Abstract
The extraction of phenolic compounds from prickly pear seeds was studied. Extraction experiments were carried out by investigating the effect of solvent nature (acetone, ethanol, methanol and water), solvent concentration (25–100%), sample to solvent ratio (0.2/10 to 0.8/10 g/ml), extraction time (30–150 min), and temperature (25–90°C) on the recovery of phenolic compounds and antioxidant activity of the extracts. Total phenolic content was assessed to determine the antioxidant compounds while ferric-reducing power and free radical scavenging activity were used to evaluate the antioxidant activity of prickly pear seed extracts. Experimental results showed that all extraction parameters had significant effects (p < 0.05) on total phenolic content and antioxidant activities of the extracts. The best conditions were: 75% acetone, 0.2 g/10 ml, 90 min, and 90°C with values of 416 mg gallic acid equivalents/100 g for total phenolic content, 237 mg ascorbic acid equivalents/100 g for ferric-reducing power, and 95% for free radical scavenging activity. Ferric-reducing and free radical scavenging potentials were found to be positively significantly correlated with phenolic content under the influence of all extraction parameters.
Résumé
L’extraction des composés phénoliques des graines de figue de barbarie a été étudiée. Les effets de la nature du solvant (acétone, éthanol, méthanol et eau), de la concentration du solvant (25–100%), du rapport échantillon/solvant (0,2/10–0,8/10 g/ml), du temps d’extraction (30–150 min) et de la température (25–90 C) sur l’extraction des composés phénoliques et l’activité anti-oxydante des extraits ont été évalués. La teneur en polyphénols totaux (PT) a été déterminée afin d’estimer la teneur en composés antioxydants, tandis que le pouvoir réducteur du fer (PR) et l’activité antiradicalaire (AAR) ont été utilisés pour évaluer l’activité antioxydante des extraits des graines de figue de barbarie. Les résultats obtenus montrent que les conditions d’extraction ont un effet significatif (p < 0,05) sur la teneur en composés phénoliques et l’activité antioxydante des extraits. Les meilleures conditions d’extraction sont les suivantes : acétone 75%, 0,2 g/10 ml, 90 min et 90 C avec des valeurs de 416 mg équivalent en acide gallique/100 g pour les polyphénols totaux, 237 mg équivalent acide ascorbique/100 g pour le pouvoir réducteur et 95% pour l’activité antiradicalaire. Les résultats montrent que le pouvoir réducteur et l’activité antiradicalaire sont positivement corrélés avec les teneurs en polyphénols totaux.
Introduction
Opuntia ficus-indica (L.) Mill., commonly known as prickly pear, is a tropical or subtropical plant, which belongs to the Cactaceae family and is mainly used for fruit production (De Cotazar and Nobel Citation1992). Originating from Mexico, it was introduced into the Mediterranean countries during the sixteenth century (Griffiths Citation2004; Vignon et al. Citation2004). Most of the 1500 species of Cactaceae are in the genus Opuntia (Hegwood Citation1990). Opuntia cactus pear fruit is a good source of natural antioxidants that can be used in foods and nutritional supplements (Joseph Citation2004). Because of its high level of adaptation to arid and semi-arid environmental conditions and its different uses, the Opuntia ficus-indica fruit is an important raw material for the industry (Habibi et al. Citation2008).
The main studies on Opuntia fruits were concerned with the chemical analysis of pulp, skin and seeds (El-Kossori et al. Citation1998). The seeds constitute about 4–7% of the edible pulp and are usually discarded as waste after extraction of the pulp (Stintzing, Schieber and Carle Citation2001).
Seeds of Opuntia ficus-indica can play a key role in determining the value of the whole fruit. For this reason, different studies have been carried out in the chemical composition of seeds. Their nutritive value was determined by Sawaya, Khaliland Al-Mohammad (Citation1983). Due to low toxicity, phytoproducts from natural sources are preferred by consumers. Consequently, some domains such as the agro-alimentary industry (food preservation) and medicine (treatment of infectious diseases caused by bacteria) are turning back to natural bioactive compounds found in plants (Guinot et al. Citation2010; Alwindy, Citation2012). Antioxidant compounds have been detected in the seeds of numerous plants, such as grapes (Kallithraka et al. Citation1995; Nawaz et al. Citation2006), tomatoes (Toor and Savage Citation2005), citrus fruits (Bocco et al. Citation1998), and red pitaya (Hylocereus polyrhizus) (Adnan, Osman, and HamidCitation2011). Soon and Barlow (Citation2004) have reported that the total phenolic content of seeds of several fruits, such as mango, avocado and jackfruit, was higher than that of their edible portions. However, studies relating to antioxidant activity of prickly pear seeds are limited.
There is no single universal method to develop a procedure suitable for extraction of all plant phenolics because of the complexity of these compounds and the possibility of their interaction with other components present in the plant matrices (Naczk and Shahidi Citation2006). Several factors can influence efficient extraction of phenolic compounds, including extraction method, solvent type and concentration, particle size of plant materials, extraction time and temperature, and solvent to solid ratio (Chirinos et al. Citation2007; Albayrak and Aksoy Citation2010; Stankovic and Topuzokiv Citation2012).
Effect of extraction process can be generally evaluated based on a one factor–one time approach, also known as single experiment, in which only one factor is variable at one time while all others are kept constant.
The availability of phenolic compounds in prickly pear seeds as an antioxidant source is documented. However, no optimal protocols have been established so far for phenolic extraction from these seeds. Hence, the aim of this work was to study the effect of solvent type, solvent concentration, sample/solvent ratio, extraction time and temperature on the recovery of phenolic compounds and antioxidant activities (ferric-reducing power and free radical-scavenging activity) of prickly pear seeds.
Materials and methods
Chemicals
Folin–Ciocalteu reagent was obtained from Biochem, Chemopharma (Montreal, Quebec); gallic acid, acetone (99.78% purity), ethanol (99.5% purity) and methanol (99.7% purity) were obtained from Prolabo (VWR International S.A.S, 94126 Fontenay-sous-Bois cedex, France); sodium carbonate, potassium ferricyanide (⩾ 99.5% purity) were purchased from Biochem, Chemopharma (82 av. du 85eme de Ligne, 58200 Cosne Sur Loire, France). Ferric chloride (⩾ 98.102% purity) was from Panreac (E-08211 Castellar del Vallès, Barcelona, Spain) and 2-2-diphenyl-1-picrylhydrazyl (DPPH) was obtained from Sigma Chemical (Sigma-Aldrich GmbH, Sternheim, Germany).
Biological material
Fresh mature prickly pear fruits (red fruits of spiny cactus, yellow fruits of spiny cactus and yellow fruits of spineless cactus) were collected in August 2010 from the same area (Bousselam, Setif, Algeria). Fruits were selected for uniformity of shape and colour, and were washed carefully, peeled with a knife and blended for a few minutes in a mixer. The seeds were separated from the pulp by washing several times with distilled water. The seeds were then lyophilized (Freeze Drier Alpha 1–4 LD plus; Christ, Osterode am Harz, Germany), ground with a crusher (IKA A 11 B; IKA, Staufen, Germany) and passed through a 500-μm sieve before analysis.
Preparation of extracts
Approximately 0.2 g of the lyophilized prickly pear seeds was weighed and extracted with 10 ml of the extracting solvent in a test tube. The mixture was shaken at a constant rate using a water bath shaker, centrifuged for 15 min at 2250 g (nüve NF 200, Ankara, Turkey), and filtered through filter paper. The filtrates were subsequently used for the determination of total phenolic compounds (TPC) and antioxidant activities: ferric reducing power (FRP) and DPPH free radical-scavenging activity (FRSA).
Effect of solvent nature
By fixing extraction time (60 min) and temperature (25°C) and sample/solvent ratio (0.2 g/10 ml), samples were extracted with 50% [volume/volume (v/v)] acetone, 50% (v/v) ethanol, 50% (v/v) methanol and water.
Effect of solvent concentration
Using the best solvent selected in the first step, samples were extracted with solvent 25%, 50% and 75% (v/v) by fixing the extraction time, temperature and sample/solvent ratio at 60 min, 25°C, and 0.2 g/10 ml, respectively.
Effect of sample/solvent ratio
Samples were extracted using the best solvent and the best solvent concentration. The extraction procedure was repeated by varying the sample/solvent ratio 0.2/10, 0.4/10, 0.6/10, and 0.8/10 g/ml, while fixing the extraction time and temperature at 60 min and 25°C.
Effect of extraction time
Samples were extracted using the best solvent, solvent concentration and sample/solvent ratio. The extracts were prepared by varying the extraction time from 30 min to 150 min (30, 60, 90, 120 and 150 min) while fixing the extraction temperature at 25°C.
Effect of extraction temperature
Using the best solvent, solvent concentration, sample/solvent ratio and extraction time, the samples were extracted at temperatures from 25 to 90°C (25, 40, 55, 70 and 90°C).
The best extraction conditions studied were selected according to the value of tree responses (TPC, FRP and FRSA).
Determination of total phenolic content (TPC)
TPC was determined using Folin–Ciocalteu reagent according to the method described by Singleton and Rossi (Citation1965), 150 μl of sample were mixed with 750 μl of Folin–Ciocalteu reagent and 600 μl of 7.5% sodium carbonate. The test tube contents were mixed and allowed to stand in the dark at room temperature for 30 min. Absorbance was measured against the blank at 750 nm with a spectrophotometer (Shimadzu Uvi-mini1240, Suzhou Jiangsu, China); TPC were expressed as mg gallic acid equivalents, GAE/100 g.
Antioxidant activity
Ferric reducing power (FRP)
The reducing power of the extracts was measured according to the method of Oyaizu (Citation1986); 1 ml of sample was mixed with 1 ml of phosphate buffer (0.2 m, pH 6.6) and 1 ml of 1% potassium ferricyanide. The mixture was incubated for 20 min. at 50°C. After incubation, 1 ml of 10% trichloroacetic acid was added to the mixture and centrifuged for 10 min at 1700 g; 1 ml of supernatant was mixed with 1 ml of distilled water and 0.2 ml of 0.1% ferric chloride. The absorbance was measured at 700 nm against the blank. Ascorbic acid was used as a standard and reducing power was expressed as ascorbic acid equivalents, mg AAE/100 g.
DPPH free radical-scavenging activity (FRSA)
The DPPH radical scavenging activity of the prickly pear seed extracts was measured according to the procedure described by Brand-Williams, Cuvelier and Berset (Citation1995); 100 μl of extract was added to 1 ml of DPPH solution. The decrease in absorbance was determined at 517 nm, after incubation for 30 min in the dark. DPPH radical solution without sample was used as a control and the DPPH percentage inhibition was calculated according to the following equation: FRSA% = [Ac – As / Ac] × 100, where Ac is the absorbance of the control, and As is the absorbance of the sample.
Statistical analyses
All data were reported as means of three replicates. The analysis of the variance at p < 0.05 was calculated using STATISTICA 5.5 to determine significant differences between the results.
Results
Calibration curves of gallic acid and ascorbic acid were constructed to measure the amount of phenolic compounds and FRP in the prickly pear seeds. Calibration equations were y = 5.59x (R 2 = 0.999) for gallic acid and y = 11.17x (R 2 = 0.996) for ascorbic acid.
Solvent type
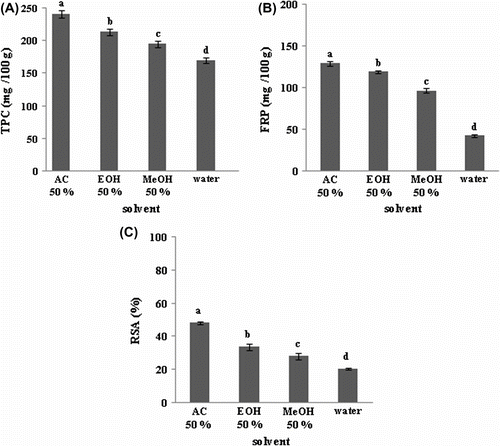
All solvents used in the present work were capable of extracting phenolic compounds (Figure ). Acetone extracts showed the highest values of TPC, FRP and FRSA (240.90 mg GAE/100 g, 129.21 mg AAE/100 g, and 48.20% respectively), followed by ethanol, methanol and water extracts under the same extraction conditions (50% for each solvent, 60 min at 25 °C). Solvent type had a significant influence (p < 0.05) on TPC and antioxidant activities FRP and FRSA.
Figure 1 Effect of the solvent type on the extraction of total phenolic compounds (A), ferric reducing power (B), and DPPH radical-scavenging activity (C) of prickly pear seeds.Figure 1. Effet du type de solvant sur la teneur en composés phénoliques (A), le pouvoir réducteur du fer (B) et l’activité antiradicalaire (C) des graines de figue de barbarie.
Solvent concentration
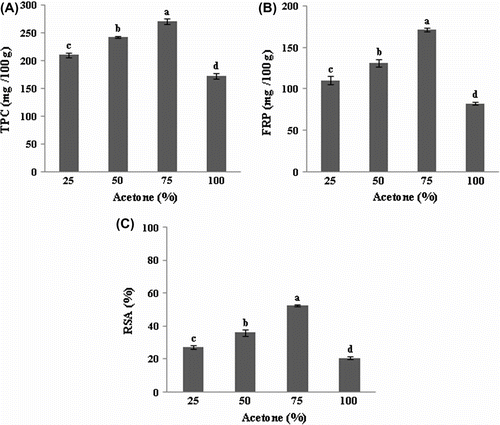
Aqueous acetone at different concentrations (25%, 50%, 75% and 100%) was employed as the extracting solvent in the present study. Acetone concentration had a significant effect (p < 0.05) on TPC and antioxidant activities FRP and FRSA of prickly pear seeds (Figure ). Antioxidant compounds in and antioxidant activity of the prickly pear seed extract increased according to the proportion of acetone in the extracting solvent; the highest value was obtained at 75% acetone with 270.61 mg GAE/100 g, 171.65 mg AAE/100 g and 52.70% for TPC, FRP and FRSA, respectively. Hence, 75% acetone was used for the determination of the effects of sample to solvent ratio, extraction time and extraction temperature on TPC, FRP and FRSA.
Figure 2 Effect of the solvent concentration on the extraction of total phenolics(A), ferric reducing power (B) and DPPH radical-scavenging activity (C) of prickly pear seeds.Figure 2. Effet de la concentration du solvant sur la teneur en composés phénoliques (A), le pouvoir réducteur du fer (B) et l’activité antiradicalaire (C) des graines de figue de barbarie.
Effect of sample/solvent ratio
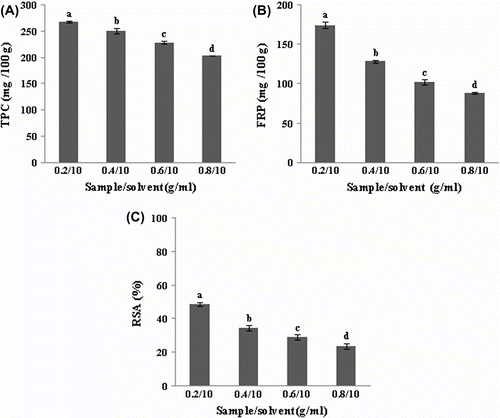
The total phenolic content and antioxidant activity of prickly pear seeds extracted by 75% acetone using four sample/solvent ratios: 0.2/10, 0.4/10, 0.6/10 and 0.8/10 g/ml are shown in Figure . Sample/solvent ratio had a significant effect (p < 0.05) on TPC and the antioxidant activities FRP and FRSA. TPC, FRP and DPPH radical-scavenging capacity decreased from 268.30 to 205.50 mg GAE/100 g, from 174.21 to 88.08 mg AAE/100 g and from 48.44 to 23.28%, respectively, with the increase of sample/solvent ratio from 0.2/10 to 0.8/10.
Figure 3 Effect of sample/solvent ratio on the extraction of total phenolics (A), ferric reducing power (B) and DPPH radical-scavenging activity (C) of prickly pear seeds.Figure 3. Effet du rapport échantillon/solvant sur la teneur en composés phénoliques (A), le pouvoir réducteur du fer (B) et l’activité antiradicalaire (C) des graines de figue de barbarie.
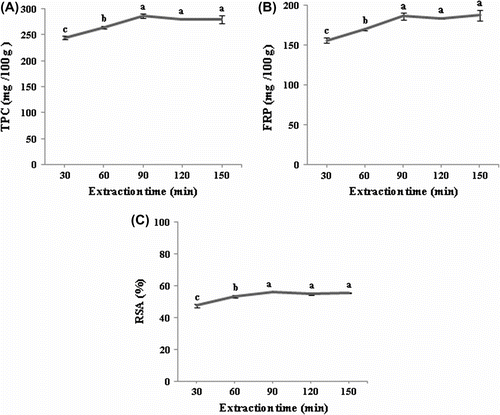
Effect of extraction time
Extraction time had a significant effect (p < 0.05) on TPC, FRP and radical scavenging activity (Figure ) of prickly pear seeds. TPC, FRP and FRSA increased when extraction time was increased from 30 to 90 min. After 90 min, further increase in process duration did not significantly (p < 0.05) improve the recovery of phenolics and antioxidant activities.
Figure 4 Effect of time on the extraction of the total phenolic compound (A), ferric reducing power (B) and DPPH radical-scavenging activity (C) of prickly pear seeds.Figure 4. Effet du temps d’extraction sur la teneur en composés phénoliques (A), le pouvoir réducteur du fer (B) et l’activité antiradicalaire (C) des graines de figue de barbarie.
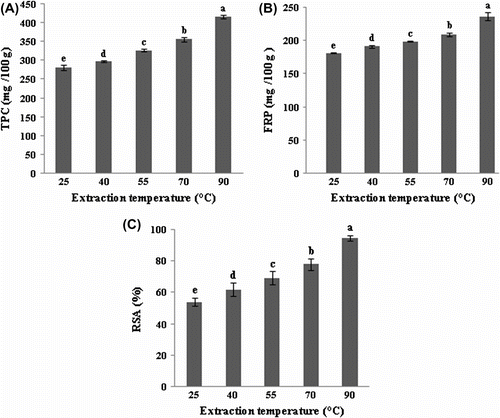
Effect of extraction temperature
As depicted in Figure , extraction temperature demonstrated a significant effect (p < 0.05) on phenolic content and antioxidant activities of prickly pear seed extracts. With the increase of temperature from 25 to 90°C, TPC, FRP and DPPH radical-scavenging capacity increased from 281.02 to 415.87 mg GAE/100 g, from 181.60 to 236.53 mg AAE/100 g and from 53.87 to 94.53%, respectively.
Figure 5 Effect of temperature on the extraction of total phenolics (A), ferric reducing power (B) and DPPH radical-scavenging activity (C) of prickly pear seeds.Figure 5. Effet de la température sur l’extraction des composés phénoliques (A), le pouvoir réducteur du fer (B) et l’activité antiradicalaire (C) des graines de figue de barbarie.
Discussion
Comparative studies have been carried out to establish the extractive efficiency of various solvents. As shown previously, acetone was the best extraction solvent. Similar results have been obtained by Amarowicz, Karamac and Chavan (Citation2001); the acetone extract of lentil seeds contained more phenolics than either methanol or ethanol extracts of lentil seeds. Furthermore, acetone allows the highest extraction of phenolic compounds from barley seeds (Liu and Yao Citation2007). Al-Farsi and Lee (Citation2008) also reported that 50% acetone was the most efficient solvent for phenolic extraction from date seeds. Solubility of phenolics is affected by the polarity of the solvent used for the extraction (Naczk and Shahidi Citation2004). According to Abaza et al. (Citation2011), extracting solvents have a significant effect on the antioxidant properties of olive leaves and the lowest radical scavenging activity is obtained in the aqueous extract.
Concerning the effect of solvent concentration, the highest TPC and antioxidant activities were obtained with 75% acetone. The lowest value was obtained with 100% acetone, indicating that combination of acetone with water was better than pure acetone. According to Turkmen, Sari and Velioglu (Citation2006) and Grujic et al. (Citation2012), mixtures of organic solvent and water have been revealed to be more efficient in extracting phenolic compounds than mono-component solvents. Turkmen, Sari and Velioglu (Citation2006) showed that higher content of polyphenols was obtained in black and mate tea extracts with an increase in polarity of the solvent used.
The increasing values of TPC and antioxidant activities resulting from the increase of sample/solvent ratio from 0.2/10 to 0.8/10, is consistent with mass transfer principles; the driving force during mass transfer is the concentration gradient between the sample and the solvent, which is greatest when a small sample/solvent ratio is used. Fan et al. (Citation2008) reported that a solid/liquid ratio of 1/32 is the best for extraction of antioxidant compounds from purple sweet potato. In the present study, a ratio of 0.2 g/10 ml was selected as the best for phenolics extraction and antioxidant activities.
The results showed a significant effect of extraction time on TPC and antioxidant activities of the extracts. Indeed, the values were increased for an extraction time from 30 to 90 min. A similar trend was shown by Hismath, Wan Aida and Ho (Citation2011) in the extraction of phenolic compounds from neem (Azadirachta indica) leaves. This observation was well explained by Fick’s second law of diffusion, which predicts a final equilibrium between the solute concentrations in the solid matrix (plant matrix) and in the bulk solution (solvent) after a certain time (Silva, Rogez, and Larondelle Citation2007). Our results revealed that an excessive time is not useful to extract more phenolic compounds from prickly pear seeds.
Our results showed that 90°C is the best extraction temperature for the phenolic compounds from prickly pear seeds. According to Gertenbach (Citation2001), the solubility of phenolic compounds increased with extraction temperature, in relation to the mass transfer rate (diffusion coefficient). Generally, a high temperature improves solubility and extraction of phenolic compounds into the solvent. Elevation of temperature would reduce the solvent viscosity, allowing better penetration of matrix particles and hence enhancing the extraction (Richter et al. Citation1996). According to Ju and Howard (Citation2003), the highest total phenolic content and antioxidant capacity of grape skin extract were obtained by extraction at 80–100°C, whereas the optimum temperature for phenolic extraction from black cohosh (Cimicifuga racemosa) was 90°C (Makhopadhyay, Luthria and Robbins Citation2006). Based on the results obtained with the effect of extraction temperature, we deduced that the phenolic compounds present in prickly pear seed extracts were heat stable. However, Chew et al. (Citation2011) reported a positive relationship between total phenolic content and extraction temperature and a negative relationship between antioxidant activity and extraction temperature. It should be noted that increasing temperature beyond a certain value can lead to decomposition of some phenolic compounds (Hismath, Wan Aida and Ho Citation2011).
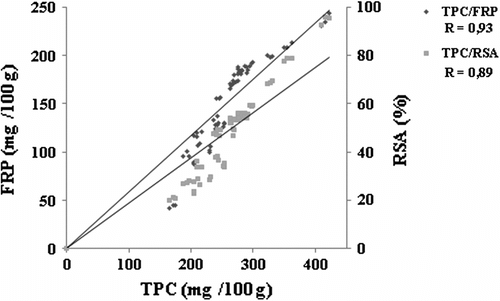
Figure showed the correlation between the phenolic content and the antioxidant activities of prickly pear seed extracts. Positive correlation was noted between the TPC and antioxidant activities FRP and FRSA of prickly pear seed extracts under different extraction parameters. This relationship suggested that the phenolic compounds of prickly pear seed extracts might be the major contributors to the analysed antioxidant activities.
Conclusions
The results of the present investigation demonstrated that all the extraction parameters (solvent nature and concentration, sample/solvent ratio, extraction time and temperature) exhibited significant effects (p < 0.05) on the extraction efficiency of TPC and the antioxidant activities FRP and FRSA of prickly pear seed extracts. It can be concluded that the best conditions for phenolic extraction and antioxidant activity were acetone 75%, sample/solvent ratio 0.2 g/10 ml, extraction time 90 min, and extraction temperature 90°C with values of 416 mg GAE/100 g, 237 mg AAE/100 g and 95% for TPC, FRP and FRSA, respectively. Under the influence of each extraction parameter, TPC contents were positively correlated with antioxidant activities FRP and FRSA. Also, these results revealed that phenolic compounds of prickly pear seed extracts kept their antioxidant activities at high temperature. Further quantitative study is needed to analyse bioactive phenolics in prickly pear seed extracts and to evaluate the contribution of each compound to the antioxidant activity, for their applications in vegetable oils, animal fats and functional foods or nutraceuticals.
References
- Abaza , L. , Ben Youssef , N. , Manai , H. , Hadada , F. M. , Methenni , K. and Zarrouk , M. 2011 . Chétoui olive leaf extracts: influence of the solvent type on phenolics and antioxidant activities . Grasas Y Aceites , 62 : 96 – 104 .
- Adnan , L. , Osman , A. and Hamid , A. A. 2011 . Antioxidant activity of different extracts of red pitaya (Hylocereus polyrhizus) seed . International Journal of Food Properties , 14 : 1171 – 1181 .
- Albayrak , B. S. and Aksoy , A. 2010 . In vitro antioxidant and antimicrobial properties of Paronychia mughlaei Chaudhri . Acta Botanica Gallica , 157 : 411 – 418 .
- Al-Farsi , M. A. and Lee , C. Y. 2008 . Optimization of phenolics and dietary fibre extraction from date seeds . Food Chemistry , 108 : 977 – 985 .
- Alwindy , S. A. 2012 . In vitro consideration the antibacterial activity of Pimpinella anisum . Internationale Pharmaceutica Sciencia , 2 : 92 – 96 .
- Amarowicz , R. , Karamac , M. and Chavan , U. 2001 . Influence of the extraction procedure on the anti-oxidative activity of lentil seed extracts in a β-carotene-linoleate model system . Grasas Y Aceites , 52 : 89 – 93 .
- Bocco , A. , Cuvelier , M. E. , Richard , H. and Berset , C. 1998 . Antioxidant activity and phenolic composition of citrus peel and seed extracts . Journal of Agriculture and Food Chemistry , 46 : 2123 – 2129 .
- Brand-Williams, W., M. E. Cuvelier, and C. Berset. 1995. Use of a Free Radical Method to Evaluate Antioxidant activity. Lebensmittel-Wissenschaft und Technologie 28:25–30.
- Chew, K. K., M. Z. Khoo, S. Y. Ng, Y. Y. Thoo, W. M. Wan Aida, and C. W. Ho. 2011. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. International Food Research Journal 18:1427–1435.
- Chirinos , R. , Rogez , H. , Campos , D. , Pedreschi , R. and Larondelle , Y. 2007 . Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruız & Pavon) tubers . Separation and Purification Technology , 55 : 217 – 225 .
- De Cotazar , V. G. and Nobel , P. S. 1992 . Biomass and Fruit Production for the Prickly Pear Cactus Opuntia ficus-indica . Journal of the American Society for Horticultural Science , 117 : 558 – 562 .
- El-Kossori , R. L. , Villaume , C. , Sauvaire , E. and Mejean , L. 1998 . Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.) . Plant Foods for Human Nutrition , 52 : 263 – 270 .
- Fan, G., Y. Han, Z. Gu, and D. Chen. 2008. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface, methodology (RSM). Lebensmittel-Wissenschaft und Technologie 41: 155–160.
- Gertenbach, D. D. 2001. Solid–liquid extraction technologies for manufacturing nutraceuticals from botanicals. In Functional Foods Biochemical and Processing Aspects, edited by J. Shi, G. Mazza, and M. Le Mague, 331–366 Boca Raton, London, New York, Washington, DC: CRC Press.
- Griffiths , P. 2004 . The origins of an important cactus crop Opuntia ficus-indica (Cactaceae) new molecular evidence . American Journal of Botany , 91 : 1915 – 1921 .
- Grujic , N. , Lepojevic , Z. , Srdjenovic , B. , Vladic , J. and Sudji , J. 2012 . Effects of different extraction methods and conditions on the phenolic composition of mate tea extracts . Molecules , 17 : 2518 – 2528 .
- Guinot , P. , Lemoine , A. , Joss , E. M. , Pelegrin , S. , Gargadennec , A. , Rapior , S. and Poucheret , P. 2010 . Evaluation of antioxidant and antiproliferative activities of dyeing plants . Acta Botanica Gallica , 157 : 37 – 43 .
- Habibi , Y. , Heux , L. , Mahrouz , M. and Vignon , R. M. 2008 . Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits . Carbohydrate Polymers , 72 : 102 – 112 .
- Hegwood , D. A. 1990 . Human Health Discoveries with Opuntia sp. (Prickly Pear) . Journal of Horticultural Science , 25 : 1515 – 1516 .
- Hismath, I., W. M. Wan Aida, and C. W. Ho. 2011. Optimization of extraction conditions for phenolic compounds from neem (Azadirachta indica) leaves. International Food Research Journal 18:931–939.
- Joseph , O. K. 2004 . Antioxidant compounds from four Opuntia cactus pear fruit varieties . Food Chemistry , 85 : 527 – 533 .
- Ju , Z. Y. and Howard , L. R. 2003 . Effects of the solvents and temperature on pressurized extraction of anthocyanins and total phenolics from dried red grape skin . Journal of Agriculture and Food Chemistry , 51 : 5207 – 5213 .
- Kallithraka , S. , Garca-Viguera , C. , Bridle , P. and Bakker , J. 1995 . Survey of solvent for the extraction of grape seed phenolics . Phytochemical Analysis , 6 : 265 – 267 .
- Liu , Q. and Yao , H. Y. 2007 . Antioxidant activities of barley seeds extracts . Food Chemistry , 102 : 732 – 737 .
- Makhopadhyay , S. , Luthria , D. L. and Robbins , D. J. 2006 . Optimization of extraction process for phenolic acids from black cohosh (Cimicifuga racemosa) by pressurized liquid extraction . Journal of the Science of Food and Agriculture , 86 : 156 – 162 .
- Naczk , M. and Shahidi , F. 2004 . Extraction and analysis of phenolics in food . Journal of Chromatography A , 1054 : 95 – 111 .
- Naczk , M. and Shahidi , F. 2006 . Phenolics in cereals, fruits and vegetables Occurrence, extraction and analysis . Journal of Pharmaceutical and Biomedical Analysis , 41 : 1523 – 1542 .
- Nawaz , H. , Shi , J. , Mittal , G. S. and Kakuda , Y. 2006 . Extraction of polyphenols from grape seeds and concentration by ultrafiltration . Separation and Purification Technology , 48 : 176 – 181 .
- Oyaizu , M. 1986 . Studies on products of browning reactions antioxidative activities of products of browning reaction prepared from glucosamine . Japanese Journal of Nutrition , 44 : 307 – 325 .
- Richter , B. E. , Jones , B. A. , Ezzell , J. L. , Poter , N. L. , Avdalovic , N. and Pohl , C. 1996 . Accelerated solvent extraction: a technique for sample preparation . Analytical Chemistry , 68 : 1033 – 1039 .
- Sawaya, W. N., J. K. Khalil, and M. N. Al-Mohammad.1983. Nutritive value prickly pears seeds, Opuntia ficus-indica. Qualitas Plantarum Plant Foods for Human Nutrition 33:91–97.
- Silva , E. M. , Rogez , H. and Larondelle , Y. 2007 . Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology . Separation and Purification Technology , 55 : 381 – 387 .
- Singleton , V. L. and Rossi , J. A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent . Americal Journal Enology and Viticulture , 16 : 144 – 158 .
- Soon , Y. and Barlow , P. J. 2004 . Antioxidant activity and phenolic content of selected fruit seeds . Food Chemistry , 88 : 411 – 417 .
- Stankovic, M. S., and M. D. Topuzokiv. 2012. In vitro antioxidant activity of extracts from leaves and fruits of common dogwood (Cornus sanguinea L.). Acta Botanica Gallica 159:79–83.
- Stintzing , F. C. , Schieber , A. and Carle , R. 2001 . Phytochemical and nutritional significance of cactus pear . European Food Research and Technology , 212 : 396 – 407 .
- Toor , R. K. and Savage , G. P. 2005 . Antioxidant activity in different fractions of tomatoes . Food Research International , 38 : 487 – 494 .
- Turkmen , N. , Sari , F. and Velioglu , Y. S. 2006 . Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods . Food Chemistry , 99 : 835 – 841 .
- Vignon , M. R. , Heux , L. , Malainine , M. E. and Mahrouz , M. 2004 . Arabinan–cellulose composite in Opuntia ficus-indica prickly pear spines . Carbohydrate Research , 339 : 123 – 131 .