Abstract
The embryo growth, endosperm degradation and in situ activity of peroxidase and esterase in seeds imbibed in distilled water or 200mM NaCl were investigated in the halophyte Crithmum maritimum L. Germination was maximal (90%) in distilled water, but was fully inhibited following seed exposure to salt. The completion of the embryo growth (ca. 2mm length) leading to the radicle emergence took 6 d in distilled, but was markedly delayed in 200mM NaCl. The endosperm degradation was markedly delayed by NaCl. Esterase and peroxidase activities were less important in NaCl-imbibed than in H2O-imbibed seeds. The adverse effect of salinity on the embryo growth and the endosperm degradation could partly explain the inhibition of seed germination observed under these circumstances.
Résumé
La croissance embryonnaire, la dégradation de l’albumen et l’activité enzymatique de l’estérase et de la pé-roxydase sont étudiés au cours de la germination des graines de Crithmum maritimum L. imbibées dans de l’eau distillée ou dans une solution de 200mM NaCl. Le pourcentage final de germination atteint 90% dans l’eau distillée, mais la salinité inhibe complètement la germination. La croissance embryonnaire est achevée après 6 jours dans l’eau distillée, mais elle est réduite en présence du sel. L’observation par microscope électronique à balayage montre une grande dégradation de l’albumen pour les graines imbibées dans l’eau distillée; par contre, la présence du sel retarde ces modifications. Les activités enzymatiques de l’estérase et de la péroxydase sont réduites par le sel. En conclusion, la salinité retarde la mobilisation des réserves et inhibe la croissance embryonnaire, ce qui pourrait expliquer l’action inhibitrice de sel sur la germination chez C. maritimum L.
Introduction
In endospermic seeds, the reserve tissues are represented by the endosperm. In these species, the embryo is often rudimentary and embedded in the endosperm, and exhibits a morphological dormancy (Nikolaeva, Citation1977; Homrichhausen et al., Citation2003). In this type of seeds, several anatomical and biochemical modifications occur during the imbibition phase, leading to the radicle emergence. The germination phase is preceded by the embryo elongation within the seeds (Nikolaeva, Citation1977; Baskin & Baskin, Citation2004), which is accompanied by the endosperm degradation (Homrichhausen et al., Citation2003). In addition, some enzymes including endo-β-mannanase, amylases, proteases, lipases, esterases, catalase and peroxidases are activated during seed imbibition (Palmiano & Juliano, Citation1973; Ben Miled & Cherif, Citation1991; Ashraf et al., Citation2002; Homrichhausen et al., Citation2003). However, these processes seem to be salt-sensitive, since salt-imbibed seeds show substantial delay in reserve mobilization (Ashraf et al., Citation2002; Sebei et al., Citation2007; Voigt et al., Citation2009).
In germinating seeds, peroxidase activity has been localized in the tegument of Brassica juncea L. (Czern.) (Le Beller et al., Citation1986), the micropylar endosperm of tomato (Morohashi, Citation2002), and the cotyledons of Brassica oleracea L. (Bellani et al., Citation2002). In the seeds of Raphanus sativus L., germination is accompanied by a release of reactive oxygen species (ROS) and high peroxidase activity (Schopfer et al., Citation2001). Thus, there is experimental evidence for the involvement of peroxidases in the reserve mobilization in seeds. Several forms of esterase have been identified in the plant tissues, among which seeds (Chandra & Toole, Citation1977; Staubmann et al., Citation1999; Fahmy et al., Citation2008), leaves (Rudolph & Stahmann, Citation1966), roots (Carino & Montgomery, Citation1968) and tubers (Hou et al., Citation1999). In Lactuca sativa L. the esterase is highly released from the germinated seeds (Chandra & Toole, Citation1977). Esterase attacks the fatty-acyl linkage of water-insoluble triacylglycerols (Fahmy et al., 2004). Thus, both the peroxidase and the esterase have been shown to be involved in the reserve mobilization.
Crithmum maritimum L. (Apiaceae), a perennial halophyte thriving among rocky coastal ecosystems, is potentially useful for economical and medicinal purposes. Indeed, its seeds are rich in oil (up to 35% DW in Tunisian accessions) with a fatty acid composition close to that of olive oil (Zarrouk et al., Citation2003). Further, its leaves display high antioxidant and antimicrobial activities (Meot-Duros et al., Citation2008; Meot-Duros & Magné, Citation2009). The seed of this species is characterized by an under-developed embryo, which achieves its growth within the seed before the germination can start. Salt response of most halophyte species is analogous to that of glycophytes at the germinative stage, showing maximal germination in salt-free media, and being strongly inhibited by increasing salinity (Debez et al., Citation2004; Tobe et al., Citation2004; Joshi et al., Citation2005; Easton & Kleindorfer, Citation2008). In previous investigations, we found that seed germination capacity of Crithmum maritimum L. was unaffected in the 0-100mM NaCl range, but was severely reduced at higher salinity levels (Atia et al., Citation2006; Meot-Duros & Magné, Citation2008). One may hypothesize that the salt-induced inhibition of germination in this halophyte could result from (i) the restriction of the embryo growth and/or (ii) the delay in seed reserve mobilization, due to the impairment of some key enzymes, such as peroxidase and esterase. Therefore, the present study focuses on the impact of salinity (200mM NaCl) on the embryo growth and the endosperm degradation, and addresses the possible implication of esterase and peroxidases in these processes.
Material and methods
Fruit harvesting, seed preparation and germination conditions
Mature fruits were collected in December 2007 from Tabarka (N-W of Tunisia, humid Mediterranean climate) and stored dry under laboratory conditions (18-23 °C) until their utilization in January 2008. Seeds, from which the spongy coat was removed, were disinfected in a 3.5% calcium hypochlorite solution for 5 mn. before beginning the germination tests. Then, 25 seeds were placed in 9 cm Petri dish on a double layer of filter paper (Filtrak type) moistened either with distilled water or with a solution containing 200mM NaCl. For each treatment, four replicates (Petri dishes) were used. Petri dishes were sealed with transparent plastic film to prevent any evaporation. The germination test was carried out in a growth chamber at 18-23 °C (night-day) temperature regime and illuminated by five lamps (OS-RAM Type, fluence of 25 μmol m-2 s-1, 400-700 nm) with 8 h-dark/16 h-light regime.
Microscopy and histochemical analysis
To monitor changes in the endosperm aspect, hand sections from dry seeds and seeds imbibed for 3 days or 6 days either in distilled water or in salt solution (200mM NaCl), were observed under scanning electron microscope (SEM) (FEI Quanta 200 type). For the study of embryo length, dry seeds or seeds imbibed for 3 days or 6 days were dissected under stereomicroscope and the embryo was isolated and fixed in a paraformalfdehyde solution (2%) containing phosphate buffer (pH 7) and stored at 4 °C. Then, the embryo length was determined under light microscope (Olympus DX41 type).
For the localization of peroxidase in the endosperm by in situ activity assay, free hand sections were incubated at 20 °C for 60 min in a O-dianisidine saturated solution and 0.01% H2O2 solution prepared in phosphate buffer (pH 6). The reaction was stopped by placing the section in distilled water (Bellani et al., Citation2002). In the case of esterase, sections from the same samples were incubated at 37 °C for 120 min in 0.015% α-naphtylacetate and 0.015% Fast Blue RR salt solutions (pH 6). To stop the reaction, the sections were placed in distilled water (Chandra and Toole Citation1977). For each enzyme, the control consisted of withdrawing the substrate; the stained sections were immediately observed and photographed under stereomicroscope (Type Leica) or under light microscope.
Results
Fruit structure and seed germination capacity
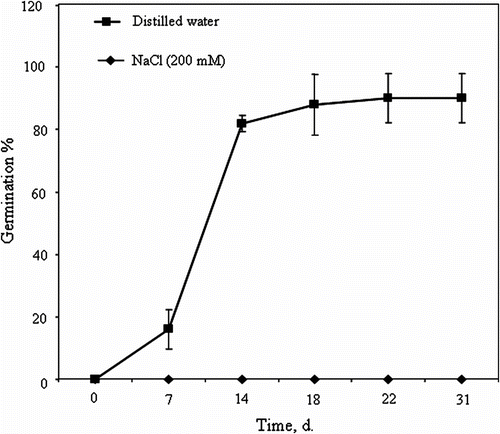
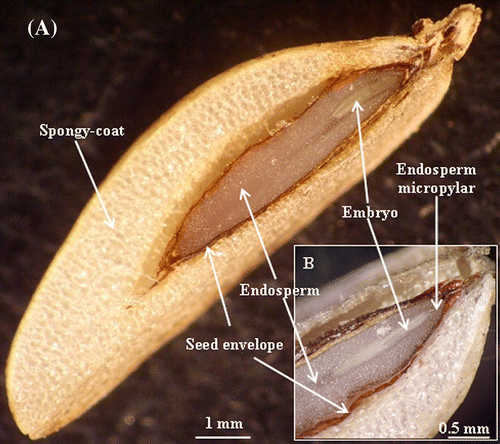
C. maritimum fruit is a schizocarp divided into two mericarps. The mericarp consists of a spongy outer coat, a secretory envelope and an endocarp delimiting a large endosperm and a rudimentary embryo located in the mi-cropylar region (Fig. and 1B). At complete fruit ripe-ning, the mesocarp is reduced to an easily-removable spongy coat. However, the endocarp and the secretory envelope, which form the seed envelope, remain firmly attached to the endosperm, i.e. fused to the seed. Therefore, in this study, the term “seed” refers to seed plus endocarp and secretory envelope (Fig. A). The germination of seeds sown in distilled water started after 7 days and reached about 82% after 14 days. After 22 days, the germination is maximal (90%) (Fig. ). In comparison, NaCl-salinity (200mM) completely inhibited the germination over time (Fig. ).
Fig. 1 Stereomicroscope micrographs of cross section of Crithmum maritimum mature fruit. (A) The mericarp is composed of a spongy outer coat, a secretory envelope, delimiting the large endosperm, and the rudimentary embryo. (B) A detailed view of the embryo region showing the endosperm micropylar and the embryo. Fig. 1 Micrographie réalisée suite à l’observation à la loupe binoculaire d’une coupe transversale du fruit de Crithmum maritimum: (A) Aspect général du fruit qui montre la graine entouré de l’enveloppe spongieuse et qui contient un large albumen et un embryon de taille réduite. (B) Détail de la partie embryonnaire montrant l’albumen micropylaire et l’embryon.
Embryo growth following imbibition in water or in NaCl
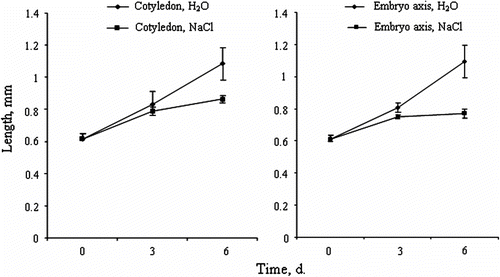
At full ripening seed (ca. 4mm long), the embryo is about 1mm long. After 3 days of seed imbibition in distilled water, the cotyledon and the embryo axis length increased from 0.58mm and 0.62mm respectively, to 0.8mm for both tissues (Fig. and 3B). For seeds imbibed in 200mM NaCl, the growth of cotyledon and the embryo axis is less important (0.75mm and 0.70mm, respectively). After 6 days of imbibition in distilled water, the cotyledon and the embryo axis length is 1.08mm and 1.05mm respectively, whereas it is only 0.85mm for the cotyledon and 0.75mm for the embryo axis in salt-imbibed seeds (Fig. and 3B).
Fig. 3 Crithmum maritimum embryo growth within the seeds during imbibition in distilled water or 200mM NaCl. (A) Cotyledon growth over time. (B) Axis growth over time (means ± SE). Fig. 3 Croissance de l’embryon à l’intérieur de la graine durant l’imbibition des graines de Crithmum maritimum en présence d’eau distillée ou d’une solution de 200mM NaCl. (A) Croissance des cotylédons au cours de temps. (B) Croissance des l’axe embryonnaire (moyennes ± ES).
Changes in endosperm aspect following imbibition in water or in NaCl
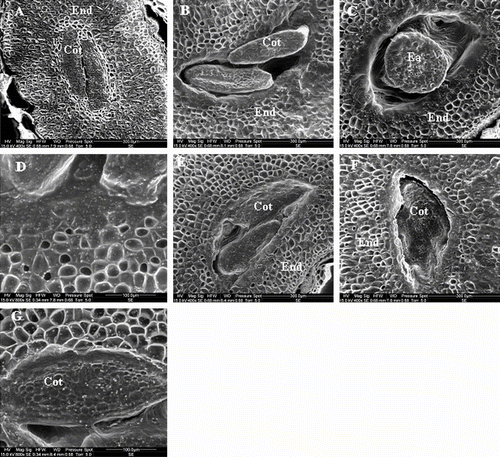
Transversal sections of a dry seed show the presence of a very large endosperm tissue surrounding the cotyledon, each cell of this tissue being filled with reserve globoids (Fig. A). After 3 days of seed imbibition in distilled water, the degradation of the endosperm is clearly visible in the region of the cotyledon, and the cell walls are hydrolysed (Fig. and 4D). In comparison, the endosperm of the seeds imbibed in NaCl remains intact (Fig. and 4G). The degradation of the endosperm surrounding the embryo is more pronounced after 6 days of seed imbibition in H2O (Fig. C), as compared to salt-imbibed seeds (Fig. F).
Fig. 4 SEM micrographs showing the endosperm degradation during imbibition: (A) Endosperm of non imbibed seeds (control). (B) Seed imbibed in distilled water for 3 days. (C) Seed imbibed in distilled water for 6 days. (D) Seed imbibed in distilled water during 3 days. (E) Seed imbibed in 200mM NaCl for 3days. (F) Seed imbibed in 200 mM NaCl for 6 days. (G) Seed imbibed in 200 mM NaCl for 3 days. All sections were realized at the same level of upper quarter of seed. Cot: cotyledon; End: endosperm; Ea: embryo axis. Fig. 4 Micrographies obtenues par microscopie électronique à balayage, illustrant la dégradation de l’albumen au cours de l’imbibition des graines de Crithmum maritimum (A) Coupe d’une graine sèche non imbibée. (B) Coupe d’une graine imbibée pendant 3 jours dans l’eau. (C) Coupe d’une graine imbibée pendant 6 jours dans l’eau. (D) Détails de la zone adjacente aux cotylédons d’une graine imbibée pendant 3 jours dans l’eau. (E) Coupe d’une graine imbibée en présence d’une solution de 200 mM NaCl pendant 3 jours. (F) Coupe d’une graine imbibée dans 200 mM NaCl pendant 6 jours. (G) Graine imbibée pendant 3 jours en présence d’une solution de 200 mM NaCl.
In situ localization of esterase and peroxidase in the endosperm
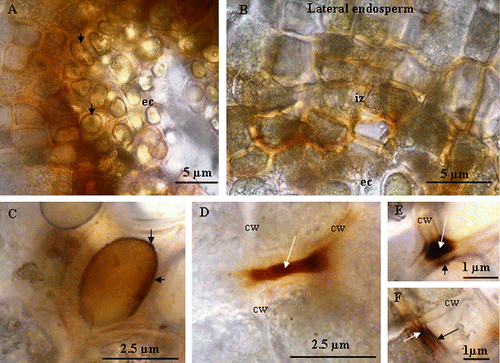
Using stereomicroscope, esterase activity is mainly detected in the degraded zone of the endosperm (Fig. A). This activity is less important in NaCl-imbibed as compared to H2O-imbibed seeds (Fig. and 5B). In the same regions, a strong peroxidase activity is also observed (Fig. C). Similar to esterase, peroxidase activity is substantially reduced in NaCl-imbibed seeds (Fig. and 5D). Light microscope (LM) observations showed that esterase activity is localized at the vicinity of the embryo cavity, i.e. the region of the endosperm degradation (Fig. A). This activity is also intense in the oil globoids (Fig. B). Light microscope observations of O-dianisidine- and H2O2-stained sections revealed a strong peroxidase activity in the oil globoids and in the intercellular space in the degraded zone of endosperm (Fig. , 7B, and 7C). In the lateral endosperm, the peroxidase activity is mainly observed in the cell wall and in the plasma membrane (Fig. , 7E, and 7F).
Fig. 5 Stereomicroscope micrographs showing the in situ activity of esterase and peroxidase. (A) Localization of esterase activity in seeds imbibed in distilled water for 6 days. ∗ embryo cavity ∗∗ lateral endosperm. (B) Localization of esterase activity in seeds imbibed in 200mM NaCl for 6 days. (C) Localization of peroxidase activity in seeds imbibed in distilled water for 6 days. (D) Localization of peroxidase activity in endosperm seeds imbibed in 200mM NaCl for 6 days. Fig. 5 Micrographies obtenues sous loupe binoculaire, montrant l’activité enzymatiques de l’ésterase et de la peroxydase. (A) Localisation de l’activité estérase dans les graines imbibées dans l’eau distillée. ∗ cavité embryonnaire, ∗∗ l’albumen latéral. (B) Localisation de l’activité estérase dans les graines imbibées dans une solution de 200 mM NaCl pendant 6 jours (C) Localisation de l’activité peroxydase dans les graines imbibées dans de l’eau distillée. ∗ cavité embryonnaire, ∗∗ l’albumen latéral. (D) Localisation de l’activité peroxydase dans les graines imbibées dans une solution de 200 mM NaCl pendant 6 jours.
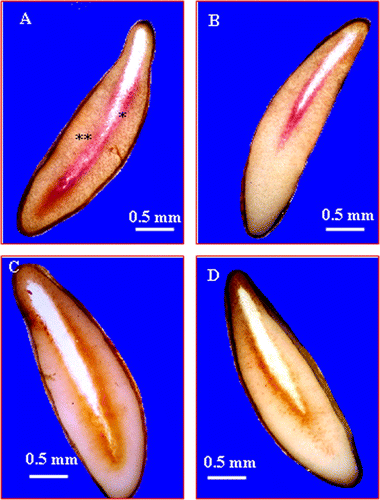
Fig. 6 (A) Light microscope micrograph of Crithmum maritimum showing the esterase activity at the vicinity of embryo cavity (ec). (B) Detail of light microscope micrograph showing the esterase activity in the oil globoid (og) at the vicinity of embryo cavity (ec). Fig. 6 Micrographie par microscope photonique, montrant l’activité estérase dans l’albumen des graines imbibées pendant 6 jours dans l’eau distillée (A) au niveau de la cavité embryonnaire (ec) et de l’albumen périphérique. (B) Micrographie par microscopie photonique montrant la localisation de l’activité estérase au niveau des globules lipidiques (og).
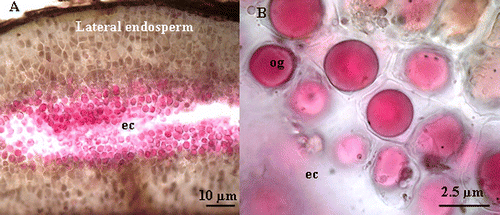
Fig. 7 Light microscope micrographs showing the peroxidase activity in the endosperm of Crithmum maritimum L. (A) localization of peroxidase activity at the vicinity of embryo cavity in the reserve globoids. (B) Localization of peroxidase activity in an intermediary zone (iz) between the lateral endosperm and the embryo cavity (ec). Note that the activity is mainly located in intercellular space. (C) Details of an oil globoid showing the peroxidase activity (arrows). (D) Localization of peroxidase activity in cell wall of lateral endosperm (arrow). (E) and (F) localization of peroxidase activity in lateral endosperm; the white arrow indicates the activity in the cell wall (cw) and the black arrow indicates the activity in the plasma membrane. Fig. 7 Micrographie obtenue par microscopie photonique, montrant l’activité de péroxydase dans l’albumen des graines imbibées pendant 6 jours dans l’eau distillée. (A) Au niveau des globules lipidiques (flèche) tout près de la cavité embryonnaire (ce) et (B) au niveau du milieu intercellulaire dans une zone intermédiaire (iz) entre la cavité embryonnaire et l’albumen périphérique. (C) Détail d’un globule lipidique montrant une forte activité peroxydasique (flèche) tout près de la cavité embryonnaire. (D) et (E) localisation de l’activité de péroxydase au niveau du milieu intercellulaire et de la paroi cellulaire de l’albumen périphérique (flèche).
Discussion
Distilled water is optimal for the germination of Crithmum maritimum, the germination percentage reaches 90% after 22 days. However, seeds sown in NaCl at 200mM fail to germinate after 31 days. These results confirm previous studies either on this species (Atia et al., Citation2006) or on other halophytes (Debez et al., Citation2004; Joshi et al., Citation2005; Easton & Kleindorfer, Citation2008). How salinity inhibits the germination is still controversial, but it is known that salt impacts this process, essentially through its osmotic and/or ionic components (Tobe et al., Citation2004; Sosa et al., Citation2005).
Several species present morphologically dormant seeds. In this case, the embryo must elongate and reach a critical length before the radicle protrusion (Nikolaeva, Citation1977). In Crithmum maritimum seeds, the embryo is underdeveloped and exhibits a morphological dormancy. Following the imbibition of Crithmum maritimum seeds in distilled water (optimal conditions for the germination), the embryo grows successfully, and reaches 2mm of length at 6 days, which is about half of the seed length. The same behaviour has been reported in carrot seeds (Homrichhausen et al., Citation2003). As seed imbibition in 200mM NaCl led to a marked delay of the embryo growth; this leads to think that supra-optimal salinity may inhibit the germination of Crithmum maritimum by extending the period required by the embryo to reach the critical level of growth, so that the germination can take place. This is of high significance for halophytes, for which any delay of this process could adversely impact the establishment capacity under salt conditions (Easton & Kleindorfer, Citation2008). In addition, when the time of imbibition in NaCl solution is prolonged, the seeds accumulate high concentrations of ions (data not shown), which may have also impaired key biochemical and physiological processes, hence delaying the mobilization of seed reserves and inhibiting the radicle emergence.
In Crithmum maritimum, the endosperm degradation is more important in H2O-imbibed than in salt-imbibed seeds. Accordingly, NaCl-salinity has been reported to delay the reserve mobilization in Linum usitatissimum L. (Sebei et al., Citation2007) and in Anacardium occidentale L. (Voigt et al., Citation2009). In addition, the most significant changes occurred in the embryo cavity, as observed in the endosperm of carrot seeds (Homrichhausen et al., Citation2003). These data strongly suggest that the embryo may at least partly control the degradation of endosperm in endospermic seeds.
Several enzymes such as amylases, proteases, phosphatases, catalase, lipases and esterases have been shown to be involved in the seed reserve mobilization (Palmiano & Juliano, Citation1973; Ben Miled & Cherif, Citation1991; Ashraf et al., Citation2002; Staubmann et al., Citation1999). In the degraded zone of Crithmum maritimum endosperm, high peroxidase activity is detected. This activity is more important in distilled water-imbibed seeds than in salt-imbibed seeds. Consistent with our findings, peroxidase activity has been also found in germinating seeds of Raphanus sativus (Shopher et al., 2001). Interestingly, a high peroxidase activity in the cotyledons of Brassica oleracea is concomitant with the initiation of reserve mobilization (Bellani et al., Citation2002). In Crithmum maritimum, the peroxidase activity is essentially localized in the cell wall of the endosperm and in the oil globoids, suggesting that this enzyme could be implicated in the endosperm degradation.
High activity of esterase, the enzyme which catalyzes the hydrolysis of carboxylic esters of short-chain fatty acids, is detected in the degraded zone of the endosperm related to oils globoids. It is noteworthy that high este-rase activity is observed in seeds of Jatropha curcas L. (Staubmann et al., Citation1999) and Cucurbita pepo L. (Fahmy et al., Citation2008). In Lactuca sativa, the seed germination is concomitant with a high release of esterase (Chandra & Toole, Citation1977). These findings strengthen the assumption of esterase involvement in the lipid reserve mobilization of C. maritimum seeds. Esterase activity is markedly impaired in salt-imbibed seeds as compared to distilled water-imbibed seeds. Thus, salt may have delayed the degradation of the endosperm by inhibiting some enzymes such as peroxidase and esterase. Similarly, Ben Miled and Cherif (Citation1991) showed that NaCl salinity delayed the reserve mobilization by decreasing the enzyme activity of catalase, isocitrate lyase and malate synthetase in germinating seeds of Medicago species when challenged with salinity.
In Crithmum maritimum seeds imbibed in distilled water (optimal medium for the germination), the embryo requires 6 days to grow before germination can start. Yet, this process appears to be salt-sensitive. In the same way, the endosperm degradation, which is more pronounced in the embryo cavity, is delayed by salt exposure. Peroxidase and esterase activities, mainly localized in the reserve tissues, may play a potent role in the endosperm degradation. NaCl salinity decreased substantially the activity of both enzymes, which may partly explain the delayed reserve mobilization, hence impairing the embryo growth and thereby the germination.
References
- Ashraf , M.Y. , Afaf , R. , Qureshi , M.S. and Naqvi , M.H. 2002 . Salinity induced changes in α-amylase and protease activities and associated metabolism in cotton varieties during germination and early seedling growth stages . Acta Physiol. Plant , 24 : 37 – 44 .
- Atia , A. , Ben Hamed , K. , Debez , A. and Abdelly , C. 2006 . “ Salt and seawater effects on the germination of Crithmum maritimum L ” . In Biosaline agriculture and salinity tolerance in plants , Edited by: Özturk , M. , Waisel , Y. , Khan , M.A. and Görk , G. 29 – 33 . Switzerland : Birkhauser Verlag .
- Baskin , J.M. and Baskin , C.C. 2004 . A classification system for seed dormancy . Seed Sci. Res , 14 : 1 – 16 .
- Bellani , L. , Guarnieri , M. and Scialabba , A. 2002 . Differences in the activity and distribution of peroxidase from three different portions of germinating Brassica oleracea seeds . Physiol. Plant , 114 : 102 – 108 .
- Ben , Miled D. and Cherif , A. 1991 . Effet du NaCl sur l’utilisation des lipides et les activités enzymatiques glyoxysomales au cours de la germination de deux espèces de Medicago . Can. J. Bot , 70 : 876 – 883 .
- Carino , L.A. and Montgomery , M.W. 1968 . Identification of some soluble esterases of the carrot (Daucus carota L.) . Phytochemistry , 7 : 1483 – 1490 .
- Chandra , G.R. and Toole , V.K. 1977 . Release of Esterase Following Germination of Lettuce Seed (Lactuca sativa L.) . Plant Physiol , 59 : 1055 – 1058 .
- Debez , A. , Ben Hamed , K. , Grignon , C. and Abdelly , C. 2004 . Effect on germination, growth and seed production of the halophyte Cakile maritima . Plant Soil , 262 : 179 – 189 .
- Easton , L.C. and Kleindorfer , S. 2008 . Effects of salinity levels and seed mass on germination in Australian species of Frankenia L. (Frankeniaceae) . Environ. Exp. Bot , 65 : 345 – 352 .
- Fahmy , A.S. , Abo-Zeid , A.Z. , Mohamed , T.M. , Ghanem , H.M. , Borai , I.H. and Mohamed , S.A. 2008 . Characterization of esterases from Cucurbita pepo cv. “Eskandrani” . Bioresource Technol , 99 : 437 – 443 .
- Homrichhausen , T.M. , Hewitt , J.R. and Nonogaki , H. 2003 . Endo-β-mannanase activity is associated with the completion of embryogenesis in imbibed carrot (Daucus carota) seeds . Seed Sci. Res , 13 : 219 – 227 .
- Hou , W.C. , Chen , H.J. , Chang , C.F. and Lin , Y.H. 1999 . Purification and properties of fatty acid esterases from yam (Dioscorea batatas Decne) tuber . Bot. Bull. Acad. Sinica , 40 : 305 – 310 .
- Joshi , A.J. , Mali , B.S. and Hinglajia , H. 2005 . Salt tolerance at germination and early growth of two forage grasses growing in marshy habitats . Environ. Exp. Bot , 54 : 267 – 274 .
- Le Beller , D. , Picque , E. and Demignot , S. 1986 . “ Peroxidase localization in the seeds of Brassica juncea ” . In molecular & physiological aspect of plant peroxidases , Edited by: Greppin , H. , Penel , C. and Gaspar , T. 451 – 454 . Geneva , Switzerland : University of Geneva .
- Meot-Duros , L. , Le Floch , G. and Magné , C. 2008 . Radical scavenging, antioxidant and antimicrobial activities of halophytic species . J. Ethnopharmacol. , 116 : 258 – 262 .
- Meot-duros , L. and Magné , C. 2008 . Effect of salinity and chemical factors on seed germination in the halophyte Crithmum maritimum L . Plant Soil , 313 : 83 – 87 .
- Meot-duros , L. and Magné , C. 2009 . Antioxidant activity and phenol content of Crithmum maritimum L. leaves . Plant Physiol. Biochem , 47 : 37 – 41 .
- Morohashi , Y. 2002 . Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radicle protrusion . J. Exp. Bot , 53 : 1643 – 1650 .
- Nikolaeva , M.G. 1977 . “ Factors controlling the seed dormancy pattern ” . In The physiology and biochemistry of seed dormancy and germination , Edited by: Kahn , A.A. 51 – 74 . Amsterdam : North-Holland .
- Palmiano , E.P. and Juliano , B.O. 1973 . Changes in the activity of some hydrolases, peroxidase, and catalase in the rice seed during germination . Plant Physiol , 52 : 274 – 277 .
- Rudolph , K. and Stahmann , M.A. 1966 . Multiple hydrolases in bean leaves (Phaseolus vulgaris L.) and the effect of the halo blight disease caused by Pseudomonas phaseolicola (Burkh.) Dowson . Plant Physiol , 41 : 389 – 394 .
- Schopfer , P. , Plachy , C. and Frahry , G. 2001 . Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid . Plant Physiol , 125 : 1591 – 1602 .
- Sebei , K. , Debez , A. , Herchi , W. , Boukhchina , S. and Kallel , H. 2007 . Germination kinetics and seed reserve mobilization in two flax (Linum usitatissimum L.) cultivars under moderate salt stress . J. Plant Biol , 50 : 447 – 454 .
- Sosa , L. , Llanes , A. , Reinoso , H. , Reginato , M. and Luna , V. 2005 . Osmotic and specific ions effect on the germination of Prospis strombulifera . Ann. Bot , 96 : 261 – 267 .
- Staubmann , R. , Ncube , I. , Gübitz , G.M. , Steiner , W. and Read , J.S. 1999 . Esterase and lipase activity in Jatropha curcas L . Seed J. Biotechnol , 75 : 117 – 126 .
- Tobe , K. , Li , X. and Omasa , K. 2004 . Effects of five different salts on seed germination and seedling growth of Haloxylon ammodendron (Chenopodiaceae) . Seed Sci. Res , 4 : 345 – 353 .
- Voigt , E.L. , Almeida , T.D. , Chagas , R.M. , Ponte , L.F.A. , Viégas , R.A. and Silveira , J.A.G. 2009 . Source–sink regulation of cotyledonary reserve mobilization during cashew (Anacardium occidentale) seedling establishment under NaCl salinity . J. Plant Physiol , 169 : 80 – 89 .
- Zarrouk , M. , El Almi , H. , Ben Youssef , N. , Sleimi , N. , Ben Miled , D. , Smaoui , A. and Abdelly , C. 2003 . “ Lipid composition of seeds of local halophyte species: Cakile maritima, Zygophyllum album and Crithmum maritimum ” . In Cash crop halophytes: Recent studies , Edited by: Lieth , H. 121 – 126 . Dordrecht : Kluwer Academic Publisher .