., Ly, K.H., & Andersson, G. (2022). Mobile technology for mental health assessment. Dialogues in Clinical Neuroscience, 18(2), 163–169. https://doi.org/10.31887/DCNS.2016.18.2/parean
- Balanda, K., Barron, S., Fahy, L., & McLaughlin, A. (2010). Making chronic conditions count: Hypertension, stroke, coronary heart disease, diabetes. A systematic approach to estimating and forecasting population prevalence on the island of Ireland. Institute of Public Health in Ireland.
- Bellomo, G., Narducci, P.L., Rondoni, F., Pastorelli, G., Stagnoni, G., Angeli, G., & Verdecchia, P. (1999). Prognostic value of 24-hour blood pressure in pregnancy. JAMA, 282(15), 1447–52. https://doi.org/10.1001/jama.282.15.1447
- Berg, C.J., Chang, J., Callaghan, W., & Whitehead, S.J. (2003). Pregnancy related mortality in the United States, 1999-1997. Obstetrics and Gynaecology, 101(2), 289–296. https://doi.org/10.1016/S0029-7844(02)02587-5
- Brown, M.A., Mangos, G., Davis, G., & Homer, C. (2005). The natural history of white coat hypertension during pregnancy. BJOG: An International Journal of Obstetrics and Gynaecology, 112(5), 601–606. https://doi.org/10.1111/j.1471-0528.2004.00516.x
- Brownson, R.C., Gurney, J.G., & Land, G.H. (1999). Evidence-based decision making in public health. Journal of Public Health Management and Practice, 5(5), 86–97. https://doi.org/10.1097/00124784-199909000-00012
- Byrne, P., & Rosen, A. (Eds.). (2014). Early intervention in psychiatry: EI of nearly everything for better mental health. John Wiley & Sons.
- Carney, S. (1997). 24-hour blood pressure monitoring: What are the benefits? Australian Prescriber, 20(1), 18–20. https://doi.org/10.18773/austprescr.1997.010
- Caulfield, B.M., & Donnelly, S.C. (2013). What is connected health and why will it change your practice? QJM: An International Journal of Medicine, 106(8), 703–707. https://doi.org/10.1093/qjmed/hct114
- Central Intelligence Agency (Ed.). (2011). The World Factbook 2011.
- Crowley, J., Heavin, C., Keenan, P., & Power, D. (2020). CDSS and DSS: Shared roots and divergent paths. Journal of Decision Systems, 29(sup1), 71–78. https://doi.org/10.1080/12460125.2020.1811446
- Dubicka, B., & Bullock, T. (2017). Mental health services for children fail to meet soaring demand. BMJ, 358. https://doi.org/10.1136/bmj.j4254
- English, F.A., Kenny, L.C., & McCarthy, F.P. (2015). Risk factors and effective management of preeclampsia. Integrated Blood Pressure Control, 8, 7. https://doi.org/10.2147/IBPC.S50641
- Finkelstein, E.A., Haaland, B.A., Bilger, M., Sahasranaman, A., Sloan, R.A., Nang, E.E.K., & Evenson, K.R. (2016). Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): A randomised controlled trial. The Lancet Diabetes & Endocrinology, 4(12), 983–995. https://doi.org/10.1016/S2213-8587(16)30284-4
- Harris, R.E. (2019). Epidemiology of chronic disease: Global perspectives. Jones & Bartlett Learning.
- Health Service Executive. (2010). Maternity and Infant Care Scheme. Accessed 08 August 2012. http://www.hse.ie/eng/services/Find_a_Service/maternity/combinedcare.html
- Jakicic, J.M., Davis, K.K., Rogers, R.J., King, W.C., Marcus, M.D., Helsel, D., Rickman, A.D., Wahed, A.S., & Belle, S.H. (2016). Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. Jama, 316(11), 1161–1171. https://doi.org/10.1001/jama.2016.12858
- Jindal, F., Jamar, R., & Churi, P. (2018). Future and challenges of internet of things. AIRCC’s International Journal of Computer Science and Information Technology, 10(2), 13–25. https://doi.org/10.5121/ijcsit.2018.10202
- Kapoor, A., Guha, S., Das, M.K., Goswami, K.C., & Yadav, R. (2020). Digital healthcare: The only solution for better healthcare during COVID-19 pandemic? Indian Heart Journal, 72(2), 61–64. https://doi.org/10.1016/j.ihj.2020.04.001
- Kelly, B.D. (2015). Revising, reforming, reframing: Report of the Expert Group on the Review of the Mental Health Act 2001 (2015). Irish Journal of Psychological Medicine, 32(2), 161–166.
- Kido, K., & Tsukamoto, K. (2020). Japan’s health care system faces a perfect storm. The International Journal of Health Planning and Management, 35(1), e210–e217. https://doi.org/10.1002/hpm.2936
- Klein, G.A. (1993). A recognition-primed decision (RPD) model of rapid decision making. In G. A. Klein, J. Orasanu, R. Calderwood, & C. E. Zsambok (Eds.), Decision making in action, models and methods (pp. 38–147). Ablex.
- Koukouris, K. (1994). Constructed case studies: Athletes’ perspectives of disengaging from organized competitive sport. Sociology of Sport Journal, 11(2), 114–139. https://doi.org/10.1123/ssj.11.2.114
- Kvedar, J., Coye, M.J., & Everett, W. (2014). Connected health: A review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Affairs, 33(2), 194–199. https://doi.org/10.1377/hlthaff.2013.0992
- Kvedar, J.C., Colman, C., & Cella, G. (2015). The internet of healthy things. Partners Healthcare Connected Health, Boston.
- Lal, A., Erondu, N.A., Heymann, D.L., Gitahi, G., & Yates, R. (2021). Fragmented health systems in COVID-19: Rectifying the misalignment between global health security and universal health coverage. The Lancet, 397(10268), 61–67. https://doi.org/10.1016/S0140-6736(20)32228-5
- Ludwig, D.S. (2011). Technology, diet, and the burden of chronic disease. Jama, 305(13), 1352–1353. https://doi.org/10.1001/jama.2011.380
- McCarthy, S., O’Raghallaigh, P., Woodworth, S., Lim, Y.L., Kenny, L.C., & Adam, F. (2016). An integrated patient journey mapping tool for embedding quality in healthcare service reform. Journal of Decision Systems, 25(sup1), 354–368. https://doi.org/10.1080/12460125.2016.1187394
- McCarthy, S., O’Raghallaigh, P., Woodworth, S., Lim, Y.Y., Kenny, L.C., & Adam, F. (2019). The “Integrated Patient Journey Map”: A design tool for embedding the pillars of quality in health information technology solutions (Preprint). JMIR Human Factors.
- Morgan, C., Webb, R.T., Carr, M.J., Kontopantelis, E., Green, J., Chew-Graham, C.A., Ashcroft, D.M. (2017). Incidence, clinical management, and mortality risk following self harm among children and adolescents: Cohort study in primary care. BMJ, 359. https://doi.org/10.1136/bmj.j4351
- Nambiar, A.R., Reddy, N., & Dutta, D. (2017, December). Connected health: Opportunities and challenges. In 2017 IEEE international conference on big data (Big Data) (pp. 1658–1662). IEEE.
- Nugent, R.A., Fathima, S.F., Feigl, A.B., & Chyung, D. (2011). The burden of chronic kidney disease on developing nations: A 21st century challenge in global health. Nephron Clinical Practice, 118(3), c269–c277. https://doi.org/10.1159/000321382
- O’Brien, E. (2008). Ambulatory blood pressure measurement: The case for implementation in primary care. Hypertension, 51(6), 1435–1441. https://doi.org/10.1161/HYPERTENSIONAHA.107.100008
- O’Raghallaigh, P., & Adam, F. (2017). A framework for designing digital health interventions. Journal of the Midwest Association for Information Systems (JMWAIS), 2017(2), 41–56. https://doi.org/10.17705/3jmwa.00030
- Payton, F., Pare, G., Le Rouge, C., & Reddy, M. (2011). Health care IT: Process, people, patients and interdisciplinary considerations. Journal of the Association for Information Systems, 12(2), I–XIII. https://doi.org/10.17705/1jais.00259
- Perry, I.J., Wilkinson, L.S., Shinton, R.A., & Beevers, D.G. (1991). Conflicting views on the measurement of blood pressure in pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology, 98(3), 241–243.
- Proudfoot, J., Ryden, C., Everitt, B., Shapiro, D.A., Goldberg, D., Mann, A., Tylee, A., Marks, I., & Gray, J.A. (2004). Clinical efficacy of computerised cognitive-behavioural therapy for anxiety and depression in primary care: Randomised controlled trial. The British Journal of Psychiatry, 185(1), 46–54. https://doi.org/10.1192/bjp.185.1.46
- Rabson, L.R. (1967). The one hundredth annual meeting of the C.M.A.: Medical care insurance and medical manpower conference. Canadian Medical Association Journal, 97(9), 484–489. PMC1923244
- Rozenblum, R., & Bates, D.W. (2013). Patient-centred healthcare, social media and the internet: The perfect storm? BMJ Quality & Safety, 22(3), 183–186. https://doi.org/10.1136/bmjqs-2012-001744
- Sibai, B.M. (1996). Treatment of hypertension in pregnant women. The New England Journal of Medicine, 335(4), 257–265.
- Staessan, J.A., Lawrie, B., Gianfranco, P., Waeber, B., & White, W. (1999). Task force IV: Clinical use of ambulatory blood pressure monitoring. Participants of the 1999 consensus conference on ambulatory blood pressure monitoring. Blood Pressure Monitoring, 4(6), 319–331. https://doi.org/10.1097/00126097-199912000-00005
- Topol, E. (2015). The patient will see you now: The future of medicine is in your hands. Basic Books.
- Waugh, J., Bosio, P., Habiba, M., Boyce, T., Shennan, A., & Halligan, A. (2001). Home monitoring of blood pressure in pregnancy at high risk of pre-eclampsia. European Journal of Obstetrics, Gynaecology and Reproductive Biology, 99(1), 109–111. https://doi.org/10.1016/S0301-2115(01)00353-0
- World Health Organization. (2003). Investing in mental health. World Health Organization. Retrieved March 30, (2003). https://apps.who.int/iris/handle/10665/42823
- Zackery, A., Zolfagharzadeh, M.M., & Hamidi, M. (2022). Policy implications of the concept of technological catch-up for the management of healthcare sector in developing countries. Journal of Health Management, 1–12. https://doi.org/10.1177/09720634221076964